Survey and Molecular Characterization of Sarcocystidae protozoa in Wild Cricetid Rodents from Central and Southern Chile
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
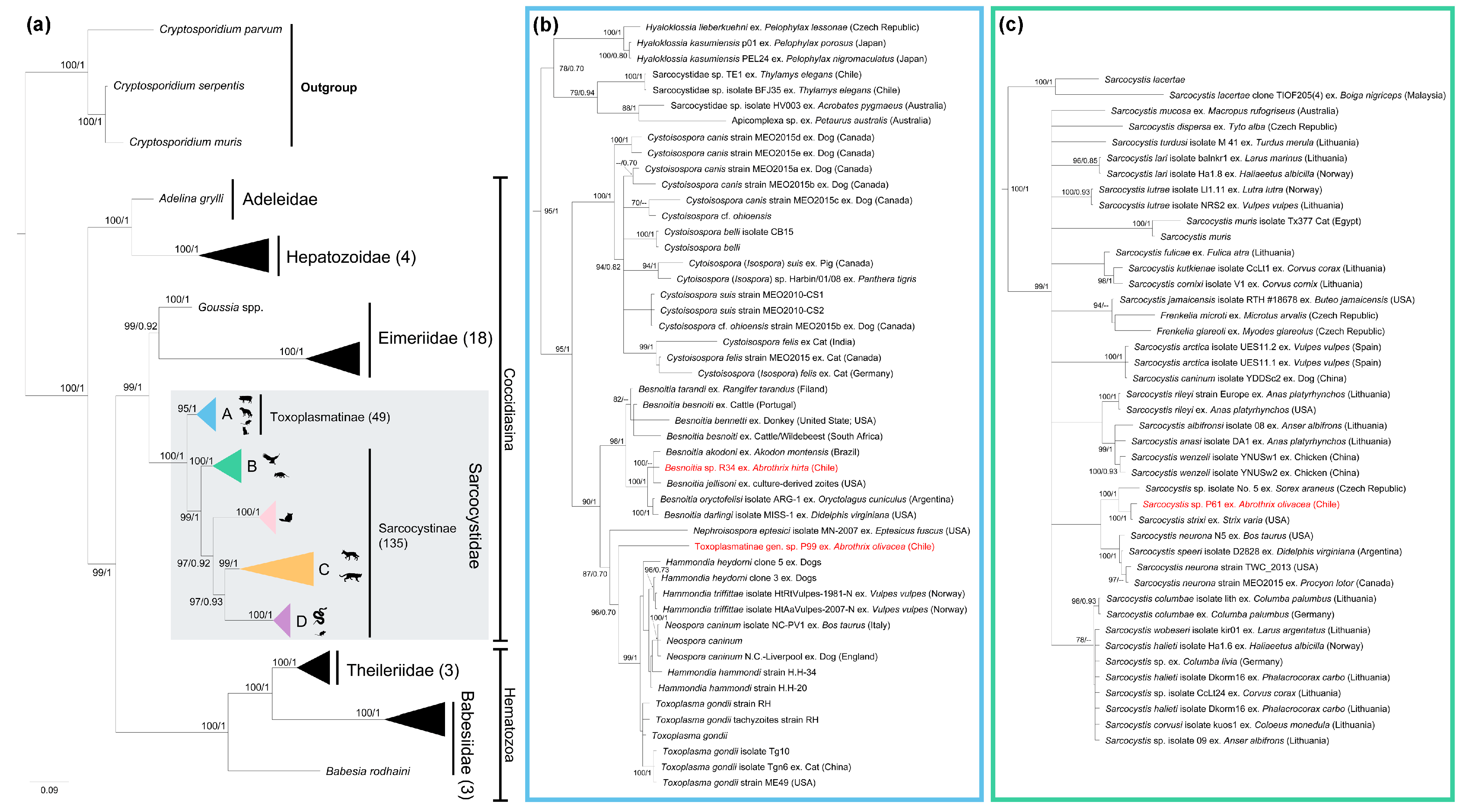
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Votýpka, J.; Modrý, D.; Oborník, M.; Šlapeta, J.; Lukeš, J. Apicomplexa. In Handbook of the Protists; Archibald, J.M., Simpson, A.G.B., Slamovits, C.H., Margulis, L., Melkonian, M., Chapman, D.J., Corliss, J.O., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–58. [Google Scholar]
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Doležel, D.; Koudela, B.; Jirků, M.; Hypša, V.; Oborník, M.; Votýpka, J.; Modrý, D.; Šlapeta, J.R.; Lukeš, J. Phylogenetic analysis of Sarcocystis spp. of mammals and reptiles supports the coevolution of Sarcocystis spp. with their final hosts. Int. J. Parasitol. 1999, 29, 795–798. [Google Scholar] [CrossRef]
- Cwirenbaum, R.; Schmidt, A.R.; Cortasa, S.A.; Corso, M.C.; Vitullo, A.D.; Dorfman, V.B.; Halperin, J. First record of an infection by tissue cyst-forming coccidia in wild vizcachas (Lagostomus maximus, Rodentia) of Argentina. Int. J. Parasitol. Parasites Wildl. 2021, 16, 52–58. [Google Scholar] [CrossRef]
- Dubey, J.P.; Sreekumar, C.; Rosenthal, B.M.; Lindsay, D.S.; Grisard, E.C.; Vitor, R.W. Biological and molecular characterization of Besnoitia akodoni n.sp. (Protozoa: Apicomplexa) from the rodent Akodon montensis in Brazil. Parassitologia 2003, 45, 61–70. [Google Scholar]
- Merino, S.; Martínez, J.; Vásquez, R.A.; Šlapeta, J. Monophyly of marsupial intraerythrocytic apicomplexan parasites from South America and Australia. Parasitology 2010, 137, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Vásquez, R.A.; Martínez, J.; Celis-Diez, J.L.; MartãNez-De La Puente, J.; Marín-Vial, P.; Sánchez-Monsalvez, I.; Peirce, M.A. A Sarcocystid Misidentified as Hepatozoon didelphydis: Molecular Data from a Parasitic Infection in the Blood of the Southern Mouse Opossum (Thylamys elegans) from Chile. J. Eukaryot. Microbiol. 2008, 55, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Santodomingo, A.M.; Thomas, R.S.; Quintero-Galvis, J.F.; Echeverry-Berrio, D.M.; La Fuente, M.C.S.-D.; Moreno-Salas, L.; Muñoz-Leal, S. Apicomplexans in small mammals from Chile, with the first report of the Babesia microti group in South American rodents. Parasitol. Res. 2022, 121, 1009–1020. [Google Scholar] [CrossRef]
- Canova, V.; Helman, E.; Robles, M.d.R.; Abba, A.M.; Moré, G. First report of Sarcocystis spp. (Apicomplexa, Sarcocystidae) in Lagostomus maximus (Desmarest, 1917) (Rodentia, Chinchillidae) in Argentina. Int. J. Parasitol. Parasites Wildl. 2023, 20, 180–186. [Google Scholar] [CrossRef] [PubMed]
