Influence of Different Light Spectra on Melatonin Synthesis by the Pineal Gland and Influence on the Immune System in Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Evolution of the Pineal Gland and How Light Influences Secretory Activity
2.1. Structure of the Pineal Gland in Chickens
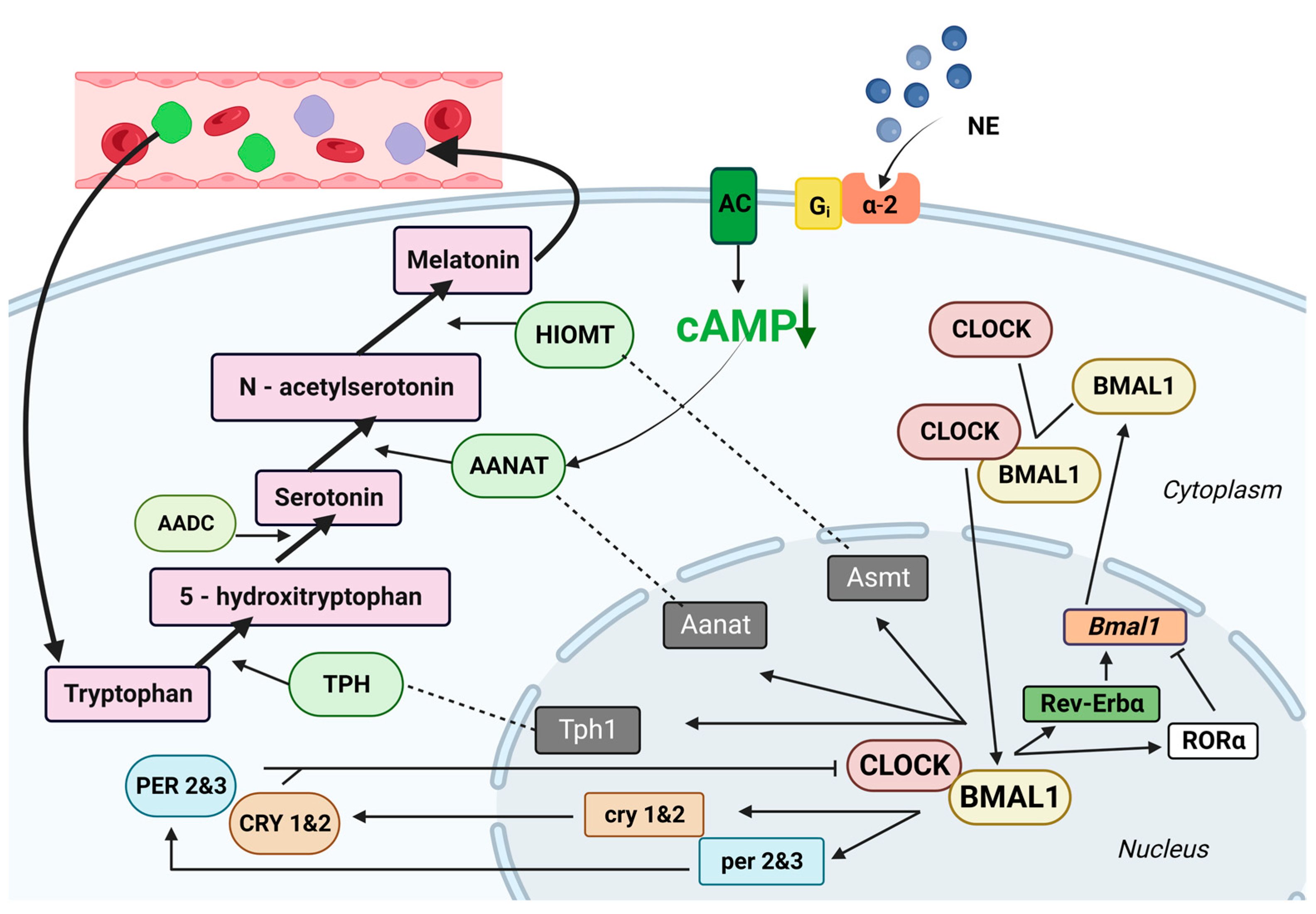
2.2. Melatonin Secretion
3. The Immune Response under the Influence of Melatonin
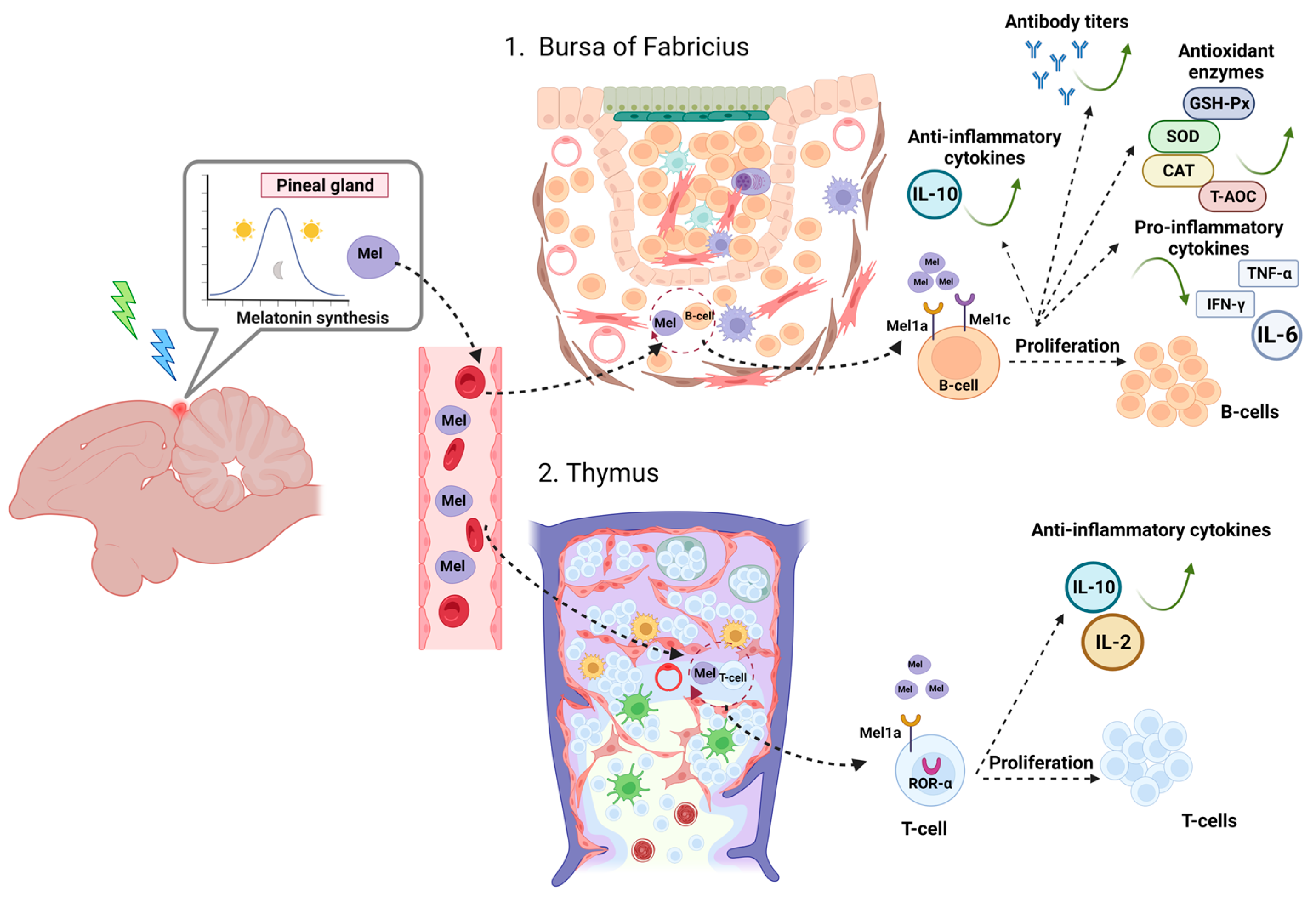
3.1. How Melatonin Influences the Activity of Primary Lymphoid Organs
3.1.1. Bursa of Fabricius
3.1.2. Thymus
3.2. How Melatonin Impacts the Activity of Secondary Lymphoid Organs
The Spleen
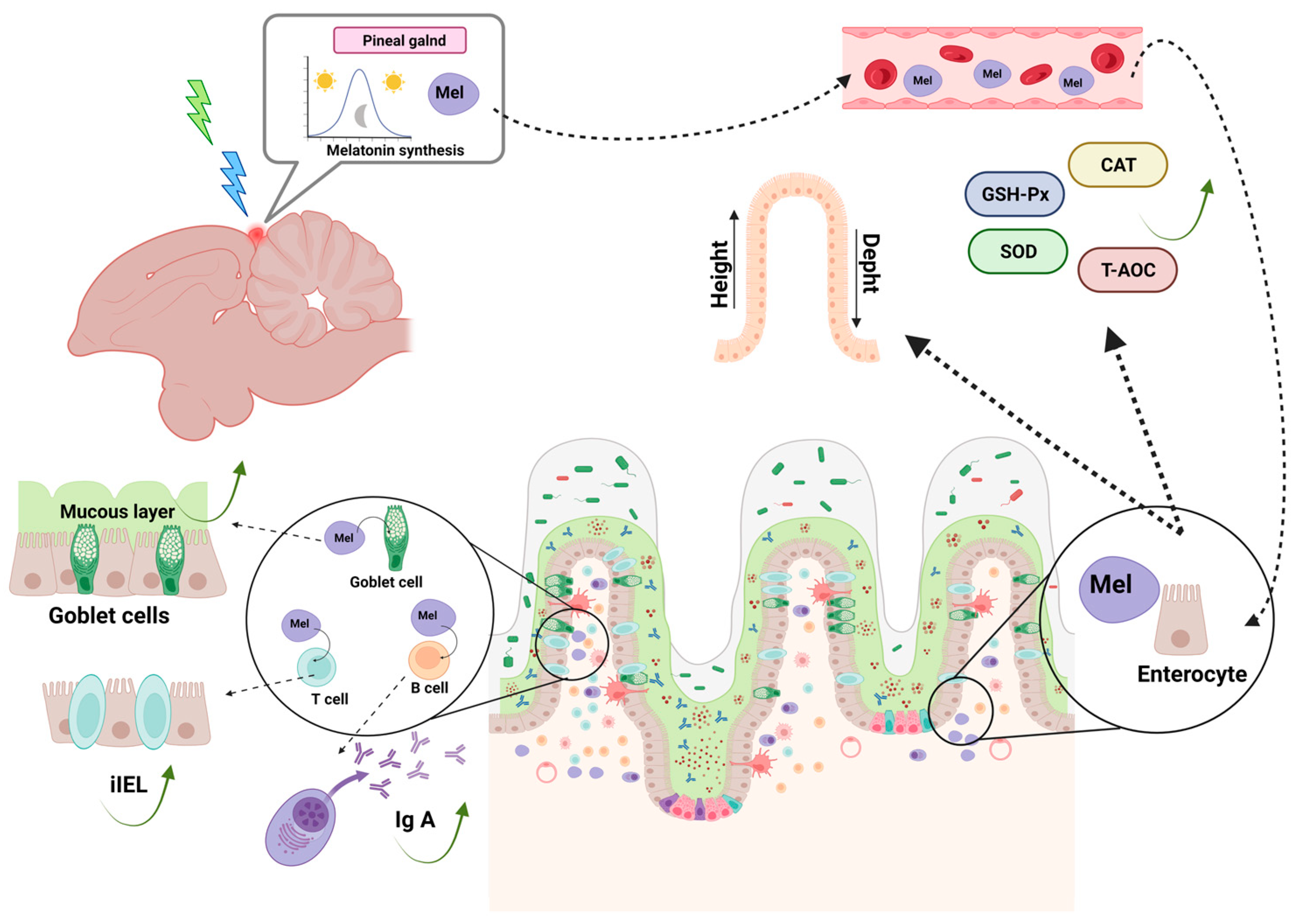
4. Intestinal Mucosa-Associated Immune System—GALT
4.1. Mechanical Barrier
4.2. Immunological Barrier
4.3. The Chemical Barrier
4.4. The Biological Barrier
5. Influence of the Activated Immune System on the Pineal Gland
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falcón, J. Nocturnal Melatonin Synthesis: How to Stop It. Endocrinology 2007, 148, 1473–1474. [Google Scholar] [CrossRef]
- Falcón, J.; Gothilf, Y.; Coon, S.L.; Boeuf, G.; Klein, D.C. Genetic, Temporal and Developmental Differences Between Melatonin Rhythm Generating Systems in the Teleost Fish Pineal Organ and Retina: Two melatonin rhythm generating systems in Teleost fish. J. Neuroendocrinol. 2003, 15, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Dodt, E.; Heerd, E. Mode of action of pineal nerve fibers in frogs. J. Neurophysiol. 1962, 25, 405–429. [Google Scholar] [CrossRef] [PubMed]
- Underwood, H. Circadian Organization in Lizards: The Role of the Pineal Organ. Science 1977, 195, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Cassone, V.M. Avian circadian organization: A chorus of clocks. Front. Neuroendocrinol. 2014, 35, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J.; Skene, D.J. Melatonin as a chronobiotic. Sleep Med. Rev. 2005, 9, 25–39. [Google Scholar] [CrossRef]
- Collin, J.-P. Differentiation and regression of the cells of the sensory line in the epiphysis cerebri. In The Pineal Gland; Churchill Livingstone: London, UK; Edinburgh, UK, 1971; pp. 79–125. [Google Scholar]
- Collin, J.-P.; Voisin, P.; Falcón, J.; Brisson, P. Evolution and environmental control of secretory processes in pineal transducers. In Functional Morphology of Neuroendocrine Systems: Evolutionary and Environmental Aspects; Springer: Berlin/Heidelberg, Germany, 1987; pp. 105–121. [Google Scholar]
- Collin, J.-P.; Oksche, A. Structural and functional relationships in the nonmammalian pineal gland. Pineal Gland 1981, 1, 27–67. [Google Scholar]
- Oksche, A. Sensory and glandular elements of the pineal organ. In The Pineal Gland; Churchill Livingstone: Edinburgh, UK, 1971. [Google Scholar]
- Oksche, A. Pinealocytes as photoneuroendocrine units of neuronal origin: Concepts and evidence. Adv. Pineal Res. 1987, 2, 1–18. [Google Scholar]
- Volrath, L. Handbuch der mikroskopischen Anatomie des Menschen Handbook of Mikroscopic Anatomy. In The Pineal Organ; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1981. [Google Scholar]
- Binkley, S. Comparative Biochemistry of the Pineal Glands of Birds and Mammals. Am. Zool. 1976, 16, 57–65. [Google Scholar] [CrossRef][Green Version]
- Binkley, S.; Macbride, S.E.; Klein, D.C.; Ralph, C.L. Regulation of Pineal Rhythms in Chickens: Refractory Period and Nonvisual Light Perception 1. Endocrinology 1975, 96, 848–853. [Google Scholar] [CrossRef]
- Binkley, S.; Riebman, J.B.; Reilly, K.B. Timekeeping by the Pineal Gland. Science 1977, 197, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Binkley, S.A.; Riebman, J.B.; Reilly, K.B. The Pineal Gland: A Biological Clock in Vitro. Science 1978, 202, 1120–1198. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, T. Circadian Rhythm of Serotonin N -Acetyltransferase Activity in Organ Culture of Chicken Pineal Gland. Science 1979, 203, 1245–1247. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, T. A circadian oscillator in cultured cells of chicken pineal gland. Nature 1979, 282, 94–96. [Google Scholar] [CrossRef]
- Deguchi, T. Endogenous Oscillator and Photoreceptor for Serotonin N-Acetyltransferase Rhythm in Chicken Pineal Gland. In Vertebrate Circadian Systems; Aschoff, J., Daan, S., Groos, G.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 164–172. [Google Scholar]
- Menaker, M. Extraretinal light perception in the sparrow. I. Entrainment of the biological clock. Proc. Natl. Acad. Sci. USA 1968, 59, 414–421. [Google Scholar] [CrossRef]
- Takahashi, J.S. Circadian Rhythms of the Isolated Chicken Pineal in Vitro. In Vertebrate Circadian Systems; Aschoff, J., Daan, S., Groos, G.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 158–163. [Google Scholar]
- Takahashi, J.S.; Hamm, H.; Menaker, M. Circadian rhythms of melatonin release from individual superfused chicken pineal glands in vitro. Proc. Natl. Acad. Sci. USA 1980, 77, 2319–2322. [Google Scholar] [CrossRef]
- Takahashi, J.S.; Menaker, M. Multiple redundant circadian oscillators within the isolated avian pineal gland. J. Comp. Physiol. A 1984, 154, 435–440. [Google Scholar] [CrossRef]
- Wainwright, S.D. Some answers to a 2000-year old question: The role(s) of the pineal gland. Rev. Pure Appl. Pharmacol. Sci. 1982, 3, 185–262. [Google Scholar]
- Wainwright, S.D.; Wainwright, L.K. Probing the chick pineal clock. Prog. Clin. Biol. Res. 1982, 92, 87–94. [Google Scholar]
- Zatz, M.; Mullen, D.A.; Moskal, J.R. Photoendocrine transduction in cultured chick pineal cells: Effect of light, dark, and potassium on the melatonin rhythm. Brain Res. 1988, 438, 199–215. [Google Scholar] [CrossRef]
- Cassone, V.M.; Menaker, M. Is the avian circadian system a neuroendocrine loop? J. Exp. Zool. 1984, 232, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Gwinner, E. Melatonin in the circadian system of birds: Model of internal resonance. In Circadian Clocks and Ecology; Hokkaido University Press: Sapporo, Japan, 1989; pp. 127–153. [Google Scholar]
- Cantwell, E.L.; Cassone, V.M. Chicken suprachiasmatic nuclei: I. Efferent and afferent connections. J. Comp. Neurol. 2006, 496, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, E.L.; Cassone, V.M. Chicken suprachiasmatic nuclei: II. Autoradiographic and immunohistochemical analysis. J. Comp. Neurol. 2006, 499, 442–457. [Google Scholar] [CrossRef]
- Cassone, V.M.; Moore, R.Y. Retinohypothalamic projection and suprachiasmatic nucleus of the house sparrow, Passer domesticus. J. Comp. Neurol. 1987, 266, 171–182. [Google Scholar] [CrossRef]
- Yoshimura, T.; Yasuo, S.; Suzuki, Y.; Makino, E.; Yokota, Y.; Ebihara, S. Identification of the suprachiasmatic nucleus in birds. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2001, 280, R1185–R1189. [Google Scholar] [CrossRef]
- Cantwell, E.L.; Cassone, V.M. Daily and circadian fluctuation in 2-deoxy [14C]-glucose uptake in circadian and visual system structures of the chick brain: Effects of exogenous melatonin. Brain Res. Bull. 2002, 57, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Cassone, V.M.; Brooks, D.S.; Kelm, T.A. Comparative Distribution of 2[125I]Iodomelatonin Binding in the Brains of Diurnal Birds: Outgroup Analysis with Turtles. Brain. Behav. Evol. 1995, 45, 241–256. [Google Scholar] [CrossRef]
- Lu, J.; Cassone, V.M. Daily melatonin administration synchronizes circadian patterns of brain metabolism and behavior in pinealectomized house sparrows, Passer domesticus. J. Comp. Physiol. A 1993, 173, 775–782. [Google Scholar] [CrossRef]
- Bailey, M.J.; Beremand, P.D.; Hammer, R.; Bell-Pedersen, D.; Thomas, T.L.; Cassone, V.M. Transcriptional Profiling of the Chick Pineal Gland, a Photoreceptive Circadian Oscillator and Pacemaker. Mol. Endocrinol. 2003, 17, 2084–2095. [Google Scholar] [CrossRef]
- Karaganis, S.P.; Kumar, V.; Beremand, P.D.; Bailey, M.J.; Thomas, T.L.; Cassone, V.M. Circadian genomics of the chick pineal gland in vitro. BMC Genom. 2008, 9, 206. [Google Scholar] [CrossRef]
- Karaganis, S.P.; Bartell, P.A.; Shende, V.R.; Moore, A.F.; Cassone, V.M. Modulation of metabolic and clock gene mRNA rhythms by pineal and retinal circadian oscillators. Gen. Comp. Endocrinol. 2009, 161, 179–192. [Google Scholar] [CrossRef]
- Bailey, M.J.; Beremand, P.D.; Hammer, R.; Reidel, E.; Thomas, T.L.; Cassone, V.M. Transcriptional Profiling of Circadian Patterns of mRNA Expression in the Chick Retina. J. Biol. Chem. 2004, 279, 52247–52254. [Google Scholar] [CrossRef]
- Bell-Pedersen, D.; Cassone, V.M.; Earnest, D.J.; Golden, S.S.; Hardin, P.E.; Thomas, T.L.; Zoran, M.J. Circadian rhythms from multiple oscillators: Lessons from diverse organisms. Nat. Rev. Genet. 2005, 6, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Yasuo, S.; Ebihara, S.; Yoshimura, T. Circadian expression of clock gene in the optic tectum of Japanese quail. Brain Res. 2004, 1005, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Yasuo, S.; Yoshimura, T. Comparative analysis of the molecular basis of photoperiodic signal transduction in vertebrates. Integr. Comp. Biol. 2009, 49, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Suzuki, Y.; Makino, E.; Suzuki, T.; Kuroiwa, A.; Matsuda, Y.; Namikawa, T.; Ebihara, S. Molecular analysis of avian circadian clock genes. Mol. Brain Res. 2000, 78, 207–215. [Google Scholar] [CrossRef]
