Influence of Cobalt Source, Folic Acid, and Rumen-Protected Methionine on Performance, Metabolism, and Liver Tissue One-Carbon Metabolism Biomarkers in Peripartal Holstein Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management and Experimental Design
2.2. Feed and Milk Sampling
2.3. Blood Collection and Analysis
2.4. Liver Biopsy and Tissue Analyses
2.5. Statistical Analysis
3. Results and Discussion
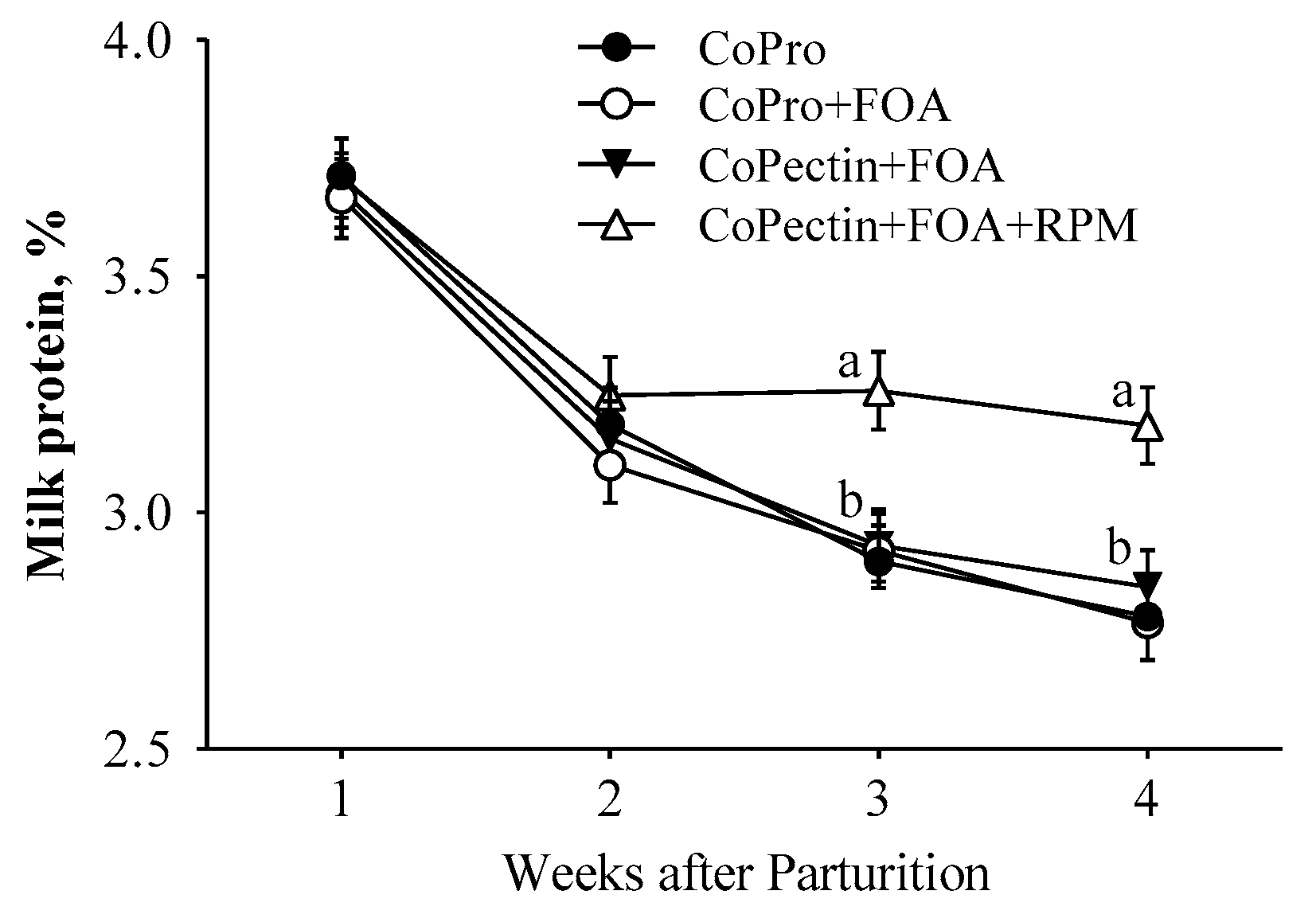
3.1. Dry Matter Intake and Milk Production
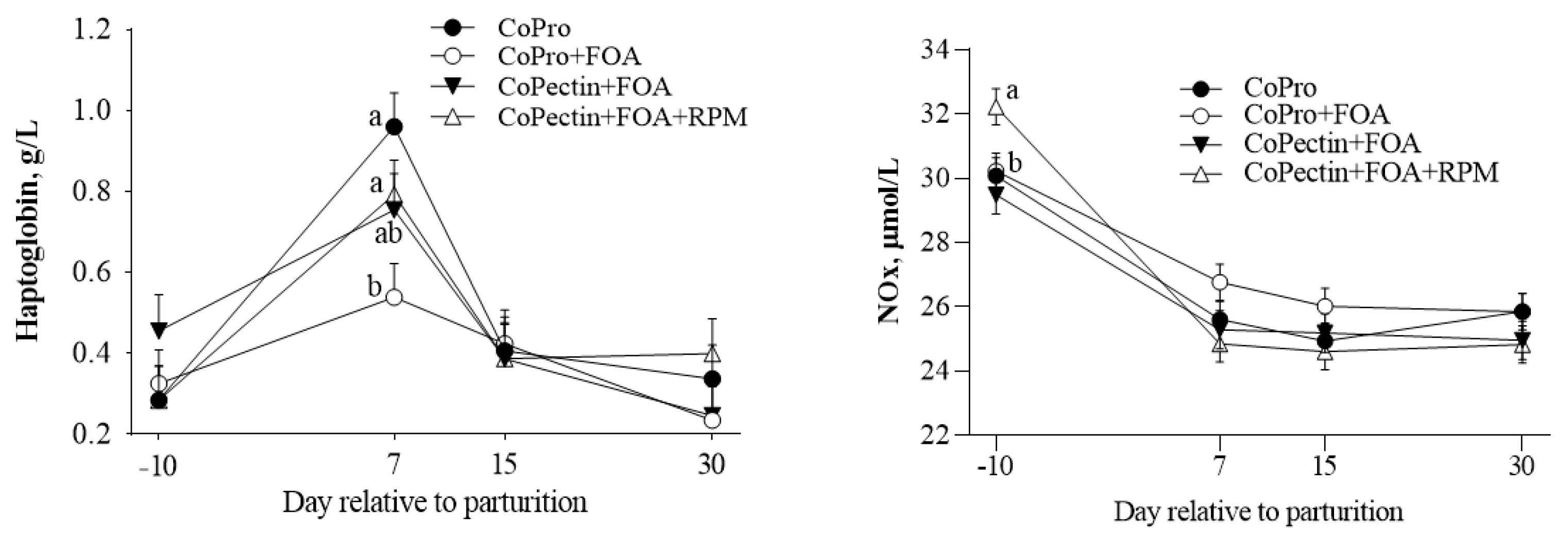
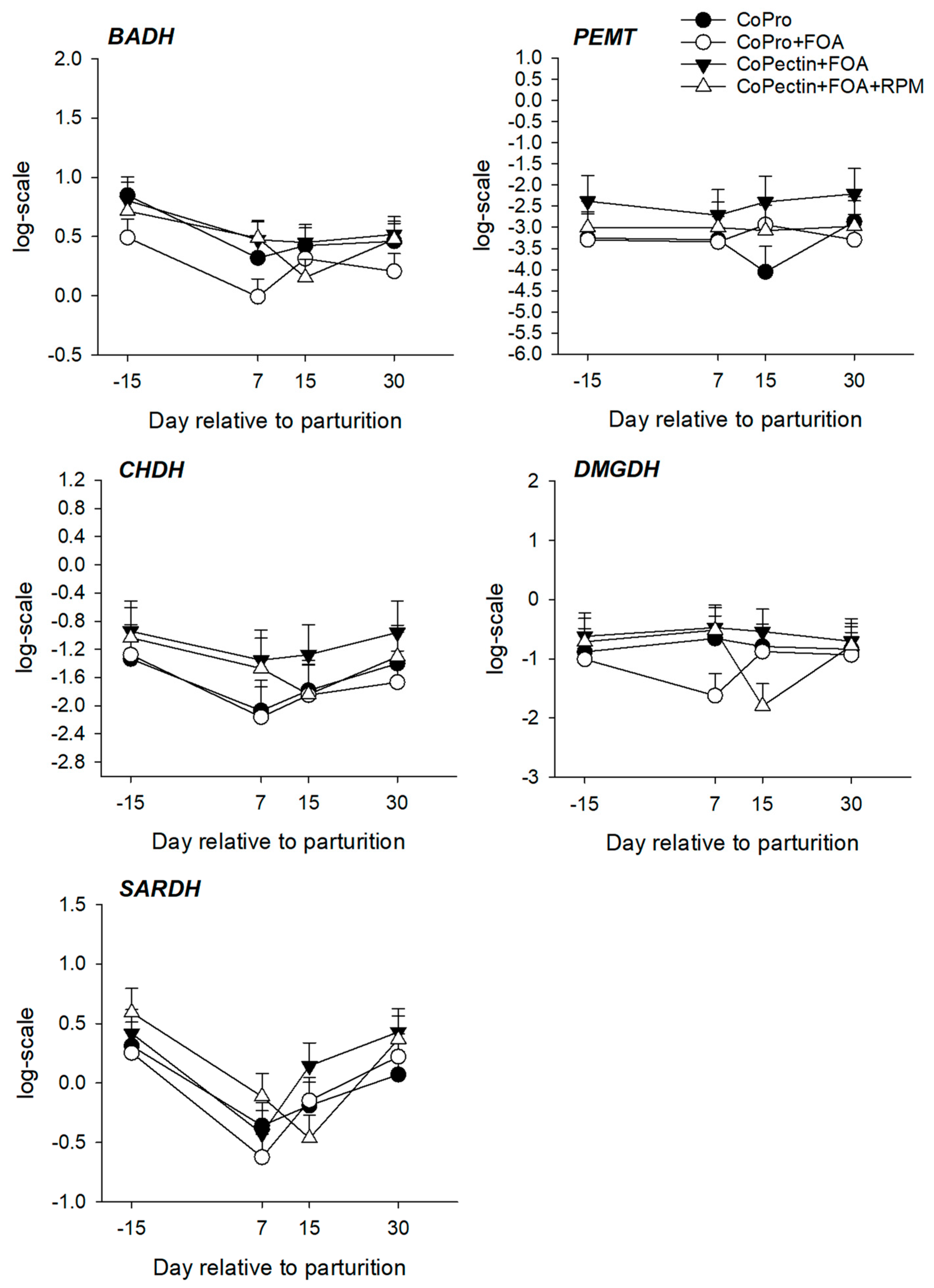
3.2. Blood Biomarkers and the Hepatic One-Carbon Metabolism Pathway
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batistel, F.; Arroyo, J.M.; Garces, C.I.M.; Trevisi, E.; Parys, C.; Ballou, M.A.; Cardoso, F.C.; Loor, J.J. Ethyl-cellulose rumen-protected methionine alleviates inflammation and oxidative stress and improves neutrophil function during the periparturient period and early lactation in Holstein dairy cows. J. Dairy Sci. 2017, 101, 480–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Bulgari, O.; Vailati-Riboni, M.; Trevisi, E.; Ballou, M.A.; Cardoso, F.C.; Luchini, D.N.; Loor, J.J. Rumen-protected methionine compared with rumen-protected choline improves immunometabolic status in dairy cows during the peripartal period. J. Dairy Sci. 2016, 99, 8956–8969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio, J.S.; Ji, P.; Drackley, J.K.; Luchini, D.; Loor, J.J. Supplemental Smartamine M or MetaSmart during the transition period benefits postpartal cow performance and blood neutrophil function. J. Dairy Sci. 2013, 96, 6248–6263. [Google Scholar] [CrossRef] [Green Version]
- Lopreiato, V.; Mezzetti, M.; Cattaneo, L.; Ferronato, G.; Minuti, A.; Trevisi, E. Role of nutraceuticals during the transition period of dairy cows: A review. J. Anim. Sci. Biotechnol. 2020, 11, 96. [Google Scholar] [CrossRef]
- McFadden, J.W.; Girard, C.L.; Tao, S.; Zhou, Z.; Bernard, J.K.; Duplessis, M.; White, H.M. Symposium review: One-carbon metabolism and methyl donor nutrition in the dairy cow. J. Dairy Sci. 2020, 103, 5668–5683. [Google Scholar] [CrossRef]