- D’Elía, G.; Canto Hernández, J.; Ossa, G.; Verde Arregoitia, L.; Bostelmann Torrealba, J.E.; Iriarte, A.; Amador, L.; Quiroga-Carmona, M.; Hurtado, N.; Cadenillas, R.; et al. Lista actualizada de los mamíferos vivientes de Chile. Boletín Mus. Nac. De Hist. Nat. 2020, 69, 67–98. [Google Scholar] [CrossRef]
- Landaeta-Aqueveque, C.; Henríquez, A.; Cattan, P.E. Introduced species: Domestic mammals are more significant transmitters of parasites to native mammals than are feral mammals. Int. J. Parasitol. 2014, 44, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Landaeta-Aqueveque, C.; Moreno Salas, L.; Henríquez, A.; Silva-de la Fuente, M.C.; González-Acuña, D. Parasites of native and invasive rodents in Chile: Ecological and human health needs. Front. Vet. Sci. 2021, 8, 643742. [Google Scholar] [CrossRef]
- Carrera-Játiva, P.D.; Torres, C.; Figueroa-Sandoval, F.; Beltrami, E.; Verdugo, C.; Landaeta-Aqueveque, C.; Acosta-Jamett, G. Gastrointestinal parasites in wild rodents in Chiloé Island-Chile. Rev. Bras. De Parasitol. Veterinária 2023, 32, e017022. [Google Scholar] [CrossRef]
- Franjola, R.; Soto, G.; Montefusco, A. Prevalencia de infección por protozoos en roedores sinantrópicos de la ciudad de Valdivia, Chile. Bol. Chil. De Parasitol. 1995, 50, 66–72. [Google Scholar]
- Correa, J.P.; Bacigalupo, A.; Yefi-Quinteros, E.; Rojo, G.; Solari, A.; Cattan, P.E.; Botto-Mahan, C. Trypanosomatid Infections among Vertebrates of Chile: A Systematic Review. Pathogens 2020, 9, 661. [Google Scholar] [CrossRef] [PubMed]
- Ruiz del Río, A. Contribución al estudio de las enfermedades parasitarias humanas transmitidas por las ratas en Concepción. Boletín De La Soc. De Biol. De Concepción 1939, 13, 47–82. [Google Scholar]
- Espinoza-Rojas, H.; Lobos-Chávez, F.; Silva-de la Fuente, M.C.; Echeverry, D.M.; Muñoz-Galaz, J.; Yáñez-Crisóstomo, C.; Oyarzún-Ruiz, P.; Ortega, R.; Sandoval, D.; Henríquez, A.; et al. Survey of Trichinella in American minks (Neovison vison Schreber, 1777) and wild rodents (Muridae and Cricetidae) in Chile. Zoonoses Public Health 2021, 68, 842–848. [Google Scholar] [CrossRef] [PubMed]
- SAG. Ley de Caza y su Reglamento. Available online: http://www.sag.cl/sites/default/files/ley_caza_edicion2012.pdf (accessed on 30 December 2022).
- Iriarte, A. Mamíferos de Chile; Lynx: Santiago, Chile, 2008. [Google Scholar]
- Iriarte, A. Guía de Campo de los Mamíferos de Chile; Flora y Fauna Ltda: Santiago, Chile, 2010; p. 216. [Google Scholar]