- Markowska, M.; Majewski, P.M.; Skwarło-Sońta, K. Avian biological clock—Immune system relationship. Dev. Comp. Immunol. 2017, 66, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Wake, K. Innervation of the avian pineal organ: A comparative study. Cell Tissue Res. 1983, 233, 237–264. [Google Scholar] [CrossRef]
- Quay, W.B.; Renzoni, A. The diencephalic relations and variability in the double structure of the epiphyseal complex of birds. Riv. Biol. 1967, 60, 9–75. [Google Scholar]
- Boya, J.; Calvo, J. Post-hatching evolution of the pineal gland of the chicken. Cells Tissues Organs 1978, 101, 1–9. [Google Scholar] [CrossRef]
- Möller, W.; Möller, G. Structural and functional differentiation of the embryonic chick pineal organ in vivo and in vitro: A scanning electron-microscopic and radioimmunoassay study. Cell Tissue Res. 1990, 260, 337–348. [Google Scholar] [CrossRef]
- Hao, C.-M.; Liao, M.; Chien, C.-L.; Peng, W.H. α-Internexin expression in photoreceptor-like cells of the developing chicken pineal gland. Res. Sq. 2021. ahead of print. [Google Scholar]
- Ko, T.-L.; Chien, C.-L.; Lu, K.-S. The expression of α-internexin and peripherin in the developing mouse pineal gland. J. Biomed. Sci. 2005, 12, 777–789. [Google Scholar] [CrossRef]
- Fejér, Z.; Röhlich, P.; Szél, Á.; Dávid, C.; Zádori, A.; João Manzano, M.; Vígh, B. Comparative ultrastructure and cytochemistry of the avian pineal organ: Comparative Pineal Ultrastructure. Microsc. Res. Tech. 2001, 53, 12–24. [Google Scholar] [CrossRef]
- Collin, J.-P.; Mirshahi, M.; Brisson, P.; Falcon, J.; Guerlotte, J.; Faure, J.P. Pineal-retinal molecular relationships: Distribution of “S-antigen” in the pineal complex. Neuroscience 1986, 19, 657–666. [Google Scholar] [CrossRef]
- Ohshima, K.; Matsuo, S. Functional morphology of the pineal gland in young chickens. Anat. Anz. 1984, 156, 407–418. [Google Scholar] [PubMed]
- Oksche, A. [Extraretinal photoreceptors in the pineal body of birds]. Arch. Anat. Histol. Embryol. Norm. Exp. 1968, 51, 495–507. [Google Scholar]
- Romieu, M.; Jullien, G. Caracterès histologiques et histophysiologiques des vésicules èpiphysaires des Gallinacés. CR Soc. Biol. 1942, 13, 628–630. [Google Scholar]
- Gonzalez, G.; Garcia, F. Ultraestructura de la glándula pineal de aves. Trab. Inst. Cajal Inv. Biol. 1966, 58, 55–67. [Google Scholar]
- Bischoff, M.B. Photoreceptoral and secretory structures in the avian pineal organ. J. Ultrastruct. Res. 1969, 28, 16–26. [Google Scholar] [CrossRef]
- Fujie, E. Ultrastructure of the Pineal Body of the Domestic Chicken, with Special Reference to the Changes Induced by Altered Photoperiods. Arch. Histol. Jpn. 1968, 29, 271–303. [Google Scholar] [CrossRef][Green Version]
- Goto, K.; Yamagata, K.; Miki, N.; Kondo, H. Direct photosensitivity of chick pinealocytes as demonstrated by visinin immunoreactivity. Cell Tissue Res. 1990, 262, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Omura, Y. Ultrastructural study of embryonic and post-hatching development in the pineal organ of the chicken (brown leghorn, Gallus domesticus). Cell Tissue Res. 1977, 183, 255–271. [Google Scholar] [CrossRef]
- Okano, T.; Takanaka, Y.; Nakamura, A.; Hirunagi, K.; Adachi, A.; Ebihara, S.; Fukada, Y. Immunocytochemical identification of pinopsin in pineal glands of chicken and pigeon. Mol. Brain Res. 1997, 50, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.Q.; Davies, S.; Dominoni, D. Hormonally mediated effects of artificial light at night on behavior and fitness: Linking endocrine mechanisms with function. J. Exp. Biol. 2018, 221, jeb156893. [Google Scholar] [CrossRef]
- Davies, W.I.L.; Turton, M.; Peirson, S.N.; Follett, B.K.; Halford, S.; Garcia-Fernandez, J.M.; Sharp, P.J.; Hankins, M.W.; Foster, R.G. Vertebrate ancient opsin photopigment spectra and the avian photoperiodic response. Biol. Lett. 2012, 8, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Hankins, M.W.; Davies, W.I.L.; Foster, R.G. The Evolution of Non-visual Photopigments in the Central Nervous System of Vertebrates. In Evolution of Visual and Non-Visual Pigments; Hunt, D.M., Hankins, M.W., Collin, S.P., Marshall, N.J., Eds.; Springer US: Boston, MA, USA, 2014; pp. 65–103. ISBN 978-1-4614-4354-4. [Google Scholar]
- Stehle, J.H.; Von Gall, C.; Korf, H.-W. Melatonin: A Clock-Output, A Clock-Input: Clock-output, clock-input. J. Neuroendocrinol. 2003, 15, 383–389. [Google Scholar] [CrossRef]
- Trivedi, A.K.; Kumar, V. Melatonin: An internal signal for daily and seasonal timing. IJEB 2014, 52, 425–437. [Google Scholar]
- Zawilska, J.B.; Lorenc, A.; Berezińska, M.; Vivien-Roels, B.; Pévet, P.; Skene, D.J. Diurnal and circadian rhythms in melatonin synthesis in the turkey pineal gland and retina. Gen. Comp. Endocrinol. 2006, 145, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Csernus, V.; Mess, B. Biorythms and pineal gland. Neuroendocrinol. Lett. 2003, 24, 401–411. [Google Scholar]
- Calislar, S.; Yeter, B.; ŞahiİN, A. Melatonin’in Kanatlı Hayvanlarda Önemi. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Ve Doğa Derg. 2018, 21, 987–997. [Google Scholar] [CrossRef]
- Natesan, A.; Geetha, L.; Zatz, M. Rhythm and soul in the avian pineal. Cell Tissue Res. 2002, 309, 35–45. [Google Scholar] [CrossRef]
- Deguchi, T. Rhodopsin-like photosensitivity of isolated chicken pineal gland. Nature 1981, 290, 706–707. [Google Scholar] [CrossRef]
- Hatakenaka, S.; Kuo, C.-H.; Miki, N. Analysis of a distinctive protein in chick retina during development. Dev. Brain Res. 1983, 10, 155–163. [Google Scholar] [CrossRef]
- Hatakenaka, S.; Kiyama, H.; Tohyama, M.; Miki, N. Immunohistochemical localization of chick retinal 24 kdalton protein (visinin) in various vertebrate retinae. Brain Res. 1985, 331, 209–215. [Google Scholar] [CrossRef]
- Doh, S.T.; Hao, H.; Loh, S.C.; Patel, T.; Tawil, H.Y.; Chen, D.K.; Pashkova, A.; Shen, A.; Wang, H.; Cai, L. Analysis of retinal cell development in chick embryo by immunohistochemistry and in ovo electroporation techniques. BMC Dev. Biol. 2010, 10, 8. [Google Scholar] [CrossRef]
- Yamagata, K.; Goto, K.; Kuo, C.-H.; Kondo, H.; Miki, N. Visinin: A novel calcium binding protein expressed in retinal cone cells. Neuron 1990, 4, 469–476. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Y.; Pan, J.; Ying, Y.; Zhou, H. A new method to manipulate broiler chicken growth and metabolism: Response to mixed LED light system. Sci. Rep. 2016, 6, 25972. [Google Scholar] [CrossRef] [PubMed]
- Farner, D.S.; King, J.R.; Parkes, K.C. Avian Biology; Academic press: New York, NY, USA; San Francisco, CA, USA; London, UK, 1971. [Google Scholar]
- Li, J.; Wang, Z.; Cao, J.; Dong, Y.L.; Chen, Y.X. Role of Monochromatic Light on Development of Cecal Tonsil in Young Broilers: Light Colour and Cecal Tonsil Development of Chick. Anat. Rec. 2014, 297, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Archer, G.S. How does red light affect layer production, fear, and stress? Poult. Sci. 2019, 98, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, J.P.T.; Thaxton, J.P.; Dozier, I.W.A.; Purswell, J.; Roush, W.B.; Branton, S.L. A Review of Lighting Programs for Broiler Production. Int. J. Poult. Sci. 2006, 5, 301–308. [Google Scholar] [CrossRef]
- Prusik, M.; Lewczuk, B.; Ziółkowska, N.; Przybylska-Gornowicz, B. Regulation of melatonin secretion in the pineal organ of the domestic duck—An in vitro study. Pol. J. Vet. Sci. 2015, 18, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Gwinner, E.; Hau, M.; Heigl, S. Melatonin: Generation and Modulation of Avian Circadian Rhythms. Brain Res. Bull. 1997, 44, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Underwood, H.; Binkley, S.; Siopes, T.; Mosher, K. Melatonin rhythms in the eyes, pineal bodies, and blood of Japanese quail (Coturnix coturnix japonica). Gen. Comp. Endocrinol. 1984, 56, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Csernus, V.; Faluhelyi, N.; Nagy, A.D. Features of the Circadian Clock in the Avian Pineal Gland. Ann. N. Y. Acad. Sci. 2005, 1040, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.G.; Davis, S. The melatonin hypothesis: Electric power and breast cancer. Environ. Health Perspect. 1996, 104, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Lewy, A.J.; Wehr, T.A.; Goodwin, F.K.; Newsome, D.A.; Markey, S.P. Light Suppresses Melatonin Secretion in Humans. Science 1980, 210, 1267–1269. [Google Scholar] [CrossRef]
