Simple Summary
Crofton weed toxicity, caused by Ageratina adenophora, has been recognized as a cause of fatal lung disease in horses for over a century. Despite its impact on horse health in many areas of the world, the toxic syndrome is poorly understood and understudied. This paper looks at the prior research on weed biology, the potential toxicology mechanisms, and the pathology in horses and other species, as well as the future directions to improve our understanding of this fatal toxic weed affecting horses.
Abstract
Crofton weed (Ageratina adenophora) is a global and highly invasive weed, with ingestion causing severe respiratory disease in horses, leading to irreversible and untreatable pulmonary fibrosis and oedema. While reports of equine pneumotoxicity remain common in Australia and New Zealand, equine pneumotoxicity may be underdiagnosed in other countries where Crofton weed is endemic but poorly differentiated. The pathogenesis of Crofton weed toxicity following ingestion has been well described in a number of different animal models, including rodents, rabbits, and goats. However, induced toxicity is organ-selective across different animal species, and these vastly differ from the pathogenesis described in horses, both clinically and after experimental exposure. Sources of variation may include species-specific susceptibility to different toxins present in the plant, different mechanistic processes of toxicity, and species differences in toxin biotransformation and bioactivation across different organs. Considering disease severity and Crofton weed’s invasiveness globally, assessing published toxicological and exposure data is necessary to advance research, identify specific toxins for horses, and possible prophylactic and therapeutic strategies. This review presents an overview of the available literature on equine toxicity, parallels between toxicity in horses and other animal species, and important aspects to be included in the future research agenda.
Keywords:
horse; lung; fibrosis; pneumonia; toxic plant; pulmonary fibrosis; dyspnea; Ageratina; Eupatorium 1. Introduction
It has been a century since Crofton weed (Ageratina adenophora, syn: Eupatorium adenophora, E. glandulosum) was identified as a plant of interest in relation to horse deaths due to pulmonary disease [1,2]. Despite this, little is known about the pathophysiological mechanisms that result in pulmonary fibrosis and the reasons why horses appear to be more susceptible than other species. Crofton weed is native to Central America but is now present in many tropical and subtropical countries as a highly invasive noxious weed, whose uncontrollable spread is characterized by allelochemical competition and alterations of soil microbial communities [3,4]. Although Crofton weed is well known for its negative impacts on ecology, agriculture, and animal health [4,5,6,7], it has diverse medicinal properties and has been used in traditional medicines and ethno-pharmacology. Some of the 34 phytochemicals and 52 volatile oils that have been isolated are currently under investigation for potential pharmacological uses [8,9,10,11,12,13,14,15].
There are no confirmative diagnostic tests or efficacious treatments for Crofton weed-associated pneumotoxicity in horses. A definitive diagnosis of the disease is not possible, and a diagnosis can be formulated only presumptively, based on the history of exposure and exclusion of diseases with similar clinicopathological findings. Crofton weed intoxication in horses causes severe respiratory disease, with clinical signs including coughing, increasingly severe exercise intolerance, tachypnoea, and adventitial respiratory sounds. [1,16,17]. The disease progresses to weight loss, respiratory distress, and cyanosis preceding death, attributed to pulmonary fibrosis, intense pulmonary oedema, and biventricular cardiac failure following hypoxia-associated cor pulmonale [1,16,17]. Histopathologically, Crofton weed induces a chronic multinodular pulmonary fibrosis with interstitial pneumonia and pulmonary oedema [1,16,17]. As terminal cases are usually diagnosed late in the disease process, Crofton weed toxicity is considered untreatable and irreversible, and euthanasia remains the most humane endpoint for affected horses. Antemortem pulmonary biopsy with histopathology will rule out other differentials such as EHV5-associated equine pulmonary multinodular fibrosis and fungal infection (such as Pneumocystis carinii) supported by PCR and serology, and could be used to produce a provisional diagnosis earlier in the disease process [18,19,20,21,22,23].