- Coleman, D.N.; Alharthi, A.S.; Liang, Y.; Lopes, M.G.; Lopreiato, V.; Vailati-Riboni, M.; Loor, J.J. Multifaceted role of one-carbon metabolism on immunometabolic control and growth during pregnancy, lactation and the neonatal period in dairy cattle. J. Anim. Sci. Biotechnol. 2021, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L.R. Vitamins in Animal and Human Nutrition; Wiley: New York, NY, USA, 2000; ISBN 9780813826301. [Google Scholar]
- Smith, R.M.; Marston, L.H.R. Production, absorption, distribution and excretion of vitamin B 12 in sheep. Br. J. Nutr. 1970, 24, 857–877. [Google Scholar] [CrossRef] [Green Version]
- National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Dairy Cattle; National Academies Press: Washington, DC, USA, 2021; ISBN 978-0-309-67777-6. [Google Scholar]
- Tiffany, M.E.; Spears, J.W.; Xi, L.; Horton, J. Influence of dietary cobalt source and concentration on performance, vitamin B12 status, and ruminal and plasma metabolites in growing and finishing steers1, 2. J. Anim. Sci. 2003, 81, 3151–3159. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, M.E.; Fellner, V.; Spears, J.W. Influence of cobalt concentration on vitamin B12 production and fermentation of mixed ruminal microorganisms grown in continuous culture flow-through fermentors1. J. Anim. Sci. 2006, 84, 635–640. [Google Scholar] [CrossRef]
- Kincaid, R.L.; Lefebvre, L.E.; Cronrath, J.D.; Socha, M.T.; Johnson, A.B. Effect of Dietary Cobalt Supplementation on Cobalt Metabolism and Performance of Dairy Cattle. J. Dairy Sci. 2003, 86, 1405–1414. [Google Scholar] [CrossRef]
- Akins, M.S.; Bertics, S.J.; Socha, M.T.; Shaver, R.D. Effects of cobalt supplementation and vitamin B12 injections on lactation performance and metabolism of Holstein dairy cows. J. Dairy Sci. 2013, 96, 1755–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.M. Folate and vitamin B 12. Proc. Nutr. Soc. 1999, 58, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Xue, G.P.; Snoswell, A.M. Regulation of methyl group metabolism in lactating ewes. Biochem. Int. 1985, 11, 381–385. [Google Scholar]