- Jin, E.; Jia, L.; Li, J.; Yang, G.; Wang, Z.; Cao, J.; Chen, Y. Effect of Monochromatic Light on Melatonin Secretion and Arylalkylamine N-Acetyltransferase mRNA Expression in the Retina and Pineal Gland of Broilers. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2011, 294, 1233–1241. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Wawrocka, M. Chick retina and pineal gland differentially respond to constant light and darkness: In vivo studies on serotoninN-acetyltransferase (NAT) activity and melatonin content. Neurosci. Lett. 1993, 153, 21–24. [Google Scholar] [CrossRef]
- Lazăr, R.; Solcan, C.; Creţu, C.; Lazăr, M.; Muntean, C.; Boişteanu, P.C. Characterization of the relations between morphology and physiological status of the pineal gland in connection with the somatic development level in turkeys reared in Romania. Arq. Bras. Med. Veterinária E Zootec. 2015, 67, 763–770. [Google Scholar] [CrossRef][Green Version]
- Reiter, R.J.; Robinson, J.O. Melatonin: Your Body’s Natural Wonder Drug; Bantam Books: New York, NY, USA, 1995; Volume 13. [Google Scholar]
- Ambriz-Tututi, M.; Rocha-González, H.I.; Cruz, S.L.; Granados-Soto, V. Melatonin: A hormone that modulates pain. Life Sci. 2009, 84, 489–498. [Google Scholar] [CrossRef]
- Barrenetxe, J.; Delagrange, P.; Martínez, J.A. Physiological and metabolic functions of melatonin. J. Physiol. Biochem. 2004, 60, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Timothy, C.; Birdsall, N.D. The biological effects and clinical uses of the pineal hormone melatonin. Aleternative Med. Rev. 1996, 1, 94–102. [Google Scholar]
- Sener, G. Karanligin hormonu: Melatonin. MARMARA Pharm. J. 2010, 3, 112–120. [Google Scholar] [CrossRef]
- Bubenik, G.A. Gastrointestinal melatonin: Localization, function, and clinical relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef]
- Csernus, V.J. The Avian Pineal Gland. Chronobiol. Int. 2006, 23, 329–339. [Google Scholar] [CrossRef]
- Lamošová, D.; Zeman, M.; Juráni, M. Influence of Melatonin on Chick Skeletal Muscle Cell Growth. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1997, 118, 375–379. [Google Scholar] [CrossRef]
- Reppert, S.M. Melatonin Receptors: Molecular Biology of a New Family of G Protein-Coupled Receptors. J. Biol. Rhythm. 1997, 12, 528–531. [Google Scholar] [CrossRef]
- Pang, C.S.; Brown, G.M.; Tang, P.L.; Cheng, K.M.; Pang, S.F. 2-[125l]lodomelatonin Binding Sites in the Lung and Heart: A Link between the Photoperiodic Signal, Melatonin, and the Cardiopulmonary System. Neurosignals 1993, 2, 228–236. [Google Scholar] [CrossRef]
- Yu, Z.-H.; Yuan, H.; Lu, Y.; Pang, S.F. [125I]Iodomelatonin binding sites in spleens of birds and mammals. Neurosci. Lett. 1991, 125, 175–178. [Google Scholar] [CrossRef]
- Lee, P.P.N.; Pang, S.F. Identification and characterization of melatonin binding sites in the gastrointestinal tract of ducks. Life Sci. 1992, 50, 117–125. [Google Scholar] [CrossRef]
- Chong, N.W.; Bernard, M.; Klein, D.C. Characterization of the Chicken SerotoninN-Acetyltransferase Gene. J. Biol. Chem. 2000, 275, 32991–32998. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.; Iuvone, P.M.; Cassone, V.M.; Roseboom, P.H.; Coon, S.L.; Klein, D.C. Avian Melatonin Synthesis: Photic and Circadian Regulation of Serotonin N-Acetyltransferase mRNA in the Chicken Pineal Gland and Retina. J. Neurochem. 2002, 68, 213–224. [Google Scholar] [CrossRef]
- Voisin, P.; Guerlotté, J.; Bernard, M.; Collin, J.P.; Cogné, M. Molecular cloning and nucleotide sequence of a cDNA encoding hydroxyindole O -methyltransferase from chicken pineal gland. Biochem. J. 1992, 282, 571–576. [Google Scholar] [CrossRef][Green Version]
- Simonneaux, V.; Ribelayga, C. Generation of the Melatonin Endocrine Message in Mammals: A Review of the Complex Regulation of Melatonin Synthesis by Norepinephrine, Peptides, and Other Pineal Transmitters. Pharmacol. Rev. 2003, 55, 325–395. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, T.; Chattoraj, A.; Ahmed, S.; Wang, M.M.; Deng, J.; Sun, X.; Borjigin, J. Posttranslational regulation of TPH1 is responsible for the nightly surge of 5-HT output in the rat pineal gland. J. Pineal Res. 2008, 45, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Brun, J.; Chazot, G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 2005, 9, 11–24. [Google Scholar] [CrossRef]
- Limbird, L.E. α2-Adrenergic systems: Models for exploring hormonal inhibition of adenylate cyclase. Trends Pharmacol. Sci. 1983, 4, 135–138. [Google Scholar] [CrossRef]
- Pratt, B.; Takahashi, J. Alpha-2 adrenergic regulation of melatonin release in chick pineal cell cultures. J. Neurosci. 1987, 7, 3665–3674. [Google Scholar] [CrossRef]
- Mishra, I.; Kumar, V. Circadian basis of seasonal timing in higher vertebrates. Biol. Rhythm Res. 2017, 48, 723–738. [Google Scholar] [CrossRef]
- Johnston, J.D.; Skene, D.J. 60 YEARS OF NEUROENDOCRINOLOGY: Regulation of mammalian neuroendocrine physiology and rhythms by melatonin. J. Endocrinol. 2015, 226, T187–T198. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.F.; Li, L.; Ayre, E.A.; Pang, C.S.; Lee, P.P.N.; Xu, R.K.; Chow, P.H.; Yu, Z.H.; Shiu, S.Y.W. Neuroendocrinology of melatonin in reproduction: Recent developments. J. Chem. Neuroanat. 1998, 14, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Smith, D.; Hardeland, R.; Yang, M.; Xu, H.; Zhang, L.; Yin, H.; Zhu, Q. Melatonin Receptor Genes in Vertebrates. Int. J. Mol. Sci. 2013, 14, 11208–11223. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L. Melatonin receptors: Are there multiple subtypes? Trends Pharmacol. Sci. 1995, 16, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Sugden, D.; Davidson, K.; Hough, K.A.; Teh, M.-T. Melatonin, Melatonin Receptors and Melanophores: A Moving Story. Pigment Cell Res. 2004, 17, 454–460. [Google Scholar] [CrossRef]
- Mailliet, F.; Ferry, G.; Vella, F.; Thiam, K.; Delagrange, P.; Boutin, J.A. Organs from mice deleted for NRH:quinone oxidoreductase 2 are deprived of the melatonin binding site MT3. FEBS Lett. 2004, 578, 116–120. [Google Scholar] [CrossRef]
- Nosjean, O.; Ferro, M.; Cogé, F.; Beauverger, P.; Henlin, J.-M.; Lefoulon, F.; Fauchère, J.-L.; Delagrange, P.; Canet, E.; Boutin, J.A. Identification of the Melatonin-binding SiteMT 3 as the Quinone Reductase 2. J. Biol. Chem. 2000, 275, 31311–31317. [Google Scholar] [CrossRef]
- Sampaio, L.D.F.S.; Mesquita, F.P.; Sousa, P.R.M.; Silva, J.L.; Alves, C.N. The melatonin analog 5-MCA-NAT increases endogenous dopamine levels by binding NRH:quinone reductase enzyme in the developing chick retina. Int. J. Dev. Neurosci. 2014, 38, 119–126. [Google Scholar] [CrossRef]
- Rivkees, S.A.; Cassone, V.M.; Weaver, D.R.; Reppert, S.M. Melatonin Receptors in Chick Brain: Characterization and Localization. Endocrinology 1989, 125, 363–368. [Google Scholar] [CrossRef]
- Stehle, J. Melatonin binding sites in brain of the 2-day-old chicken: An autoradiographic localisation. J. Neural Transm. 1990, 81, 83–89. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Marcus Leo, M.D.; Subramani, J.; Anish, D.; Sudhagar, M.; Ahmed, K.A.; Saxena, M.; Tyagi, J.S.; Sastry, K.V.H.; Saxena, V.K. Expression analysis of melatonin receptor subtypes in the ovary of domestic chicken. Vet. Res. Commun. 2009, 33, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Takaku, M.; Zou, L.; Gu, A.; Chou, W.; Zhang, G.; Wu, B.; Kong, Q.; Thomas, S.Y.; Serody, J.S.; et al. Reversing SKI–SMAD4-mediated suppression is essential for TH17 cell differentiation. Nature 2017, 551, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Melatonin Nuclear Receptors Mediate Green-and-Blue-Monochromatic-Light-Combinations-Inhibited B Lymphocyte Apoptosis in the Bursa of Chickens via Reducing Oxidative Stress and Nfκb Expression. Antioxidants 2022, 11, 748. [Google Scholar] [CrossRef]
- Cutando, A.; Aneiros-Fernández, J.; López-Valverde, A.; Arias-Santiago, S.; Aneiros-Cachaza, J.; Reiter, R.J. A new perspective in Oral health: Potential importance and actions of melatonin receptors MT1, MT2, MT3, and RZR/ROR in the oral cavity. Arch. Oral Biol. 2011, 56, 944–950. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Effect of the melatonin nuclear receptor RORα on monochromatic light-induced T-lymphocyte proliferation in chicken thymus. Immunol. Lett. 2019, 213, 21–29. [Google Scholar] [CrossRef]
- Ding, S.; Lin, N.; Sheng, X.; Zhao, Y.; Su, Y.; Xu, L.; Tong, R.; Yan, Y.; Fu, Y.; He, J.; et al. Melatonin stabilizes rupture-prone vulnerable plaques via regulating macrophage polarization in a nuclear circadian receptor RORα-dependent manner. J. Pineal Res. 2019, 67, e12581. [Google Scholar] [CrossRef]
- Farez, M.F.; Calandri, I.L.; Correale, J.; Quintana, F.J. Anti-inflammatory effects of melatonin in multiple sclerosis. BioEssays 2016, 38, 1016–1026. [Google Scholar] [CrossRef]
- Liu, H.; Aramaki, M.; Fu, Y.; Forrest, D. Retinoid-Related Orphan Receptor β and Transcriptional Control of Neuronal Differentiation. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 227–255. [Google Scholar]