Despite confirmation of Crofton weed as the cause of fatal pneumotoxicity in horses [16,17], the phytotoxins involved and pathophysiological mechanisms remain unclear. Equine Crofton weed pneumotoxicity—also known as “Crofton Weed Poisoning”, “Numinbah Horse Sickness”, and “Tallebudgera Disease”—was originally attributed to a possible allergic response or to the hematogenous distribution of an unknown phytotoxin after ingestion [16,17]. In contrast to horses, Crofton weed ingestion is predominantly linked to hepatotoxicity in other animal species, which has been associated with terpenoids [16,24,25,26,27,28,29]. As different organs are affected across several animal species, specific target organ phytotoxins, as well as different routes of exposure, have been proposed [7,14,25,26,30]. However, the possible role of species-specific metabolic bioactivation of Crofton weed toxins as a basis for organ-selective toxicity has not been investigated, but remains plausible.
Equine Crofton weed toxicity research ceased in the 1980s, but other disciplines have extensively investigated Crofton weed biology, control, phytochemical properties, potential for pharmaceutical development, and toxicity in different animal species [3,4,14]. This review presents an overview of the available literature on Crofton weed toxicity in horses, toxicity in other animal species and parallels with the equine disease, and possible prophylactic and therapeutic strategies.
2. Crofton Weed Identification, Morphology, and Impact
2.1. Identification of Crofton Weed
Field identification is paramount, as currently, prevention is the only cure. Once identified within paddocks, horse owners should be advised to prevent any grazing of infiltrated pastures by fencing off infested areas, and rehabilitating quarantined paddocks with the use of approved herbicides, mechanical control, and planting competitive replacement pastures [31,32]. In China and India, the invasive nature of this species has threatened native biodiversity and resulted in significant research into control strategies [3,5,33,34,35]. Despite successful biological control documented in Hawaii during the 1920s, the biological control strategies applied in Australia were not successful [31,32,36,37,38]. Once the plant is established in grazing areas, physical and chemical control have limited effectiveness considering rapid seed dispersal, plant regrowth, and negative impacts of pesticides on native flora and fauna [31,32,38]. In addition to the cited literature, there are many online resources that have weed control advice, specific to each region, which are being constantly updated as new chemical and rehabilitation protocols emerge. Owners should be advised to seek the latest, local information for both Ageratina spp. control methods.
Also known as Eupatory, Sticky Snakeroot, Catweed, Banmara, Maui Pakami, Sándara, Flor de Espuma, and Mexican Devil, A. adenophora is a member of the Asteraceae family. The plant is a perennial large herb or under-shrub with multiple erect glandular and hairy burgundy stems reaching a height of 1–2 m (Figure 1A,B). Its leaves grow in an opposite arrangement, are 2.5 to 5 cm long, and are dark green, broad, trowel-shaped with serrated margins, and burgundy petioles (Figure 1C,D). The foliage is distinctly and highly aromatic when disturbed or crushed. Flowers are 5–8 mm wide and grow in white clusters of disc florets, appearing as small, dense heads at the ends of branches during spring. Small leaf-like structures called bracts surround the flowers. Although Crofton weed is apomictic (able to produce female clones from asexual seed formation) it is a prolific pollen producer. A mature plant can produce 100,000 to 1,000,000 seeds per year, which are very light (25,000 seeds/g), slender, angular, 2 mm long, and almost black, with fine white hairs at the tip [31,32,35,39]. Crofton weed and the less prolific Mist weed (Ageratina riparia, syn: Eupatorium riparia), which is commonly found along waterways, are sometimes mistaken for each other due to their flowers being almost identical. However, when it is not flowering, Mist weed is often not recognized at all, as it is far less visible than the erect Crofton weed, but evidence indicates that both are toxic when consumed [17]. While the flowers and odor of A. riparia are very similar to those of A. adenophora, A. riparia stems can be pale green rather than burgundy, have a short (<50 cm height) prostrate habit, and have leaves with an elliptic shape. (Figure 1E,F) [32].