- Graulet, B.; Matte, J.J.; Desrochers, A.; Doepel, L.; Palin, M.-F.; Girard, C.L. Effects of Dietary Supplements of Folic Acid and Vitamin B12 on Metabolism of Dairy Cows in Early Lactation. J. Dairy Sci. 2007, 90, 3442–3455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girard, C.L.; Matte, J.J. Dietary Supplements of Folic Acid During Lactation: Effects on the Performance of Dairy Cows. J. Dairy Sci. 1998, 81, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Drackley, J.K. Biology of Dairy Cows During the Transition Period: The Final Frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Fiore, E.; Perillo, L.; Piccione, G.; Gianesella, M.; Bedin, S.; Armato, L.; Giudice, E.; Morgante, M. Effect of combined acetylmethionine, cyanocobalamin and α-lipoic acid on hepatic metabolism in high-yielding dairy cow. J. Dairy Res. 2016, 83, 438–441. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle, 7th Revised ed.; National Academies Press: Washington, DC, USA, 2001; ISBN 0-309-51521-1. [Google Scholar]
- Lopreiato, V.; Ghaffari, M.H.; Cattaneo, L.; Ferronato, G.; Alharthi, A.S.; Piccioli-Cappelli, F.; Loor, J.J.; Trevisi, E.; Minuti, A. Suitability of rumination time during the first week after calving for detecting metabolic status and lactation performance in simmental dairy cows: A cluster-analytic approach. Ital. J. Anim. Sci. 2021, 20, 1909–1923. [Google Scholar] [CrossRef]
- Bionaz, M.; Trevisi, E.; Calamari, L.; Librandi, F.; Ferrari, A.; Bertoni, G. Plasma paraoxonase, health, inflammatory conditions, and liver function in transition dairy cows. J. Dairy Sci. 2007, 90, 1740–1750. [Google Scholar] [CrossRef] [Green Version]
- Trevisi, E.; Amadori, M.; Cogrossi, S.; Razzuoli, E.; Bertoni, G. Metabolic stress and inflammatory response in high-yielding, periparturient dairy cows. Res. Vet. Sci. 2012, 93, 695–704. [Google Scholar] [CrossRef]
- Zhou, Z.; Vailati-Riboni, M.; Trevisi, E.; Drackley, J.K.; Luchini, D.N.; Loor, J.J. Better postpartal performance in dairy cows supplemented with rumen-protected methionine compared with choline during the peripartal period. J. Dairy Sci. 2016, 99, 8716–8732. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, K.; Lopreiato, V.; Liang, Y.; Trevisi, E.; Osorio, J.S.; Xu, C.; Loor, J.J. Hepatic metabolomics and transcriptomics to study susceptibility to ketosis in response to prepartal nutritional management. J. Anim. Sci. Biotechnol. 2019, 10, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, D.N.; Alharthi, A.; Lopreiato, V.; Trevisi, E.; Miura, M.; Pan, Y.