- Jetten, A.M.; Cook, D.N. (Inverse) Agonists of Retinoic Acid–Related Orphan Receptor γ: Regulation of Immune Responses, Inflammation, and Autoimmune Disease. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 371–390. [Google Scholar] [CrossRef]
- Kasal, D.N.; Bendelac, A. Multi-transcription factor reporter mice delineate early precursors to the ILC and LTi lineages. J. Exp. Med. 2021, 218, e20200487. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.; Álvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J. Melatonin: Buffering the Immune System. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef]
- Dzhagalov, I.; Giguère, V.; He, Y.-W. Lymphocyte Development and Function in the Absence of Retinoic Acid-Related Orphan Receptor α. J. Immunol. 2004, 173, 2952–2959. [Google Scholar] [CrossRef]
- Lardone, P.J.; Guerrero, J.M.; Fernández-Santos, J.M.; Rubio, A.; Martín-Lacave, I.; Carrillo-Vico, A. Melatonin synthesized by T lymphocytes as a ligand of the retinoic acid-related orphan receptor: Melatonin, natural ligand of RORα. J. Pineal Res. 2011, 51, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Lardone, P.J.; Carrillo-Vico, A.; Molinero, P.; Rubio, A.; Guerrero, J.M. A novel interplay between membrane and nuclear melatonin receptors in human lymphocytes: Significance in IL-2 production. Cell. Mol. Life Sci. 2009, 66, 516–525. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhao, Y.; Xu, L.; Gao, L.; Su, Y.; Lin, N.; Pu, J. The nuclear melatonin receptor ROR α is a novel endogenous defender against myocardial ischemia/reperfusion injury. J. Pineal Res. 2016, 60, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Lin, J.-X.; Leonard, W.J. Interleukin-2 at the Crossroads of Effector Responses, Tolerance, and Immunotherapy. Immunity 2013, 38, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mauriño, S.; Gonzalez-Haba, M.G.; Calvo, J.R.; Goberna, R.; Guerrero, J.M. Involvement of nuclear binding sites for melatonin in the regulation of IL-2 and IL-6 production by human blood mononuclear cells. J. Neuroimmunol. 1998, 92, 76–84. [Google Scholar] [CrossRef]
- Ren, W.; Liu, G.; Chen, S.; Yin, J.; Wang, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Li, T.; et al. Melatonin signaling in T cells: Functions and applications. J. Pineal Res. 2017, 62, e12394. [Google Scholar] [CrossRef]
- Wang, R.-X.; Liu, H.; Xu, L.; Zhang, H.; Zhou, R.-X. Melatonin downregulates nuclear receptor RZR/RORγ expression causing growth-inhibitory and anti-angiogenesis activity in human gastric cancer cells in vitro and in vivo. Oncol. Lett. 2016, 12, 897–903. [Google Scholar] [CrossRef]
- Jetten, A.M.; Takeda, Y.; Slominski, A.; Kang, H.S. Retinoic acid-related orphan receptor γ (RORγ): Connecting sterol metabolism to regulation of the immune system and autoimmune disease. Curr. Opin. Toxicol. 2018, 8, 66–80. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.; Takeda, Y.; Janjetovic, Z.; Brożyna, A.A.; Skobowiat, C.; Wang, J.; Postlethwaite, A.; Li, W.; Tuckey, R.C.; et al. RORα and ROR γ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014, 28, 2775–2789. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.-K.; Hobrath, J.V.; Oak, A.S.W.; Tang, E.K.Y.; Tieu, E.W.; Li, W.; Tuckey, R.C.; Jetten, A.M. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORα and RORγ. J. Steroid Biochem. Mol. Biol. 2017, 173, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Agez, L.; Laurent, V.; Pévet, P.; Masson-Pévet, M.; Gauer, F. Melatonin affects nuclear orphan receptors mRNA in the rat suprachiasmatic nuclei. Neuroscience 2007, 144, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, J. The Pineal Gland: A Neurochemical Transducer: Chemical signals from nerves regulate synthesis of melatonin and convey information about internal clocks. Science 1974, 184, 1341–1348. [Google Scholar] [CrossRef]
- Deguchi, T.; Axelrod, J. Control of Circadian Change of Serotonin N -Acetyltransferase Activity in the Pineal Organ by the β-Adrenergic Receptor. Proc. Natl. Acad. Sci. USA 1972, 69, 2547–2550. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.C.; Weller, J.L. Indole Metabolism in the Pineal Gland: A Circadian Rhythm in N -Acetyltransferase. Science 1970, 169, 1093–1095. [Google Scholar] [CrossRef]
- Bernard, M.; Voisin, P.; Guerlotté, J.; Collin, J.-P. Molecular and cellular aspects of hydroxyindole-O-methyltransferase expression in the developing chick pineal gland. Dev. Brain Res. 1991, 59, 75–81. [Google Scholar] [CrossRef]
- Kawashima, K. Improvement of radioimmunoassay for serum and tissue melatonin. J. Pharmacobiodyn. 1982, 5, 5–25. [Google Scholar]
- Rollag, M.D.; Niswender, G.D. Radioimmunoassay of Serum Concentrations of Melatonin in Sheep Exposed to Different Lighting Regimens. Endocrinology 1976, 98, 482–489. [Google Scholar] [CrossRef]
- Liou, S.S.; Cogburn, L.A.; Biellier, H.V. Photoperiodic regulation of plasma melatonin levels in the laying chicken (Gallus domesticus). Gen. Comp. Endocrinol. 1987, 67, 221–226. [Google Scholar] [CrossRef]
- Cockrem, J.F.; Follett, B.K. Circadian rhythm of melatonin in the pineal gland of the Japanese quail (Coturnix coturnix japonica). J. Endocrinol. 1985, 107, 317–324. [Google Scholar] [CrossRef]
- Oshima, I.; Yamada, H.; Sato, K.; Ebihara, S. The phase relationship between the circadian rhythms of locomotor activity and circulating melatonin in the pigeon (Columba livia). Gen. Comp. Endocrinol. 1987, 67, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Doi, O.; Ohno, A.; Kaneda, T.; Iwasawa, A.; Nakamura, T. Immunohistochemical Demonstration and Daily Rhythms of Melatonin in the Chicken Pineal Gland. Jpn. Poult. Sci. 1995, 32, 90–98. [Google Scholar] [CrossRef][Green Version]
- McFarland, L.Z.; Wilson, W.O.; Winget, C.M. Response of the Chicken Pineal Gland, Blood and Reproductive Organs to Darkness. Poult. Sci. 1969, 48, 903–907. [Google Scholar] [CrossRef]
- Winget, C.M. Morphological and biochemical changes associated with a change in photoperiod (Gallus domesticus). Am. Zool. 1966, 6, 506. [Google Scholar]
- Milcu, S.M.; Postelnicu, D.; Ionescu, G. Observations on the structure of the pineal body in relation to the phases of the sexual cycle in hens. Stud. Cercet. Endocrinol. 1964, 15, 485–486. [Google Scholar]
- Ralph, C.L. Structure and Alleged Functions of Avian Pineals. Am. Zool. 1970, 10, 217–235. [Google Scholar] [CrossRef][Green Version]
- Lane, K.B.; Ralph, C.L.; Gilbertt, S. Lack of correlation between sexual maturation and pineal cytology in Japanese quail. Anat. Rec. 1969, 163, 215. [Google Scholar]
- Ralph, C.L.; Lane, K.B. Morphology of the pineal body of wild house sparrows (Passer domesticus) in relation to reproduction and age. Can. J. Zool. 1969, 47, 1205–1208. [Google Scholar] [CrossRef]
- Chen, Q.; Wei, W. Effects and mechanisms of melatonin on inflammatory and immune responses of adjuvant arthritis rat. Int. Immunopharmacol. 2002, 2, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-I.; Dadakhujaev, S.; Ryu, H.; im Kim, T.; Kim, E.K. Melatonin protects against oxidative stress in granular corneal dystrophy type 2 corneal fibroblasts by mechanisms that involve membrane melatonin receptors: Melatonin protects GCD2 corneal fibroblasts. J. Pineal Res. 2011, 51, 94–103. [Google Scholar] [CrossRef]
- Lardone, P.J.; Rubio, A.; Cerrillo, I.; Gómez-Corvera, A.; Carrillo-Vico, A.; Sanchez-Hidalgo, M.; Guerrero, J.M.; Fernandez-Riejos, P.; Sanchez-Margalet, V.; Molinero, P. Blocking of melatonin synthesis and MT1 receptor impairs the activation of Jurkat T cells. Cell. Mol. Life Sci. 2010, 67, 3163–3172. [Google Scholar] [CrossRef]
- Li, J.; Cao, J.; Wang, Z.; Dong, Y.; Chen, Y. Melatonin plays a critical role in inducing B lymphocyte proliferation of the bursa of Fabricius in broilers via monochromatic lights. J. Photochem. Photobiol. B 2015, 142, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cao, Q.; Cheng, Y.; Zhao, D.; Wang, Z.; Yang, H.; Wu, Q.; You, L.; Wang, Y.; Lin, Y.; et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. USA 2018, 115, E2960–E2969. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Kyoung Seo, E.; Lee, J.; Young Lee, J. Suppression of Splenic Lymphocyte Proliferation by Eucommia ulmoides and Genipin. Chem. Biodivers. 2015, 12, 538–546. [Google Scholar] [CrossRef]
- González Maglio, D.H.; Paz, M.L.; Leoni, J. Sunlight Effects on Immune System: Is There Something Else in addition to UV-Induced Immunosuppression? BioMed Res. Int. 2016, 2016, 1934518. [Google Scholar] [CrossRef]
- Dubocovich, M.L. Molecular pharmacology regulation and function of mammalian melatonin receptors. Front. Biosci. 2003, 8, d1093–d1108. [Google Scholar] [CrossRef] [PubMed]
- Leon, J.; Acuña-Castroviejo, D.; Sainz, R.M.; Mayo, J.C.; Tan, D.-X.; Reiter, R.J. Melatonin and mitochondrial function. Life Sci. 2004, 75, 765–790. [Google Scholar] [CrossRef]
- Lacoste, B.; Angeloni, D.; Dominguez-Lopez, S.; Calderoni, S.; Mauro, A.; Fraschini, F.; Descarries, L.; Gobbi, G. Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. J. Pineal Res. 2015, 58, 397–417. [Google Scholar] [CrossRef]