Figure 1.
Examples of different life stages and growth habits of A. Adenophora (A–D) and A. riparia (E,F). Plates (A,B) show the erect burgundy stems, with opposite arrangements of bright to dark green leaves. Plates (C,D) show the rhomboid or trowel-shaped leaves with serrated edges and demonstrate its prolific growth creating a continuous hedge of A. Adenophora. Plates (E,F) demonstrate the spear-shaped leaves with toothed edges and a pointed tip, prostrate growth habit, and reduced biomass of A. riparia. Plates (B,E) show the similarity between the flower morphologies of both species.
2.2. Pollen Morphology and Investigation as a Potential Alveolar Mechanical Irritant
Many of the plants of the family Eupatorium have had their pollen morphology characterized, with 11 of the 192 species exhibiting short-spined pollen typical of plants associated with allergic responses [40,41]. However, the pollen from A. adenophora and A. riparia has not been characterized by a Scanning Electron Microscopy (SEM). The Environmental Analysis Laboratory at Southern Cross University, Lismore, was contracted by the authors to perform SEM of A. adenophora and A. riparia pollen samples collected from Bentley, Northern NSW. Twelve pollen samples from A. adenophora and A. riparia were prepared via desiccation, attachment to SEM stubs, and examined under a low vacuum. A Hitachi TM4000 desktop SEM was used to evaluate and record the images. No differences between the Crofton weed and the Mist weed pollen were noted, with both being spherical to ovoid and with one colpi. Pollen ranged from 15–22 µm in size, with continuous spikes across the surface (Figure 2A–D). The barbellate achenes (seed capsules: ~1–2 mm) had sharp barbs across the surface of the seed and papillae (Figure 2E,F), and all the samples contained fungal hyphae that were intertwined with the reproductive structures (Figure 2A,B).
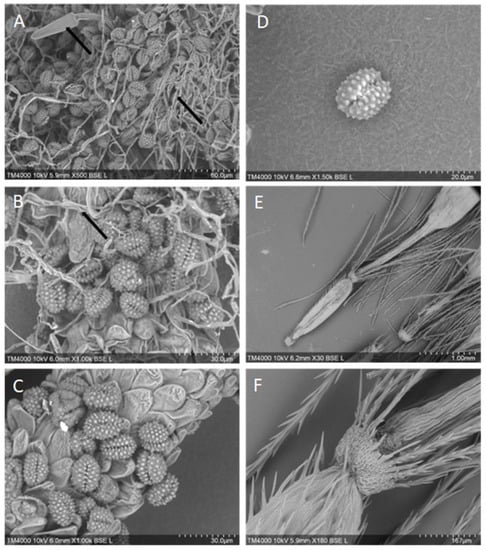
Figure 2.
Pollen and achene morphology of Crofton weed, A. adenophora (A,B), and Mist weed, A. riparia (C–F), using scanning electron microscopy. Plates (A,B) Note the presence of fungal fruiting bodies and hyphae intertwined amongst the pollen granules, as indicated by arrows. Plates (C,D) note pollen morphology are very similar to that of other Ageratina spp. [40]. Plates (E,F) barbed achenes.
Due to the predominant timing of the clinical signs of equine pneumotoxicity falling 6–10 weeks after the onset of the flowering season [11], combined with the high volume of flowers, seeds, and pollen produced, the role of the floral components and or pollen inhalation in pneumotoxicity has been considered. In 1958, one report from China attributed the death of 200 horses as a result of asthma brought on by pollen from Crofton weed flowers [42]. However, as Crofton weed pollen is greater than 5 µm in size, it is too large to enter the alveoli, and therefore, a direct mechanical cause of the pulmonary damage in horses is considered unlikely. Additionally, feeding trials using non-flowering plants and intragastric tubes feeding across different species have demonstrated that pollen inhalation is not necessary to elicit toxicity [16,17,43,44].