-X.; Loor, J.J. Choline supply during negative nutrient balance alters hepatic cystathionine β-synthase, intermediates of the methionine cycle and transsulfuration pathway, and liver function in Holstein cows. J. Dairy Sci. 2019, 102, 8319–8331. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Garrow, T.A.; Dong, X.; Luchini, D.N.; Loor, J.J. Hepatic Activity and Transcription of Betaine-Homocysteine Methyltransferase, Methionine Synthase, and Cystathionine Synthase in Periparturient Dairy Cows Are Altered to Different Extents by Supply of Methionine and Choline. J. Nutr. 2017, 147, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Girard, C.L.; Lapierre, H.; Matte, J.J.; Lobley, G.E. Effects of Dietary Supplements of Folic Acid and Rumen-Protected Methionine on Lactational Performance and Folate Metabolism of Dairy Cows. J. Dairy Sci. 2005, 88, 660–670. [Google Scholar] [CrossRef]
- Batistel, F.; Arroyo, J.M.; Bellingeri, A.; Wang, L.; Saremi, B.; Parys, C.; Trevisi, E.; Cardoso, F.C.; Loor, J.J. Ethyl-cellulose rumen-protected methionine enhances performance during the periparturient period and early lactation in Holstein dairy cows. J. Dairy Sci. 2017, 100, 7455–7467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patton, R.A. Effect of rumen-protected methionine on feed intake, milk production, true milk protein concentration, and true milk protein yield, and the factors that influence these effects: A meta-analysis. J. Dairy Sci. 2010, 93, 2105–2118. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Loor, J.J. Gene Networks Driving Bovine Mammary Protein Synthesis during the Lactation cycle. Bioinform. Biol. Insights 2011, 5, BBI.S7003. [Google Scholar] [CrossRef]
- Wang, F.; van Baal, J.; Ma, L.; Loor, J.J.; Wu, Z.L.; Dijkstra, J.; Bu, D.P. Short communication: Relationship between lysine/methionine ratios and glucose levels and their effects on casein synthesis via activation of the mechanistic target of rapamycin signaling pathway in bovine mammary epithelial cells. J. Dairy Sci. 2019, 102, 8127–8133. [Google Scholar] [CrossRef]
- Jones, P.; Lucock, M.; Scarlett, C.J.; Veysey, M.; Beckett, E.L. Folate and Inflammation—Links between folate and features of inflammatory conditions. J. Nutr. Intermed. Metab. 2019, 18, 100104. [Google Scholar] [CrossRef]