- Dzhagalov, I.; Zhang, N.; He, Y.-W. The roles of orphan nuclear receptors in the development and function of the immune system. Cell. Mol. Immunol. 2004, 1, 401–407. [Google Scholar] [PubMed]
- Gupta, S.; Haldar, C.; Ahmad, R. Photoperiodic regulation of nuclear melatonin receptor RORα in lymphoid organs of a tropical rodent Funambulus pennanti: Role in seasonal oxidative stress. J. Photochem. Photobiol. B 2015, 142, 141–153. [Google Scholar] [CrossRef]
- Currier, N.L.; Sun, L.Z.-Y.; Miller, S.C. Exogenous melatonin: Quantitative enhancement in vivo of cells mediating non-specific immunity. J. Neuroimmunol. 2000, 104, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Kang, J.; Fan, W.; He, H.; Huang, F. ROR: Nuclear Receptor for Melatonin or Not? Molecules 2021, 26, 2693. [Google Scholar] [CrossRef]
- Poon, A.M.S.; Liu, Z.M.; Pang, C.S.; Brown, G.M.; Pang, S.F. Evidence for a Direct Action of Melatonin on the Immune System. Neurosignals 1994, 3, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Farez, M.F.; Mascanfroni, I.D.; Méndez-Huergo, S.P.; Yeste, A.; Murugaiyan, G.; Garo, L.P.; Balbuena Aguirre, M.E.; Patel, B.; Ysrraelit, M.C.; Zhu, C.; et al. Melatonin Contributes to the Seasonality of Multiple Sclerosis Relapses. Cell 2015, 162, 1338–1352. [Google Scholar] [CrossRef]
- Ciriaco, E.; Píñera, P.P.; Díaz-Esnal, B.; Laurà, R. Age-related changes in the avian primary lymphoid organs (thymus and bursa of Fabricius): Aging of Avian Primary Lymphoid Organs. Microsc. Res. Tech. 2003, 62, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Kliger, C.A.; Gehad, A.E.; Hulet, R.M.; Roush, W.B.; Lillehoj, H.S.; Mashaly, M.M. Effects of photoperiod and melatonin on lymphocyte activities in male broiler chickens. Poult. Sci. 2000, 79, 18–25. [Google Scholar] [CrossRef]
- Moore, C.B.; Siopes, T.D. Effects of Lighting Conditions and Melatonin Supplementation on the Cellular and Humoral Immune Responses in Japanese Quail Coturnix coturnix japonica. Gen. Comp. Endocrinol. 2000, 119, 95–104. [Google Scholar] [CrossRef]
- Scott, R.P.; Siopes, T.D. Light color: Effect on blood cells, immune function and stress status in turkey hens. Comp. Biochem. Physiol. A Physiol. 1994, 108, 161–168. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin mediates monochromatic light–induced proliferation of T/B lymphocytes in the spleen via the membrane receptor or nuclear receptor. Poult. Sci. 2020, 99, 4294–4302. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, J.; Wang, Z.; Dong, Y.; Chen, Y. Effect of a combination of green and blue monochromatic light on broiler immune response. J. Photochem. Photobiol. B 2014, 138, 118–123. [Google Scholar] [CrossRef]
- Moore, C.B.; Siopes, T.D. Melatonin enhances cellular and humoral immune responses in the Japanese quail (Coturnix coturnix japonica) via an opiatergic mechanism. Gen. Comp. Endocrinol. 2003, 131, 258–263. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin receptor subtypes Mel1a and Mel1c but not Mel1b are associated with monochromatic light-induced B-lymphocyte proliferation in broilers. Domest. Anim. Endocrinol. 2013, 45, 206–215. [Google Scholar] [CrossRef]
- Chen, F.; Reheman, A.; Cao, J.; Wang, Z.; Dong, Y.; Zhang, Y.; Chen, Y. Effect of melatonin on monochromatic light-induced T-lymphocyte proliferation in the thymus of chickens. J. Photochem. Photobiol. B 2016, 161, 9–16. [Google Scholar] [CrossRef]
- Xie, D.; Wang, Z.X.; Dong, Y.L.; Cao, J.; Wang, J.F.; Chen, J.L.; Chen, Y.X. Effects of Monochromatic Light on Immune Response of Broilers. Poult. Sci. 2008, 87, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. A Green and Blue Monochromatic Light Combination Therapy Reduces Oxidative Stress and Enhances B-Lymphocyte Proliferation through Promoting Melatonin Secretion. Oxid. Med. Cell. Longev. 2021, 2021, 5595376. [Google Scholar] [CrossRef] [PubMed]
- Vishwas, D.K.; Haldar, C. Photoperiodic induced melatonin regulates immunity and expression pattern of melatonin receptor MT1 in spleen and bone marrow mononuclear cells of male golden hamster. J. Photochem. Photobiol. B 2013, 128, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Haldar, C. Reciprocal interaction between melatonin receptors (Mel1a, Mel1b, and Mel1c) and androgen receptor (AR) expression in immunoregulation of a seasonally breeding bird, Perdicula asiatica: Role of photoperiod. J. Photochem. Photobiol. B 2013, 122, 52–60. [Google Scholar] [CrossRef]
- Venegas, C.; García, J.A.; Doerrier, C.; Volt, H.; Escames, G.; López, L.C.; Reiter, R.J.; Acuña-Castroviejo, D. Analysis of the daily changes of melatonin receptors in the rat liver. J. Pineal Res. 2013, 54, 313–321. [Google Scholar] [CrossRef]
- Jimenez-Jorge, S.; Jimenez-Caliani, A.J.; Guerrero, J.M.; Naranjo, M.C.; Lardone, P.J.; Carrillo-Vico, A.; Osuna, C.; Molinero, P. Melatonin synthesis and melatonin-membrane receptor (MT1) expression during rat thymus development: Role of the pineal gland. J. Pineal Res. 2005, 39, 77–83. [Google Scholar] [CrossRef]
- Sánchez-Hidalgo, M.; Guerrero Montávez, J.M.; Carrascosa-Salmoral, M.D.P.; Naranjo Gutierrez, M.D.C.; Lardone, P.J.; de la Lastra Romero, C.A. Decreased MT1 and MT2 melatonin receptor expression in extrapineal tissues of the rat during physiological aging. J. Pineal Res. 2009, 46, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R.; Cassone, V.M.; Godson, C.; Kolakowski, L.F. Melatonin receptors are for the birds: Molecular analysis of two receptor subtypes differentially expressed in chick brain. Neuron 1995, 15, 1003–1015. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Henkel, T. Function and activation of NF-kappaB in the immune system. Annu. Rev. Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef]
- Covert, M.W.; Leung, T.H.; Gaston, J.E.; Baltimore, D. Achieving stability of lipopolysaccharide-induced NF-κB activation. Science 2005, 309, 1854–1857. [Google Scholar] [CrossRef]
- DM, R. The NF-kappa B activation pathway: A paradigm in information transfer from membrane to nucleus. Sci STKE 1999, 1999, RE1. [Google Scholar]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Basak, S.; Kim, H.; Kearns, J.D.; Tergaonkar, V.; O’Dea, E.; Werner, S.L.; Benedict, C.A.; Ware, C.F.; Ghosh, G.; Verma, I.M.; et al. A Fourth IκB Protein within the NF-κB Signaling Module. Cell 2007, 128, 369–381. [Google Scholar] [CrossRef]
- Delerive, P.; Monté, D.; Dubois, G.; Trottein, F.; Fruchart-Najib, J.; Mariani, J.; Fruchart, J.; Staels, B. The orphan nuclear receptor RORα is a negative regulator of the inflammatory response. EMBO Rep. 2001, 2, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jia, Y.; Sun, Q.; Dong, H.; Zhao, R. White light emitting diode induces autophagy in hippocampal neuron cells through GSK-3-mediated GR and RORα pathways. Aging 2019, 11, 1832–1849. [Google Scholar] [CrossRef] [PubMed]
- Mühlbauer, E.; Bazwinsky-Wutschke, I.; Wolgast, S.; Labucay, K.; Peschke, E. Differential and day-time dependent expression of nuclear receptors RORα, RORβ, RORγ and RXRα in the rodent pancreas and islet. Mol. Cell. Endocrinol. 2013, 365, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kharwar, R.K.; Haldar, C. Daily variation in antioxidant enzymes and lipid peroxidation in lungs of a tropical bird Perdicula asiatica: Role of melatonin and nuclear receptor RORα. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2012, 162, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Litvinenko, G.I.; Shurlygina, A.V.; Gritsyk, O.B.; Mel’nikova, E.V.; Tenditnik, M.V.; Avrorov, P.A.; Trufakin, V.A. Effects of Melatonin on Morphological and Functional Parameters of the Pineal Gland and Organs of Immune System in Rats During Natural Light Cycle and Constant Illumination. Bull. Exp. Biol. Med. 2015, 159, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Effects of Different Monochromatic Light Combinations on Cecal Microbiota Composition and Cecal Tonsil T Lymphocyte Proliferation. Front. Immunol. 2022, 13, 849780. [Google Scholar] [CrossRef]
- Haldar, C.; Ahmad, R. Photoimmunomodulation and melatonin. J. Photochem. Photobiol. B 2010, 98, 107–117. [Google Scholar] [CrossRef]
- Yellon, S.M.; Fagoaga, O.R.; Nehlsen-Cannarella, S.L. Influence of photoperiod on immune cell functions in the male Siberian hamster. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1999, 276, R97–R102. [Google Scholar] [CrossRef]
- Brainard, G.C.; Vaughan, M.K.; Reiter, R.J. Effect of Light Irradiance and Wavelength on the Syrian Hamster Reproductive System*. Endocrinology 1986, 119, 648–654. [Google Scholar] [CrossRef]
- Vaughan, M.K.; Hubbard, G.B.; Champney, T.H.; Vaughan, G.M.; Little, J.C.; Reiter, R.J. Splenic hypertrophy and extramedullary hematopoiesis induced in male Syrian hamsters by short photoperiod or melatonin injections and reversed by melatonin pellets or pinealectomy. Am. J. Anat. 1987, 179, 131–136. [Google Scholar] [CrossRef]