2.3. Geographic Distribution of Crofton Weed Toxicity in Horses
During the 1920s “Blowing disease” was anecdotally associated with Maui Pamakani, the local Hawaiian common name for Crofton weed introduced in about 1860 in the Maui Island [1,2,36] The etiology of the described disease in Hawaii was never confirmed, as feeding and pollen inhalation trials were negative; however, the descriptions were identical with the first reports of Numinbah Horse Sickness in Australia during the 1950s [1]. Pamakani was postulated to cause the death of a large number of horses pasturing above certain altitudes (600 m above sea level) in Maui [1,2]. However, despite its presence, it was reported to cause no injuries to horses on Hawaiian islands other than Maui [2]. Interestingly, reports declined during weeding periods, and apparently, they ceased after the establishment of biocontrol by a stem gall fly (Procecidochares utilis) in 1945 [1,36,37,45,46].
In Australia, the plant escaped domestication in Sydney in 1904, and by the 1940s, it had established itself as a weed throughout coastal New South Wales (NSW) and Southeast Queensland. Accordingly, the first reports of the disease in Australia were documented in 1941 in the extreme northern part of NSW and southern Queensland [1]. Preceding World War II, Crofton weed toxicity was commonly suspected in Australian Draught Horses working in the valleys and mountains of south-eastern Queensland and north-eastern NSW, and from the year 1948, reports continued to increase [1,16]. In 1952, P. utilis was unsuccessfully introduced as Crofton weed biocontrol in Australia, and by 1954, the NSW Institute of Inspectors of Stock yearbook was reporting outbreaks of “Numinbah Horse Sickness”, estimating that during the decade preceding the report, hundreds of horses had died from this disease [1,25,37]. By the 1970s, respiratory problems increased in regions where Crofton weed was common [16]. Farms infested with the plant in Australia sporadically reported cases of suspected Crofton weed pneumotoxicosis in the summer, affecting horses of all ages and occurring a minimum of two months after plant ingestion in the spring [47].
As a confirmative diagnosis is not possible, the actual prevalence and worldwide distribution of Crofton weed intoxication in horses are largely unknown. Outside Australia, it has been suggested that the disease is also present in New Zealand, possibly China, and the Himalayas [25,47,48]. However, despite the wide distribution and reported invasiveness of Crofton weed in Burma, Fiji, India, Jamaica, Malaysia, Nepal, Pakistan, Singapore, South Africa, Spain, Sri Lanka, Thailand, the Philippines, the Pacific Islands, the United States, and Vietnam, pneumotoxicity in horses is not commonly observed or officially documented, and reports are mostly anecdotal [4,15,32,39,49,50,51]. However, in some of these countries, local differences in horse husbandry and population size might also lead to reduced exposure, even when the plant is present.
Despite the wide distribution and invasiveness of Crofton weed [4,15,32,39,49,50,51], pneumotoxicity in horses seems to be limited to some specific locations, and to our knowledge, it has not been reported in countries where the plant is native. This may be explained by specific factors impacting the concentration of phytotoxins that are present in the plant across different geographic locations. For instance, climate, soil, and other environmental variables influence the synthesis and content of phytochemicals, which are known to vary with geographical region in several plant species [52,53]. Indeed, the allelopathic activity of volatile organic compounds and the foliar concentration of various terpenes, which are part of the defensive arsenal of A. adenophora, are different between plants from native and non-native locations [54,55]. A comparative assessment of phytotoxins in plants collected from regions where the disease is present and absent is necessary to compare the concentrations of specific toxins and identify the reasons why horses might be at a higher risk of developing toxicity in certain geographic areas.