- Zhou, Z.; Vailati-Riboni, M.; Luchini, D.N.; Loor, J.J. Methionine and choline supply during the periparturient period alter plasma amino acid and one-carbon metabolism profiles to various extents: Potential role in hepatic metabolism and antioxidant status. Nutrients 2016, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Fiore, E.; Lisuzzo, A.; Laghi, L.; Harvatine, K.J.; Mazzotta, E.; Alterisio, M.C.; Ciaramella, P.; Zhu, C.; Contiero, B.; Faillace, V.; et al. Serum metabolomics assessment of etiological processes predisposing ketosis in water buffalo during early lactation. J. Dairy Sci. 2023, 106, 3465–3476. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.S.; Trevisi, E.; Li, C.; Drackley, J.K.; Socha, M.T.; Loor, J.J. Supplementing Zn, Mn, and Cu from amino acid complexes and Co from cobalt glucoheptonate during the peripartal period benefits postpartal cow performance and blood neutrophil function. J. Dairy Sci. 2016, 99, 1868–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio, J.S.; Trevisi, E.; Ji, P.; Drackley, J.K.; Luchini, D.; Bertoni, G.; Loor, J.J. Biomarkers of inflammation, metabolism, and oxidative stress in blood, liver, and milk reveal a better immunometabolic status in peripartal cows supplemented with Smartamine M or MetaSmart. J. Dairy Sci. 2014, 97, 7437–7450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Jimenez, S.; Haerr, K.J.; Trevisi, E.; Loor, J.J.; Cardoso, F.C.; Osorio, J.S. Prepartal standing behavior as a parameter for early detection of postpartal subclinical ketosis associated with inflammation and liver function biomarkers in peripartal dairy cows. J. Dairy Sci. 2018, 101, 8224–8235. [Google Scholar] [CrossRef]
- Preynat, A.; Lapierre, H.; Thivierge, M.C.; Palin, M.F.; Matte, J.J.; Desrochers, A.; Girard, C.L. Effects of supplements of folic acid, vitamin B12, and rumen-protected methionine on whole body metabolism of methionine and glucose in lactating dairy cows. J. Dairy Sci. 2009, 92, 677–689. [Google Scholar] [CrossRef] [Green Version]



| Diets | ||
|---|---|---|
| Item | Close-Up | Lactation |
| Ingredient (% of DM) | ||
| Corn silage | 37.47 | 41.12 |
| Ground shelled corn | 11.60 | 23.95 |
| Wheat straw | 24.00 | 2.30 |