- Blatchford, R.A.; Klasing, K.C.; Shivaprasad, H.L.; Wakenell, P.S.; Archer, G.S.; Mench, J.A. The effect of light intensity on the behavior, eye and leg health, and immune function of broiler chickens. Poult. Sci. 2009, 88, 20–28. [Google Scholar] [CrossRef]
- Demas, G.E.; Nelson, R.J. Photoperiod and Temperature Interact to Affect Immune Parameters in Adult Male Deer Mice: (Peromyscus manicuiatus). J. Biol. Rhythm. 1996, 11, 94–102. [Google Scholar] [CrossRef]
- Prendergast, B.J.; Yellon, S.M.; Tran, L.T.; Nelson, R.J. Photoperiod Modulates the Inhibitory Effect of In Vitro Melatonin on Lymphocyte Proliferation in Female Siberian Hamsters. J. Biol. Rhythm. 2001, 16, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Physiological crosstalk between the AC/PKA and PLC/PKC pathways modulates melatonin-mediated, monochromatic-light-induced proliferation of T-lymphocytes in chickens. Cell Tissue Res. 2017, 369, 555–565. [Google Scholar] [CrossRef]
- Rozenboim, I.; El Halawani, M.E.; Kashash, Y.; Piestun, Y.; Halevy, O. The effect of monochromatic photostimulation on growth and development of broiler birds. Gen. Comp. Endocrinol. 2013, 190, 214–219. [Google Scholar] [CrossRef]
- Xie, D.; Li, J.; Wang, Z.X.; Cao, J.; Li, T.T.; Chen, J.L.; Chen, Y.X. Effects of monochromatic light on mucosal mechanical and immunological barriers in the small intestine of broilers. Poult. Sci. 2011, 90, 2697–2704. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Effects of Monochromatic Light on Proliferation Response of Splencyte in Broilers. Anat. Histol. Embryol. 2008, 37, 332–337. [Google Scholar] [CrossRef]
- Drazen, D.L.; Nelson, R.J. Melatonin Receptor Subtype MT2 (Mel 1b) and Not mt1 (Mel 1a) Is Associated with Melatonin-Induced Enhancement of Cell-Mediated and Humoral Immunity. Neuroendocrinology 2001, 74, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, S.; Ueda, E.; Sakaue, M.; Patel, D.D.; Medvedev, A.; Zhang, F.; Jetten, A.M. Retinoid-related orphan receptor γ (RORγ) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc. Natl. Acad. Sci. USA 2000, 97, 10132–10137. [Google Scholar] [CrossRef]
- Santori, F.R.; Huang, P.; van de Pavert, S.A.; Douglass, E.F.; Leaver, D.J.; Haubrich, B.A.; Keber, R.; Lorbek, G.; Konijn, T.; Rosales, B.N.; et al. Identification of Natural RORγ Ligands that Regulate the Development of Lymphoid Cells. Cell Metab. 2015, 21, 286–298. [Google Scholar] [CrossRef]
- He, Y.-W.; Deftos, M.L.; Ojala, E.W.; Bevan, M.J. RORγt, a Novel Isoform of an Orphan Receptor, Negatively Regulates Fas Ligand Expression and IL-2 Production in T Cells. Immunity 1998, 9, 797–806. [Google Scholar] [CrossRef]
- Eberl, G.; Littman, D.R. Thymic Origin of Intestinal αß T Cells Revealed by Fate Mapping of RORγt+ Cells. Science 2004, 305, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Chairakaki, A. The role of intestinal microbial/immune cell interactions in patients with Inflammatory Bowel Disease. Ann. Gastroenterol. 2009, 22, 239–241. [Google Scholar]
- Lotz, M.; Menard, S.; Hornef, M. Innate immune recognition on the intestinal mucosa. Int. J. Med. Microbiol. 2007, 297, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.V.; O’Farrelly, C.; Feighery, C.; Murchan, P.; Leonard, N.; Fulton, G.; O’Morain, C.; Keane, F.B.V.; Tanner, W.A. Impaired gut barrier function in malnourished patients: Impaired gut barrier function in malnourished patients. Br. J. Surg. 1996, 83, 1288–1291. [Google Scholar] [CrossRef]
- Fasina, Y.O.; Classen, H.L.; Garlich, J.D.; Black, B.L.; Ferket, P.R.; Uni, Z.; Olkowski, A.A. Response of Turkey Poults to Soybean Lectin Levels Typically Encountered in Commercial Diets. 2. Effect on Intestinal Development and Lymphoid Organs. Poult. Sci. 2006, 85, 870–877. [Google Scholar] [CrossRef]
- Uni, Z.; Gal-Garber, O.; Geyra, A.; Sklan, D.; Yahav, S. Changes in Growth and Function of Chick Small Intestine Epithelium Due to Early Thermal Conditioning. Poult. Sci. 2001, 80, 438–445. [Google Scholar] [CrossRef]
- Ding, L.-A.; Li, J.-S. Gut in diseases: Physiological elements and their clinical significance. World J. Gastroenterol. 2003, 9, 2385. [Google Scholar] [CrossRef]
- Franco, J.; Murakami, A.; Natali, M.; Garcia, E.; Furlan, A. Influence of delayed placement and dietary lysine levels on small intestine morphometrics and performance of broilers. Rev. Bras. Ciênc. Avícola 2006, 8, 233–241. [Google Scholar] [CrossRef]
- Mekbungwan, A.; Yamauchi, K.; Sakaida, T. Intestinal Villus Histological Alterations in Piglets fed Dietary Charcoal Powder Including Wood Vinegar Compound Liquid. Anat. Histol. Embryol. J. Vet. Med. Ser. C 2004, 33, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hu, C.; Xia, M.; Zhan, X.; Wang, M. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003, 82, 1030–1036. [Google Scholar] [CrossRef]
- Tasaki, I.; Takahashi, N. Absorption of Amino Acids from the Small Intestine of Domestic Fowl. J. Nutr. 1966, 88, 359–364. [Google Scholar] [CrossRef]
- Bao, H.; She, R.; Liu, T.; Zhang, Y.; Peng, K.S.; Luo, D.; Yue, Z.; Ding, Y.; Hu, Y.; Liu, W.; et al. Effects of pig antibacterial peptides on growth performance and intestine mucosal immune of broiler chickens. Poult. Sci. 2009, 88, 291–297. [Google Scholar] [CrossRef]
- Awad, W.A.; Ghareeb, K.; Abdel-Raheem, S.; Böhm, J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009, 88, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; She, R.; Wang, K.; Bao, H.; Zhang, Y.; Luo, D.; Hu, Y.; Ding, Y.; Wang, D.; Peng, K. Effects of Rabbit Sacculus Rotundus Antimicrobial Peptides on the Intestinal Mucosal Immunity in Chickens. Poult. Sci. 2008, 87, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, Y. Intraepithelial lymphocytes in birds. Adv. Host Def. Mech. 1994, 37, 163–174. [Google Scholar]
- Duncker, S.C.; Lorentz, A.; Schroeder, B.; Breves, G.; Bischoff, S.C. Effect of orally administered probiotic E. coli strain Nissle 1917 on intestinal mucosal immune cells of healthy young pigs. Vet. Immunol. Immunopathol. 2006, 111, 239–250. [Google Scholar] [CrossRef]
- Sim, G.-K. Intraepithelial Lymphocytes and the Immune System. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 1995; pp. 297–343. [Google Scholar]
- Bäck, O. Studies on the lymphocytes in the intestinal epithelium of the chicken: 1. Ontogeny. Acta Pathol. Microbiol. Scand. A Pathol. 2009, 80, 84–90. [Google Scholar] [CrossRef]
- Vervelde, L.; Jeurissen, S.H.M. Postnatal development of intra-epithelial leukocytes in the chicken digestive tract: Phenotypical characterization in situ. Cell Tissue Res. 1993, 274, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.I.; Akter, S.H.; Islam, M.N.; Karim, M.R.; Islam, M.R.; Kon, Y. The Effect of Selenium and Vitamin E on the Lymphocytes and Immunoglobulin-containing Plasma cells in the Lymphoid organ and Mucosa-Associated Lymphatic Tissues of Broiler Chickens. Anat. Histol. Embryol. J. Vet. Med. Ser. C 2007, 37, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lian, G.; Gong, X. Enhancement of Mucosal Immune Responses in Chickens by Oral Administration of Cysteamine. Poult. Sci. 2007, 86, 1323–1328. [Google Scholar] [CrossRef]
- Hanson, L.A. Breastfeeding Provides Passive and Likely Long-Lasting Active Immunity. Ann. Allergy Asthma Immunol. 1998, 81, 523–537. [Google Scholar] [CrossRef]
- Lammers, A.; Wieland, W.H.; Kruijt, L.; Jansma, A.; Straetemans, T.; Schots, A.; den Hartog, G.; Parmentier, H.K. Successive immunoglobulin and cytokine expression in the small intestine of juvenile chicken. Dev. Comp. Immunol. 2010, 34, 1254–1262. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Yang, Q. Effect of compound mucosal immune adjuvant on mucosal and systemic immune responses in chicken orally vaccinated with attenuated Newcastle-disease vaccine. Vaccine 2007, 25, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Kilic, U.; Yilmaz, B.; Ugur, M.; Yüksel, A.; Reiter, R.J.; Hermann, D.M.; Kilic, E. Evidence that membrane-bound G protein-coupled melatonin receptors MT1 and MT2 are not involved in the neuroprotective effects of melatonin in focal cerebral ischemia: Role of melatonin receptors in brain injury. J. Pineal Res. 2012, 52, 228–235. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Z.; Cao, J.; Dong, Y.; Wang, T.; Chen, Y. Effects of monochromatic light stimuli on the development and Muc2 expression of goblet cells in broiler small intestines during embryogenesis. Poult. Sci. 2014, 93, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Specian, R.D.; Oliver, M.G. Functional biology of intestinal goblet cells. Am. J. Physiol.-Cell Physiol. 1991, 260, C183–C193. [Google Scholar] [CrossRef] [PubMed]
- McDole, J.R.; Wheeler, L.W.; McDonald, K.G.; Wang, B.; Konjufca, V.; Knoop, K.A.; Newberry, R.D.; Miller, M.J. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 2012, 483, 345–349. [Google Scholar] [CrossRef]