3. Phytotoxicity
3.1. Crofton Weed Phytochemical Analysis
Crofton weed is rich in bioactive phytochemicals such as benzofuran, coumarins, flavonoids, phenolic acids, phenylpropanoids, polysaccharides, quinic acid, chromene derivatives, sterols, alkaloids, and mono-, sesqui-, di-, and tri-terpenoids [5,14,15,56,57,58]. A 2020 review identified 34 phytochemicals and 52 volatile oils that were isolated from Crofton weed [14]. There is a plethora of research investigating pharmacological applications for Crofton weed’s chemical extracts and phytotoxins, including its antimicrobial, anti-inflammatory, anti-pyretic, wound-healing, anti-oxidant, analgesic, anti-tumor, anti-viral, insecticidal, larvicidal, and acaricidal activities [14,59,60,61]. Additionally, differential production and activity of allelopathic phytochemicals between plant specimens from native and non-native regions have been documented [54,55]. Pyrrolizidine alkaloids may potentially play a role in Crofton weed toxicity in horses [47]. Food safety concerns resulting from pyrrolizidine alkaloid contamination during the production of honey and pollen products have facilitated significant research confirming the presence of these phytochemicals in the pollen of many plants in Ageratina genus [15,62,63,64,65]. Although one phytochemical study reported the isolation of alkaloids from Crofton weed leaves, the type of alkaloid detected in that study was not further characterized [57].
3.2. Feeding Trials in Horses
The results of feeding trials were congruent with the current and historical reports from horse owners and veterinarians that disease outbreaks tend to occur in late spring through summer, corresponding to the time coincident with and after Crofton weed’s annual flowering season. The first feeding trial confirming the suspected etiology of Crofton weed toxicity in horses was published in 1979 [16]. Two horses were fed whole plant materials, including the flowering heads (Table 1). One horse was fed Crofton weed over eight months and developed the clinical signs and severe pathological pulmonary changes that are typically observed in field cases [16]. The second horse was fed the plant over 42 days, and although no clinical abnormalities were observed, less severe pulmonary pathological changes were also documented at necropsy [16]. The findings of this first trial supported a possible allergic reaction, and although no microbiological growth was detected from the pulmonary samples, a secondary infectious process was suspected, and the negative cultures were attributed to antibiotic treatment that was administered prior to euthanasia [16]. Because the horses were fed in deep troughs, which might have facilitated the inhalation of all small particles including pollen, pneumotoxicity following pollen inhalation was not ruled out [16].

Table 1.
Toxicity-related findings after experimental exposure to Crofton weed, plant extracts, and purified toxins in several animal species.
In a subsequent study published by the same research group, the toxicity of the closely related plant Mist weed (A. riparia) was determined utilizing two horses fed plant material that was picked during its flowering season [66]. Mist weed induced clinical signs and necropsy findings similar to Crofton weed, suggesting a common phytotoxic mechanism [66]. The two species also share a common geographic distribution and some habitats, though Crofton weed is far more prevalent due to its ability to colonize open, moist regions, while Mist weed rarely colonizes beyond a riparian zone [33,34]. Furthermore, Crofton weed is an erect, multi-stemmed shrub that forms dense, tangled bushes of up to 2.0 m height, producing large quantities of biomass compared to the comparatively low biomass of the prostrate Mist weed [32,33,34]. While this trial confirmed Mist weed’s toxicity in horses, there have been no case reports of spontaneous disease linked to Mist weed, and this may be due to a lack of readily ingestible volumes or its co-habitation with the more abundant Crofton weed.