- Muñoz-Pedreros, A. Orden Rodentia. In Mamíferos de Chile, 2nd ed.; Muñoz-Pedreros, A., Yáñez, J., Eds.; Editorial CEA: Valdivia, Chile, 2009; pp. 93–157. [Google Scholar]
- Birkenheuer, A.J.; Levy, M.G.; Breitschwerdt, E.B. Development and Evaluation of a Seminested PCR for Detection and Differentiation of Babesia gibsoni (Asian Genotype) and B. canis DNA in Canine Blood Samples. J. Clin. Microbiol. 2003, 41, 4172–4177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oosthuizen, M.C.; Zweygarth, E.; Collins, N.E.; Troskie, M.; Penzhorn, B.L. Identification of a Novel Babesia sp. from a Sable Antelope (Hippotragus niger Harris, 1838). J. Clin. Microbiol. 2008, 46, 2247–2251. [Google Scholar] [CrossRef] [Green Version]
- Greay, T.L.; Zahedi, A.; Krige, A.-S.; Owens, J.M.; Rees, R.L.; Ryan, U.M.; Oskam, C.L.; Irwin, P.J. Endemic, exotic and novel apicomplexan parasites detected during a national study of ticks from companion animals in Australia. Parasites Vectors 2018, 11, 197. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT Multiple sequence alignment software version 7: Improvements in oerformance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Criscuolo, A.; Gribaldo, S. BMGE (Block Mapping and Gathering with Entropy): A new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol. Biol. 2010, 10, 210. [Google Scholar] [CrossRef] [Green Version]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef]
- Rannala, B.; Yang, Z. Probability distribution of molecular evolutionary trees: A new method of phylogenetic inference. J. Mol. Evol. 1996, 43, 304–311. [Google Scholar] [CrossRef]
- Yang, Z.; Rannala, B. Bayesian phylogenetic inference using DNA sequences: A Markov Chain Monte Carlo Method. Mol. Biol. Evol. 1997, 14, 717–724. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [Green Version]
- Huelsenbeck, J.P.; Rannala, B. Frequentist properties of Bayesian posterior probabilities of phylogenetic trees under simple and complex substitution models. Syst. Biol. 2004, 53, 904–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Silvestro, D.; Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2012, 12, 335–337. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.K.; von Dohlen, A.R.; Mowery, J.D.; Scott, D.; Cerqueira-Cézar, C.K.; Rosenthal, B.M.; Dubey, J.P.; Lindsay, D.S. Sarcocystis strixi n. sp. from a Barred Owl (Strix varia) definitive host and Interferon Gamma gene knockout mice as experimental intermediate host. J. Parasitol. 2017, 103, 768–777. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J.T.; Holmdahl, O.J.M.; Ryce, C.; Njenga, J.M.; Harper, P.A.W.; Morrison, D.A. Molecular phylogeny of Besnoitia and the genetic relationships among Besnoitia of cattle, wildebeest and goats. Protist 2000, 151, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Minh, B.Q.; Nguyen, M.A.T.; Von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [Green Version]