- Deplancke, B.; Gaskins, H.R. Microbial modulation of innate defense: Goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001, 73, 1131S–1141S. [Google Scholar] [CrossRef] [PubMed]
- Forder, R.E.A.; Howarth, G.S.; Tivey, D.R.; Hughes, R.J. Bacterial Modulation of Small Intestinal Goblet Cells and Mucin Composition During Early Posthatch Development of Poultry. Poult. Sci. 2007, 86, 2396–2403. [Google Scholar] [CrossRef]
- Forstner, J.; Oliver, M.; Sylvester, F. Production, structure and biologic relevance of gastrointestinal mucins. Infect. Gastrointest. Tract 1995, 1995, 71–88. [Google Scholar]
- Dekker, J.; Rossen, J.W.A.; Büller, H.A.; Einerhand, A.W.C. The MUC family: An obituary. Trends Biochem. Sci. 2002, 27, 126–131. [Google Scholar] [CrossRef]
- Habte, H.H.; Mall, A.S.; de Beer, C.; Lotz, Z.E.; Kahn, D. The role of crude human saliva and purified salivary MUC5B and MUC7 mucins in the inhibition of Human Immunodeficiency Virus type 1 in an inhibition assay. Virol. J. 2006, 3, 99. [Google Scholar] [CrossRef]
- Göttke, M.; Keller, K.; Chadee, K.; Göettke, M.; Belley, A. Intestinal mucins in colonization and host defense against pathogens. Am. J. Trop. Med. Hyg. 1999, 60, 10–15. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Uni, Z.; Smirnov, A.; Sklan, D. Pre- and posthatch development of goblet cells in the broiler small intestine: Effect of delayed access to feed. Poult. Sci. 2003, 82, 320–327. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.A.C.L.; Monteiro, M.P.C.; Robbs, P.G.; Leite, S.G.F. Cellular automata cryptographic model based on bi-directional toggle rules. Aquac. Int. 1999, 7, 261–275. [Google Scholar] [CrossRef]
- Podolsky, D.K. Regulation of intestinal epithelial proliferation: A few answers, many questions. Am. J. Physiol.-Gastrointest. Liver Physiol. 1993, 264, G179–G186. [Google Scholar] [CrossRef] [PubMed]
- Engberg, R.M.; Hedemann, M.S.; Leser, T.D.; Jensen, B.B. Effect of zinc bacitracin and salinomycin on intestinal microflora and performance of broilers. Poult. Sci. 2000, 79, 1311–1319. [Google Scholar] [CrossRef]
- Lee, K.-W.; Ho Hong, Y.; Lee, S.-H.; Jang, S.I.; Park, M.-S.; Bautista, D.A.; Donald Ritter, G.; Jeong, W.; Jeoung, H.-Y.; An, D.-J.; et al. Effects of anticoccidial and antibiotic growth promoter programs on broiler performance and immune status. Res. Vet. Sci. 2012, 93, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Rubel, M.d.Z.U.; Beg, M.d.A.; Saiful Islam, K.B.M.; Begum, M.; Patoary, M.d.M.U. Effect of Dietary Supplement of Algae (Spirulina platensis) as an Alternative to Antibiotics on Growth Performance and Health Status of Broiler Chickens. Int. J. Poult. Sci. 2019, 18, 576–584. [Google Scholar] [CrossRef]
- Skelly, A.N.; Sato, Y.; Kearney, S.; Honda, K. Mining the microbiota for microbial and metabolite-based immunotherapies. Nat. Rev. Immunol. 2019, 19, 305–323. [Google Scholar] [CrossRef]
- Strus, M.; Pakosz, K.; Gościniak, H.; Przondo-Mordarska, A.; Rozynek, E.; Pituch, H.; Meisel-Mikołajczyk, F.; Heczko, P.B. Antagonistic activity of Lactobacillus bacteria strains against anaerobic gastrointestinal tract pathogens (Helicobacter pylori, Campylobacter coli, Campylobacter jejuni, Clostridium difficile). Med. Dosw. Mikrobiol. 2001, 53, 133–142. [Google Scholar]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Liu, J.; Stewart, S.N.; Robinson, K.; Yang, Q.; Lyu, W.; Whitmore, M.A.; Zhang, G. Linkage between the intestinal microbiota and residual feed intake in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Ravachol, J.; Borne, R.; Meynial-Salles, I.; Soucaille, P.; Pagès, S.; Tardif, C.; Fierobe, H.-P. Combining free and aggregated cellulolytic systems in the cellulosome-producing bacterium Ruminiclostridium cellulolyticum. Biotechnol. Biofuels 2015, 8, 114. [Google Scholar] [CrossRef]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef]
- Meijer, K.; de Vos, P.; Priebe, M.G. Butyrate and other short-chain fatty acids as modulators of immunity: What relevance for health? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Scott, N.A.; Andrusaite, A.; Andersen, P.; Lawson, M.; Alcon-Giner, C.; Leclaire, C.; Caim, S.; Le Gall, G.; Shaw, T.; Connolly, J.P.R.; et al. Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Sci. Transl. Med. 2018, 10, eaao4755. [Google Scholar] [CrossRef]
- Lenoir, M.; Martín, R.; Torres-Maravilla, E.; Chadi, S.; González-Dávila, P.; Sokol, H.; Langella, P.; Chain, F.; Bermúdez-Humarán, L.G. Butyrate mediates anti-inflammatory effects of Faecalibacterium prausnitzii in intestinal epithelial cells through Dact3. Gut Microbes 2020, 12, 1826748. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Smorodchenko, A.; Hess, C.; Aschenbach, J.R.; Molnár, A.; Dublecz, K.; Khayal, B.; Pohl, E.E.; Hess, M. Increased intracellular calcium level and impaired nutrient absorption are important pathogenicity traits in the chicken intestinal epithelium during Campylobacter jejuni colonization. Appl. Microbiol. Biotechnol. 2015, 99, 6431–6441. [Google Scholar] [CrossRef]
- Dolka, B.; Chrobak-Chmiel, D.; Makrai, L.; Szeleszczuk, P. Phenotypic and genotypic characterization of Enterococcus cecorum strains associated with infections in poultry. BMC Vet. Res. 2016, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Raikhlin, N.; Kvetnoy, I. Lightening effect of the extract of human appendix mucosa on frog skin melanophores. Bull Exp. Biol. Med. 1974, 8, 114–116. [Google Scholar]
- Bubenik, G.A.; Blask, D.E.; Brown, G.M.; Maestroni, G.J.M.; Pang, S.F.; Reiter, R.J.; Viswanathan, M.; Zisapel, N. Prospects of the Clinical Utilization of Melatonin. Neurosignals 1998, 7, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.H.; Lee, P.N.; Poon, A.M.S.; Shiu, S.Y.W.; Pang, S.E. The Gastrointestinal System: A Site of Melatonin Paracrine Action1. In Frontiers of Hormone Research; Pang, S.F., Reiter, R.J., Tang, P.L., Eds.; Karger Publishers: Basel, Switzerland, 1996; pp. 123–132. [Google Scholar]
- Lee, P.P.N.; Hong, G.X.; Pang, S.F. Melatonin in the Gastrointestinal Tract. In Role of Melatonin and Pineal Peptides in Neuroimmunomodulation; Fraschini, F., Reiter, R.J., Eds.; Springer US: Boston, MA, USA, 1991; pp. 127–136. [Google Scholar]
- Martin, M.T.; Azpiroz, F.; Malagelada, J.R. Melatonin and the gastrointestinal tract. Therapie 1998, 53, 453–458. [Google Scholar]
- Kachi, T.; Kurushima, M. Pineal-digestive organ relations: Physiological and pathophysiological significance of melatonin in the digestive system. Hirosaki Med. J. 2000, 51, 93–108. [Google Scholar]
- Bubenik, G.A.; Brown, G.M.; Grota, L.J. Immunohistological localization of melatonin in the rat digestive system. Experientia 1977, 33, 662–663. [Google Scholar] [CrossRef]
- Erspamer, V.; Asero, B. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 1952, 169, 800–801. [Google Scholar] [CrossRef]
- Holloway, W.; Grota, L.; Brown, G. Determination of immunoreactive melatonin in the colon of the rat by immunocytochemistry. J. Histochem. Cytochem. 1980, 28, 255–262. [Google Scholar] [CrossRef]
- Vakkuri, O.; Rintamäki, H.; Leppäluoto, J. Presence of immunoreactive melatonin in different tissues of the pigeon (Columba livia). Gen. Comp. Endocrinol. 1985, 58, 69–75. [Google Scholar] [CrossRef]
- Huether, G. Melatonin Synthesis in the Gastrointestinal Tract and the Impact of Nutritional Factors on Circulating Melatonin. Ann. N. Y. Acad. Sci. 1994, 719, 146–158. [Google Scholar] [CrossRef]
- Lee, P.P.N.; Pang, S.F. Melatonin and Its Receptors in the Gastrointestinal Tract. Neurosignals 1993, 2, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A.; Brown, G.M. Pinealectomy Reduces Melatonin Levels in the Serum but Not in the Gastrointestinal Tract of Rats. Neurosignals 1997, 6, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Huether, G.; Poeggeler, B.; Reimer, A.; George, A. Effect of tryptophan administration on circulating melatonin levels in chicks and rats: Evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci. 1992, 51, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J.; Frith, R.G.; Phillipou, G.; Matthews, C.D.; Seamark, R.F. A Specific Radioimmunoassay for Melatonin in Biological Tissue and Fluids and its Validation by Gas Chromatography-Mass Spectrometry. Endocrinology 1977, 101, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Yaga, K.; Reiter, R.J.; Richardson, B.A. Tryptophan loading increases daytime serum melatonin levels in intact and pinealectomized rats. Life Sci. 1993, 52, 1231–1238. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Ayles, H.L.; Friendship, R.M.; Brown, G.M.; Ball, R.O. Relationship between melatonin levels in plasma and gastrointestinal tissues and the incidence and severity of gastric ulcers in pigs. J. Pineal Res. 1998, 24, 62–66. [Google Scholar] [CrossRef]