| Canola meal | 11.67 | 3.25 |
| Soybean meal | 6.30 | 13.12 |
| Alfalfa hay | - | 8.70 |
| Soyhulls | - | 0.36 |
| Soychlor® 1 | 3.37 | - |
| Corn gluten feed | 2.80 | 2.50 |
| Mineral–vitamin mix | 1.19 | 3.26 |
| ProvAAL2 AADvantage® 2 | 0.47 | 0.73 |
| Biotin 3 | 0.10 | 0.08 |
| AjiPro-L® 4 | 0.06 | 0.06 |
| Availa® Dairy 5 | 0.05 | 0.06 |
| Rumensin® 6 | 0.19 | 0.02 |
| Calcium sulfate | 0.53 | 0.12 |
| Magnesium oxide | 0.10 | 0.12 |
| Salt | 0.10 | 0.25 |
| Chemical composition | ||
| DM, % | 42.88 | 47.26 |
| CP, % of DM | 14.60 | 17.00 |
| NDF, % of DM | 39.15 | 21.50 |
| ADF, % of DM | 31.08 | 16.76 |
| NFC, % of DM | 27.68 | 46.83 |
| Starch, % of DM | 17.17 | 29.46 |
| Crude fat, % of DM | 2.53 | 2.74 |
| NEL, Mcal/kg of DM | 1.37 | 1.65 |
| NEL allowable milk, kg/d | - | 37.82 |
| MP allowable milk, kg/d | - | 40.55 |
| RDP, % of DM | 8.92 | 10.54 |
| RUP, % of DM | 5.68 | 6.46 |
| RDP required, g/d | 1061 | 2435 |
| RDP supplied, g/d | 1116 | 2581 |
| RDP balance, g/d | 49 | 145 |
| RUP required, g/d | 168 | 1043 |
| RUP supplied, g/d | 676 | 1583 |
| RUP balance, g/d | 503 | 540 |
| MP required, g/d | 776 | 2341 |
| MP supplied, g/d | 1188 | 2803 |
| MP balance, g/d | 413 | 463 |
| Ca | 0.66 | 1.00 |
| P | 0.33 | 0.35 |
| Na | 0.12 | 0.45 |
| Cl | 0.78 | 0.68 |
| Mg | 0.45 | 0.38 |
| K | 1.36 | 1.45 |
| S | 0.33 | 0.20 |
| Item | Treatment | SEM 1 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CoPro | CoPro + FOA | CoPectin + FOA | CoPectin + FOA + RPM | Trt | Time | Trt × Time | ||
| Total, no. | 19 | 17 | 18 | 18 | ||||
| Close-up period | ||||||||
| DMI, kg/d | 12.9 | 13.0 | 13.2 | 13.3 | 0.6 | 0.98 | <0.01 | 0.24 |
| Fresh period | ||||||||
| DMI, kg/d | 15.9 | 15.8 | 15.9 | 15.9 | 0.8 | 0.99 | <0.01 | 0.99 |
| Milk, kg/d | 40.7 | 39.0 | 39.7 | 40.8 | 1.9 | 0.87 | <0.01 | 0.25 |
| Body condition score | 3.01 | 3.02 | 2.99 | 2.99 | 0.10 | 0.99 | <0.01 | 0.46 |
| Milk composition, % | ||||||||
| Fat | 3.65 | 3.65 | 3.62 | 3.94 | 0.16 | 0.41 | <0.01 | 0.40 |
| Protein | 3.14 b | 3.11 b | 3.15 b | 3.35 a | 0.07 | 0.08 | <0.01 | 0.02 |
| Lactose | 4.82 | 4.86 | 4.83 | 4.85 | 0.04 | 0.87 | <0.01 | 0.81 |
| Total solids | 12.5 | 13.4 | 11.9 | 11.9 | 0.76 | 0.48 | 0.18 | 0.31 |