A second Crofton weed feeding trial hypothesized that the disease was caused by either the (1) ingestion of flowers, (2) inhalation of pollen, or (3) an increased concentration of phytotoxins in the foliage during plant flowering [17]. To test these hypotheses, ten horses were fed 3–4 kg of Crofton weed daily. Two horses were fed the flowering plant only (for 50 and 90 days, one by consumption and the other by the administration of blended plant materials via the stomach tube, respectively). Four horses received non-flowering plants only (for 93, 164,164, and 327 days), and another four horses were fed both flowering and non-flowering plants (for 165, 415, 442, and 442 days) [17]. While the sample size and variability in days of feeding excluded the use of statistical analysis, the data generally supported several key assumptions, which are as follows: (1) non-flowering plants were also toxic, though at a reduced level than flowering plants; (2) necropsy findings were more severe with prolonged feeding times and when the diet included flowering plants; (3) early lung lesions in the absence of clinical signs were detected after only 57 days in horses that were fed flowering plants; and (4) once the pulmonary lesions occurred, the damage appeared to be irreversible and cumulative with re-exposure [17]. Based on the single horse fed by stomach tube having widely distributed focal pulmonary lesions after 90 days and the results from the necropsies performed on all ten horses, it was concluded that hematogenous dissemination of an unknown toxin was more likely than inhalation [17]. No further equine feeding trial expanded on these conclusions, and it is unknown if any signs of inflammation occur in peripheral blood with Crofton weed toxicity, which may confuse its antemortem diagnosis with other infectious causes of pulmonary oedema and fibrosis in horses. Additional research using different animal models has continued to explore Crofton weed toxicity, the involved phytotoxins, and the mechanisms of toxicity (Table 1).
3.3. Postmortem Findings of Equine Crofton Weed Toxicity
Crofton weed induces interstitial pneumonia characterized by multinodular pulmonary fibrosis in horses. The necropsy findings from early feeding trials confirmed that chronic lesions developed into extensive and severe fibrosis, which appeared in the gross tissue as white nodular lesions that did not collapse and generalized throughout the lung [16,17]. The normal pulmonary architecture was effaced by fibrous tissue, with few alveoli remaining and less severe areas displaying a proliferation of type II pneumocytes [17]. In the 1979 feeding trial, the disease was suspected to be triggered by an allergic response, due to proteinaceous fluid present in the alveoli and vascular damage resulting from a loss of capillary integrity, progressing to a secondary infection and abscessation [16]. However, the histopathologic findings from the 1985 trial described interstitial pneumonia with type II pneumocyte hyperplasia, clusters of interalveolar macrophages with lymphocytes infiltrating within interlobular septa, and perivascular fascia. In addition to the pulmonary changes, a single study described cardiac dilation with slight hydropericardium, after Crofton weed ingestion in horses, with other abnormalities attributed to heavy parasite infestation [1]
It is now believed that circulating absorbed phytotoxins such as pyrrolizidine alkaloids and ketones originating from the ingestion of Crofton weed could trigger interstitial pneumonia in horses. Other differentials for interstitial pneumonia in horses include lungworm infections, Influenza virus, Hendra virus, and toxins, such as Paraquat and 3-methylindole, while differentials for pulmonary fibrosis include Equine Herpesvirus 5 (EHV 5)-associated equine multinodular pulmonary fibrosis silicosis and fungal pneumonia, such as from Pneumocystis carinii [18,19,20,21,22,23,67,68]. Most infectious causes would likely have clinicopathological evidence of inflammation in the peripheral blood such as a combination of some of the following: anemia of chronic infection, hyper or hypo-gammaglobulinemia, hypalbuminemia, hyperfibrinogenemia, low serum iron concentrations, elevated serum amyloid A concentrations, leukocytosis, toxic changes in leukocytes, and possibly the presence of band neutrophils. Although unlikely, it is unknown whether Crofton weed pneumotoxicity causes any changes in serum biochemistry or hematology. Terminally, there would likely be hypoxemia and a respiratory acidosis.
3.4. Species Differences in Crofton Weed Organ-Selective Toxicity
Crofton weed induces organ-selective toxicity across different animal species, which is possibly mediated by different phytotoxins or different species-specific metabolic mechanisms. A multispecies feeding trial performed in 1979 included rabbits (n = 2), sheep (n = 2), and rats (n = 4) [16]. Both rabbits were fed Crofton weed for nine months and developed pulmonary microscopic changes similar to early lesions observed in horses, while sheep and rats did not display any pulmonary abnormalities during pathologic examination; however, it is not definitively stated if other organs were examined for microscopic changes [16]. Although that study did not identify any abnormalities in sheep and rats, subsequent trials reported specific target organs across different species (Table 1). Specifically, the oral intake or intragastric administration of Crofton weed or its extracts lead to renal, splenic, and liver toxicity in goats; liver, spleen, and intestinal toxicity in rodents; and negative impacts in the digestive function of cattle (Table 1) [16,17,24,25,27,28,29,44,51,69,70,71,72,73,74,75,76,77].