- Girini, J.M.; Palacio, F.X.; Zelaya, P.V. Predictive modeling for allopatric Strix (Strigiformes: Strigidae) owls in South America: Determinants of their distributions and ecological niche-based processes. J. Field Ornithol. 2017, 88, 1–15. [Google Scholar] [CrossRef]
- Pavez, E.F. Descripción de las especies de aves rapaces de Chile. In Aves Rapaces de Chile, 2nd ed.; Muñoz-Pedreros, A., Rau, J., Yáñez, J., Eds.; CEA Ediciones: Valdivia, Chile, 2019; pp. 45–166. [Google Scholar]
- Malatji, M.P.; Tembe, D.; Mukaratirwa, S. An update on epidemiology and clinical aspects of besnoitiosis in livestock and wildlife in sub-Saharan Africa: A systematic review. Parasite Epidemiol. Control 2023, 21, e00284. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, H. Besnoitia. In Parasitic Protozoa of Farm Animals and Pets; Florin-Christensen, M., Schnittger, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 169–185. [Google Scholar]
- Olias, P.; Schade, B.; Mehlhorn, H. Molecular pathology, taxonomy and epidemiology of Besnoitia species (Protozoa: Sarcocystidae). Infect. Genet. Evol. 2011, 11, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.V.; Chobotar, B.; Oaks, E.C.; Hammond, D.M. Besnoitia jellisoni (Sporozoa: Toxoplasmea) in rodents from Utah and California. J. Parasitol. 1968, 54, 545–549. [Google Scholar] [CrossRef]
- Mazzoni Baldini, M.H.; Pinto Sandoval, E.D.; Barbanti Duarte, J.M. Assessment of transplacental transmission of Neospora caninum and Toxoplasma gondii in Neotropical deer: An estimative based on serology. Vet. Parasitol. 2022, 303, 109677. [Google Scholar] [CrossRef]
- Meireles, L.R.; Machado Bezerra, E.C.; Queiroz Andrade, J.; Cassiano, L.A.; Pena, H.F.J.; Farias Alves, B.; Vieira Francisco, R.P.; De Andrade, H.F. Isolation and characterization of Toxoplasma gondii isolates from human congenital toxoplasmosis cases reveal a new virulent genotype in São Paulo, Brazil. Parasitol. Res. 2022, 121, 3223–3228. [Google Scholar] [CrossRef] [PubMed]
- Zanet, S.; Poncina, M.; Ferroglio, E. Congenital transmission of Neospora caninum in wild ungulates and foxes. Front. Vet. Sci. 2023, 10, 1109986. [Google Scholar] [CrossRef]