- Kopin, I.J.; Pare, C.M.B.; Axelrod, J.; Weissbach, H. The Fate of Melatonin in Animals. J. Biol. Chem. 1961, 236, 3072–3075. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Pang, S.F.; Cockshut, J.R.; Smith, P.S.; Grovum, L.W.; Friendship, R.M.; Hacker, R.R. Circadian variation of portal, arterial and venous blood levels of melatonin in pigs and its relationship to food intake and sleep: Circadian rhythm of melatonin in the pig GIT. J. Pineal Res. 2000, 28, 9–15. [Google Scholar] [CrossRef]
- Huether, G. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 1993, 49, 665–670. [Google Scholar] [CrossRef]
- Huether, G.; Hajak, G.; Reimer, A.; Poeggeler, B.; Blömer, M.; Rodenbeck, A.; Rüther, E. The metabolic fate of infusedl-tryptophan in men: Possible clinical implications of the accumulation of circulating tryptophan and tryptophan metabolites. Psychopharmacology 1992, 109, 422–432. [Google Scholar] [CrossRef]
- Messner, M.; Huether, G.; Lorf, T.; Ramadori, G.; Schwörer, H. Presence of melatonin in the human hepatobiliary-gastrointestinal tract. Life Sci. 2001, 69, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A.; Pang, S.F.; Hacker, R.R.; Smith, P.S. Melatonin concentrations in serum and tissues of porcine gastrointestinal tract and their relationship to the intake and passage of food. J. Pineal Res. 1996, 21, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Krieger, D.T.; Hauser, H.; Krey, L.C. Suprachiasmatic Nuclear Lesions Do Not Abolish Food-Shifted Circadian Adrenal and Temperature Rhythmicity. Science 1977, 197, 398–399. [Google Scholar] [CrossRef] [PubMed]
- Roky, R.; Kapás, L.; Taishi, P.; Fang, J.; Krueger, J.M. Food Restriction Alters the Diurnal Distribution of Sleep in Rats. Physiol. Behav. 1999, 67, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A.; Ball, R.O.; Pang, S.-F. The effect of food deprivation on brain and gastrointestinal tissue levels of tryptophan, serotonin, 5-hydroxyindoleacetic acid, and melatonin. J. Pineal Res. 1992, 12, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A.; Hacker, R.R.; Brown, G.M.; Bartos, L. Melatonin concentrations in the luminal fluid, mucosa, and muscularis of the bovine and porcine gastrointestinal tract. J. Pineal Res. 1999, 26, 56–63. [Google Scholar] [CrossRef]
- Menendez-Pelaez, A.; Poeggeler, B.; Reiter, R.J.; Barlow-Walden, L.; Pablos, M.I.; Tan, D. Nuclear localization of melatonin in different mammalian tissues: Immunocytochemical and radioimmunoassay evidence. J. Cell. Biochem. 1993, 53, 373–382. [Google Scholar] [CrossRef]
- Lee, P.P.N.; Shiu, S.Y.W.; Chow, P.H.; Pang, S.F. Regional and Diurnal Studies of Melatonin and Melatonin Binding Sites in the Duck Gastro-lntestinal Tract. Neurosignals 1995, 4, 212–224. [Google Scholar] [CrossRef]
- Skwarlo-Sonta, K. Functional connections between the pineal gland and immune system. Acta Neurobiol. Exp. 1996, 56, 341–358. [Google Scholar]
- Skwarlo-Sonta, K. Reciprocal interdependence between pineal gland and avian immune system. Neuroendocrinol. Lett. 1999, 20, 151–156. [Google Scholar] [PubMed]
- Skwarlo-Sonta, K. Melatonin in immunity: Comparative aspects. Neuroendocrinol. Lett. 2002, 23, 61–66. [Google Scholar] [PubMed]
- Janković, B.D.; Knežević, Z.; Kojić, L.; Nikolić, V. Pineal Gland and Immune System: Immune Functions in the Chick Embryo Pinealectomized at 96 Hours of Incubation a. Ann. N. Y. Acad. Sci. 1994, 719, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Rosołowska-Huszcz, D.; Tnaela, M.; Jagura, M.; Stȩpień, D.; Skwarło-Sońta, K. Pineal influence on the diurnal rhythm of nonspecific immunity indices in chickens. J. Pineal Res. 1991, 10, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Youbicier-Simo, B.; Boudard, F.; Mekaouche, M.; Baylé, J.; Bastide, M. A role for bursa fabricii and bursin in the ontogeny of the pineal biosynthetic activity in the chicken. J. Pineal Res. 1996, 21, 35–43. [Google Scholar] [CrossRef]
- Di Stefano, A.; Paulesu, L. Inhibitory effect of melatonin on production of IFNγ or TNFα in peripheral blood mononuclear cells of some blood donors. J. Pineal Res. 1994, 17, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Blalock, J.E. Human lymphocyte production of corticotropin and endorphin-like substances: Association with leukocyte interferon. Proc. Natl. Acad. Sci. USA 1981, 78, 7530–7534. [Google Scholar] [CrossRef]
- Calvo, J.R.; Rafii-EI-ldrissi, M.; Pozo, D.; Guerrero, J.M. Immunomodulatory role of melatonin: Specific binding sites in human and rodent lymphoid cells. J. Pineal Res. 1995, 18, 119–126. [Google Scholar] [CrossRef]
- Finocchiaro, L.M.E.; Nahmod, V.E.; Launay, J.M. Melatonin biosynthesis and metabolism in peripheral blood mononuclear leucocytes. Biochem. J. 1991, 280, 727–731. [Google Scholar] [CrossRef]
- Lissoni, P.; Barni, S.; Tancini, G.; Rovelli, F.; Ardizzoia, A.; Conti, A.; Maestroni, G.J.M. A Study of the Mechanisms Involved in the Immunostimulatory Action of the Pineal Hormone in Cancer Patients. Oncology 1993, 50, 399–402. [Google Scholar] [CrossRef]
- Maestroni, G.J.M. T-Helper-2 lymphocytes as a peripheral target of melatonin. J. Pineal Res. 1995, 18, 84–89. [Google Scholar] [CrossRef]
- Guerrero, J.M.; Reiter, R.J. A Brief Survey of Pineal Gland-Immune System Interrelationships. Endocr. Res. 1992, 18, 91–113. [Google Scholar] [CrossRef]
- Shafer, L.L.; McNulty, J.A.; Young, M.R.I. Assessment of melatonin’s ability to regulate cytokine production by macrophage and microglia cell types. J. Neuroimmunol. 2001, 120, 84–93. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Takahashi, J.S. Use of 2-[125I]iodomelatonin to characterize melatonin binding sites in chicken retina. Proc. Natl. Acad. Sci. USA 1987, 84, 3916–3920. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.; Ferreira, A.C.F.; Skorupa, A.L.; Afeche, S.C.; Cipolla-Neto, J.; Costa Rosa, L.F.B.P. Tryptophan consumption and indoleamines production by peritoneal cavity macrophages. J. Leukoc. Biol. 2004, 75, 1116–1121. [Google Scholar] [CrossRef]
- Turkowska, E.; Rai, S.; Majewski, P.; Skwarło-Sońta, K. Diurnal and seasonal changes in IL-6 and IL-18 gene expression in blood leukocytes of male chickens with experimental peritonitis: The impact of lighting conditions and melatonin. J. Anim. Feed Sci. 2013, 22, 149–157. [Google Scholar] [CrossRef]
- Barassin, S.; Saboureau, M.; Kalsbeek, A.; Bothorel, B.; Vivien-Roels, B.; Malan, A.; Buijs, R.M.; Guardiola-Lemaitre, B.; Pévet, P. Interindividual differences in the pattern of melatonin secretion of the Wistar rat. J. Pineal Res. 1999, 27, 193–201. [Google Scholar] [CrossRef]
- Berczi, I.; Chow, D.A.; Sabbadini, E.R. Neuroimmunoregulation and natural immunity. Domest. Anim. Endocrinol. 1998, 15, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, H.O.; del Rey, A.; Sorkin, E. Lymphokine-containing supernatants from con A-stimulated cells increase corticosterone blood levels. J. Immunol. 1981, 126, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Blalock, J.E. Production of Peptide Hormones and Neurotransmitters by the Immune System1. Neuroimmunoendocrinology 1992, 52, 1–24. [Google Scholar]
- Maes, M.; Verkerk, R.; Delmeire, L.; Van Gastel, A.; van Hunsel, F.; Scharpé, S. Serotonergic markers and lowered plasma branched-chain-amino acid concentrations in fibromyalgia. Psychiatry Res. 2000, 97, 11–20. [Google Scholar] [CrossRef] [PubMed]
- PARFITT, A.; WELLER, J.L.; KLEIN, D.C.; SAKAI, K.K.; MARKS, B.H. Blockade by ouabain or elevated potassium ion concentration of the adrenergic and adenosine cyclic 3′, 5′-monophosphate-induced stimulation of pineal serotonin N-acetyltransferase activity. Mol. Pharmacol. 1975, 11, 241–255. [Google Scholar] [PubMed]
- Rivier, C. Influence of Immune Signals on the Hypothalamic-Pituitary Axis of the Rodent. Front. Neuroendocrinol. 1995, 16, 151–182. [Google Scholar] [CrossRef] [PubMed]
- Cogburn, L.A.; Glick, B. Lymphopoiesis in the chicken pineal gland. Am. J. Anat. 1981, 162, 131–142. [Google Scholar] [CrossRef]
- Cogburn, L.A.; Glick, B. Functional lymphocytes in the chicken pineal gland. J. Immunol. Baltim. Md 1950 1983, 130, 2109–2112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horodincu, L.; Solcan, C. Influence of Different Light Spectra on Melatonin Synthesis by the Pineal Gland and Influence on the Immune System in Chickens. Animals 2023, 13, 2095. https://doi.org/10.3390/ani13132095
Horodincu L, Solcan C. Influence of Different Light Spectra on Melatonin Synthesis by the Pineal Gland and Influence on the Immune System in Chickens. Animals. 2023; 13(13):2095. https://doi.org/10.3390/ani13132095
Chicago/Turabian StyleHorodincu, Loredana, and Carmen Solcan. 2023. "Influence of Different Light Spectra on Melatonin Synthesis by the Pineal Gland and Influence on the Immune System in Chickens" Animals 13, no. 13: 2095. https://doi.org/10.3390/ani13132095
APA StyleHorodincu, L., & Solcan, C. (2023). Influence of Different Light Spectra on Melatonin Synthesis by the Pineal Gland and Influence on the Immune System in Chickens. Animals, 13(13), 2095. https://doi.org/10.3390/ani13132095