The phytochemicals 9-oxo-10, 11-dehydroagerophorone (Euptox A), 2-deoxo-2-(acetyloxy)-9-oxo-ageraphorone (DAOA) and 9-oxoagerophorone (OA) have been identified as the major phytotoxins in A. adenophora, and have been demonstrated to induce hepatotoxicity in rats and mice [25,43,71,76]. Euptox A hepatotoxicity in rodents has been demonstrated after toxin purification [25,43,56,71,76]. Furthermore, the oral administration of Euptox A was associated not only with hepatotoxicity, but also with spleen and intestinal toxicity in rodents [43,76]. Oxidative stress and inflammation were postulated as the main mechanistic processes leading to animal disease after Crofton weed ingestion [7,26]. Further mechanistic studies in mice revealed that Euptox A induces cell cycle arrest and apoptosis in hepatocytes via the accumulation of reactive oxygen species (ROS) and in splenocytes autophagy by disrupting the p38 MAPK- and PI3K/Akt/mTOR-mediated pathways [43,44]. These findings are like those of feeding trials in goats, demonstrating the induction of cell cycle arrest, apoptosis, and autophagy in renal cells, splenocytes, and hepatocytes, highlighting the possible role of Euptox A and other terpenes behind the observed toxicity in goats [27,28,29]. The role of Euptox A and other terpenes as responsible for the toxic effects of Crofton weed in horses has not been investigated.
Considering the presence of alkaloids in Crofton weed and the reported pneumotoxicity of these phytochemicals in horses after the ingestion of alkaloid-rich plants [19,78], pyrrolizidine alkaloids have also been anecdotally suspected to play a role in equine Crofton weed pneumotoxicity [47]. Similar to Euptox A, pyrrolizidine alkaloids typically induce hepatotoxicity in rodent models [79]. Pharmacokinetics studies of ingested pyrrolizidine alkaloids in mice and rats, demonstrated preferential bioactivation in the liver, resulting in hepatotoxicity and secondary pneumotoxicity after the migration of liver-derived dehydro-pyrrolizidine alkaloids and the intrapulmonary formation of pyrrole–protein adducts [78,79,80,81]. Species differences in the concentration of biotransformative enzymes in the liver and lungs of horses and other animal species may explain the differences in organ toxicity between species [82,83,84,85,86]. As observed with other toxicities in horses, a predominant cytochrome P450-mediated pulmonary bioactivation of phytotoxins in the Clara cells, also known as club cells or bronchiolar exocrine cells, of horses might be a possible driver for Crofton weed-associated pulmonary-selective toxicity [78,85,86,87].
4. Discussion
4.1. Gaps in Clinical Diagnostics
It is likely that chronic long-term ingestion of Crofton weed would result in a gradual progression of clinical signs in horses. The toxic dose required to induce subtle clinical signs is unknown. Published feed trials that recorded pathological findings utilized generous doses of weed. Changes in hematology and serum biochemistry have not been investigated, but knowledge of any bloodwork changes (or lack thereof) resulting from Crofton weed pneumotoxicity would improve diagnostic capacity. Changes in arterial blood gas analysis (at rest and after controlled exercise), thoracic ultrasound, and radiographs after controlled experimental exposure would be useful to confirm the severity of interstitial pneumonia antemortem. Fluid from a transtracheal wash or bronchoalveolar lavage can be utilized for cytology, microbiological cultures, and PCR and are important in the work-up of clinical cases and samples that should be collected during experimental exposure. Despite some risk of pulmonary hemorrhage, an antemortem lung biopsy can be useful for providing samples for histopathology, cytology, microbiological cultures, and PCR to help determine the presence of etiological agents [18,67,88,89]. Although the biopsy findings would not be pathognomonic for Crofton weed toxicity, the documentation of the progressive disease after experimental exposure would be useful when paired with other clinical parameters. In clinical cases where Crofton weed exposure has occurred, histopathology could indicate a decreased likelihood of other differentials, which may have a better prognosis with an earlier, specific therapy.