- Wünschmann, A.; Wellehan, J.F.X.; Armien, A.; Bemrick, W.J.; Barnes, D.; Averbeck, G.A.; Roback, R.; Schwabenlander, M.; D’Almeida, E.; Joki, R.; et al. Renal infection by a new coccidian genus in big brown bats (Eptesicus fuscus). J. Parasitol. 2010, 96, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.D.; Silva, D.A.O.; Ferro, E.A.V.; Mineo, J.R. Acquired and congenital ocular Toxoplasmosis experimentally induced in Calomys callosus (Rodentia, Cricetidae). Mem. Do Inst. Oswaldo Cruz 1999, 94, 103–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinchilla, M.; Guerrero, O.M.; Reyes, L.; Abrahams, E. Susceptibility of the rat Sigmodon hispidus (Rodentia: Cricetidae) to Toxoplasma gondii (Eucoccidia: Sarcocystidae). Rev. De Biol. Trop. 1996, 44, 265–268. [Google Scholar]
- Grisard, E.C.; Elsaid, M.M.A.; Tafuri, W.L.; Lima, J.D.; Pinto, C.J.C.; Steindel, M.; Vitor, R.W.A. Besnoitia sp. (Protozoa: Toxoplasmatinae) from Akodon montensis (Rodentia: Cricetidae) in Santa Catarina State, Brazil. J. Parasitol. 1997, 83, 314–316. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyarzún-Ruiz, P.; Thomas, R.S.; Santodomingo, A.M.; Uribe, J.E.; Ardila, M.M.; Echeverry, D.M.; Muñoz-Leal, S.; Silva-de la Fuente, M.C.; Loyola, M.; Palma, C.J.; et al. Survey and Molecular Characterization of Sarcocystidae protozoa in Wild Cricetid Rodents from Central and Southern Chile. Animals 2023, 13, 2100. https://doi.org/10.3390/ani13132100
Oyarzún-Ruiz P, Thomas RS, Santodomingo AM, Uribe JE, Ardila MM, Echeverry DM, Muñoz-Leal S, Silva-de la Fuente MC, Loyola M, Palma CJ, et al. Survey and Molecular Characterization of Sarcocystidae protozoa in Wild Cricetid Rodents from Central and Southern Chile. Animals. 2023; 13(13):2100. https://doi.org/10.3390/ani13132100
Chicago/Turabian StyleOyarzún-Ruiz, Pablo, Richard S. Thomas, Adriana M. Santodomingo, Juan E. Uribe, Marlon M. Ardila, Diana M. Echeverry, Sebastián Muñoz-Leal, María C. Silva-de la Fuente, Marco Loyola, Cristina J. Palma, and et al. 2023. "Survey and Molecular Characterization of Sarcocystidae protozoa in Wild Cricetid Rodents from Central and Southern Chile" Animals 13, no. 13: 2100. https://doi.org/10.3390/ani13132100
APA StyleOyarzún-Ruiz, P., Thomas, R. S., Santodomingo, A. M., Uribe, J. E., Ardila, M. M., Echeverry, D. M., Muñoz-Leal, S., Silva-de la Fuente, M. C., Loyola, M., Palma, C. J., Landaeta-Aqueveque, C., & Henríquez, A. (2023). Survey and Molecular Characterization of Sarcocystidae protozoa in Wild Cricetid Rodents from Central and Southern Chile. Animals, 13(13), 2100. https://doi.org/10.3390/ani13132100