| SCC 2 × 1000 | 57.8 | 36.1 | 51.1 | 35.9 | 15.2 | 0.48 | 0.31 | 0.56 |
| MUN 3, mg/dL | 12.0 | 12.1 | 12.5 | 11.8 | 0.50 | 0.83 | 0.05 | 0.68 |
| Item | Treatment | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CoPro | CoPro + FOA | CoPectin + FOA | CoPectin + FOA + RPM | Trt | Time | Trt × Time | ||
| Total, no. | 16 | 16 | 16 | 16 | ||||
| Energy metabolism | ||||||||
| Glucose, mmol/L | 3.95 | 3.92 | 3.97 | 3.97 | 0.08 | 0.96 | <0.01 | 0.55 |
| Fatty acids, mmol/L | 0.69 | 0.62 | 0.58 | 0.70 | 0.07 | 0.57 | <0.01 | 0.64 |
| Urea, mmol/L | 5.30 | 5.60 | 5.38 | 5.31 | 0.27 | 0.81 | <0.01 | 0.37 |
| β-hydroxybutyrate, mmol/L | 0.82 | 0.77 | 0.65 | 0.66 | 0.09 | 0.44 | <0.01 | 0.59 |
| Inflammation | ||||||||
| Ceruloplasmin, µmol/L | 2.95 | 2.95 | 3.04 | 2.91 | 0.12 | 0.88 | <0.01 | 0.96 |
| Albumins, g/L | 35.8 | 35.9 | 35.8 | 35.6 | 0.51 | 0.97 | 0.02 | 0.85 |
| Haptoglobin, g/L | 0.50 | 0.38 | 0.46 | 0.47 | 0.05 | 0.42 | <0.01 | 0.07 |
| Myeloperoxidase, U/L | 497 | 498 | 475 | 474 | 21.1 | 0.73 | <0.01 | 0.62 |
| Zn, µmol/L | 12.6 | 12.5 | 12.7 | 12.7 | 0.67 | 0.99 | <0.01 | 0.10 |
| Liver function | ||||||||
| Cholesterol, mmol/L | 2.77 | 2.95 | 3.17 | 3.08 | 0.18 | 0.40 | <0.01 | 0.99 |
| AST/GOT, U/L | 103 | 111 | 110 | 104 | 6.81 | 0.76 | <0.01 | 0.60 |
| GGT, U/L | 22.9 | 23.0 | 27.6 | 22.6 | 2.12 | 0.28 | <0.01 | 0.89 |
| Total bilirubin, µmol/L | 5.67 | 4.10 | 4.38 | 4.80 | 0.58 | 0.21 | <0.01 | 0.74 |
| Alkaline phosphatase, U/L | 58.5 | 51.7 | 57.3 | 56.1 | 6.65 | 0.88 | <0.01 | 0.97 |
| Paraoxonase, U/mL | 82.0 | 85.5 | 84.9 | 73.8 | 4.43 | 0.18 | <0.01 | 0.67 |
| Retinol, µg/mL | 27.2 | 32.1 | 30.1 | 30.5 | 2.16 | 0.39 | <0.01 | 0.11 |
| Antioxidant status | ||||||||
| ROMt, mg H2O2/dL | 14.9 | 14.7 | 15.5 | 14.6 | 0.45 | 0.54 | <0.01 | 0.99 |
| NOx, µmol/L | 26.6 | 27.2 | 26.2 | 26.6 | 0.39 | 0.34 | <0.01 | 0.01 |
| NO2, µmol/L | 4.42 | 4.70 | 4.81 | 4.89 | 0.29 | 0.65 | <0.01 | 0.10 |
| NO3, µmol/L | 22.2 | 22.5 | 21.4 | 21.7 | 0.38 | 0.17 | <0.01 | 0.65 |
| FRAP, µmol/L | 125 | 123 | 129 | 128 | 4.22 | 0.72 | <0.01 | 0.34 |
| Tocopherol, µg/mL | 3.29 | 3.64 | 3.62 | 3.87 | 0.24 | 0.41 | <0.01 | 0.27 |
| β-carotene, mg/dL | 0.19 | 0.21 | 0.17 | 0.21 | 0.02 | 0.39 | <0.01 | 0.99 |
| Gene 1 | Treatment | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CoPro | CoPro + FOA | CoPectin + FOA | CoPectin + FOA + RPM | Trt | Day | Trt × Day | ||
| Met and folic acid cycle and trans-sulfuration | ||||||||
| BHMT | 0.86 a | 0.53 b | 0.87 a | 0.69 ab | 0.11 | 0.03 | 0.08 | 0.12 |
| CBS | 0.93 | 0.87 | 0.94 | 0.90 | 0.07 | 0.88 | 0.01 | 0.39 |
| MAT1A | 0.02 | 0.02 | 0.04 | 0.02 | 0.02 | 0.90 | 0.20 | 0.37 |
| MTR | 1.06 | 1.00 | 1.20 | 1.09 | 0.07 | 0.19 | <0.01 | 0.22 |
| SAHH | 0.03 | 0.04 | 0.07 | 0.05 | 0.04 | 0.82 | 0.09 | 0.81 |
| Choline metabolism | ||||||||
| BADH | 1.43 | 1.19 | 1.48 | 1.37 | 0.11 | 0.20 | <0.01 | 0.53 |
| PEMT | 0.10 | 0.11 | 0.19 | 0.12 | 0.07 | 0.62 | 0.60 | 0.61 |
| CHDH | 0.32 | 0.30 | 0.46 | 0.38 | 0.11 | 0.63 | <0.01 | 0.97 |
| DMGDH | 0.58 | 0.46 | 0.67 | 0.52 | 0.11 | 0.42 | 0.83 | 0.37 |
| SARDH | 0.97 | 0.95 | 1.10 | 1.07 | 0.10 | 0.56 | <0.01 | 0.33 |
| Riboflavin and cobalamin metabolism | ||||||||
| MTHFR | 1.25 | 1.17 | 1.26 | 1.19 | 0.07 | 0.75 | <0.01 | 0.52 |
| MTRR | 1.60 | 1.59 | 1.62 | 1.51 | 0.06 | 0.64 | <0.01 | 0.66 |
| MMUT | 0.97 | 0.92 | 0.97 | 0.94 | 0.05 | 0.85 | <0.01 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopreiato, V.; Alharthi, A.S.; Liang, Y.; Elolimy, A.A.; Bucktrout, R.; Socha, M.T.; Trevisi, E.; Loor, J.J. Influence of Cobalt Source, Folic Acid, and Rumen-Protected Methionine on Performance, Metabolism, and Liver Tissue One-Carbon Metabolism Biomarkers in Peripartal Holstein Cows. Animals 2023, 13, 2107. https://doi.org/10.3390/ani13132107
Lopreiato V, Alharthi AS, Liang Y, Elolimy AA, Bucktrout R, Socha MT, Trevisi E, Loor JJ. Influence of Cobalt Source, Folic Acid, and Rumen-Protected Methionine on Performance, Metabolism, and Liver Tissue One-Carbon Metabolism Biomarkers in Peripartal Holstein Cows. Animals. 2023; 13(13):2107. https://doi.org/10.3390/ani13132107
Chicago/Turabian StyleLopreiato, Vincenzo, Abdulrahman S. Alharthi, Yusheng Liang, Ahmed A. Elolimy, Ryan Bucktrout, Mike T. Socha, Erminio Trevisi, and Juan J. Loor. 2023. "Influence of Cobalt Source, Folic Acid, and Rumen-Protected Methionine on Performance, Metabolism, and Liver Tissue One-Carbon Metabolism Biomarkers in Peripartal Holstein Cows" Animals 13, no. 13: 2107. https://doi.org/10.3390/ani13132107