4.2. Areas Requiring Further Research
Crofton weed pneumotoxicity in horses has been known for over a century. Although it is a recognized differential for interstitial pneumonia, no case series has been published [20,22]. It is unknown whether the removal of subclinically affected horses from contaminated pastures would lead to a possible cessation of disease progression. Additional therapeutic strategies have not been investigated. Although experimental disease induction in early feeding trials was successful [16,17,66], the responsible toxins in horses have not yet been identified.
The characteristic seasonal behavior of Crofton weed pneumotoxicity in horses has led to the consideration of two major non-mutually exclusive hypotheses to explain the higher toxicity of flowering plants: (1) a higher concentration of toxins in the leaves during flowering, and (2) the possible effect of pollen as a mechanical irritant during the flowering season. The first hypothesis is supported by the typical accumulation of specific phytochemicals in the foliage of several plants during or around the flowering period [90,91,92]. Specifically, the concentration of allelochemicals and defensive compounds in invasive plants varies across different reproductive stages and parts of the plant [55,93]. The pollen of various plants in the genus Ageratina typically contains toxic compounds such as pyrrolizidine alkaloids [65,87,93]. Therefore, an increased concentration of phytotoxins in the leaves and pollen might contribute to toxicity if the whole flowering plant is ingested, or if there is a substantial amount of pollen accumulated on the leaves. A comparative phytochemical analysis of different parts of the plant possibly involved in animal toxicity (e.g., leaves, flowers, and pollen) during flowering and non-flowering periods would be informative.
As it is only possible to investigate the effects of toxins on a limited number of animal species and exposure scenarios (Table 1), translating the research conducted in model species into spontaneous disease in horses is challenging. Reproducing pneumotoxicity in other animal models has been unsuccessful, as Crofton weed induces organ-selective toxicoses across different animal species [16,17,24,25,27,28,29,44,51,69,70,71,72,73,74,75,76,77]. The role of terpenes in Crofton weed pneumotoxicity in horses has not been investigated; however, the identification of phytotoxins, including terpenes and alkaloids, in blood is possible and might be a suitable screening method for future research in cases where plant toxicity is suspected [94]. Additionally, the role of the intestinal microbiome of horses and the potential for microbial biotransformation of plant compounds into toxins have not been investigated. Microbial biotransformation has been identified as having a role in other plant toxicities, and it is noted that the only other species that presents with pulmonary abnormalities after the ingestion of Crofton weed is the rabbit, which is another hind-gut fermenter (Table 1).
Crofton weed toxicity in horses displays similar clinicopathological findings to Crotalariosis equorum “Jaagsiekte” [47], a respiratory disease of horses in South Africa and Northern Australia following the ingestion of Crotarlaria spp. Similar to equine Crofton weed pneumotoxicity, Crotalariosis equorum is characterized by interstitial pneumonia and the proliferation of pulmonary Clara cells, suggesting intoxication by pyrrolizidine alkaloids, possibly bioactivated via the cytochrome P450 monooxygenase system by Clara cells located in the terminal bronchioles [78]. Our current understanding of the cytochrome P450-related biotransformation processes in horses is still incomplete. However, important differences in the concentration of biotransformative enzymes in the liver and lungs of horses and other animal species in response to different drugs and xenobiotics have been identified [82,83,84,85,86]. Though the exact bioactivation mechanisms of pyrrolizidine alkaloids and sesquiterpenoids in horses have not been characterized, a predominant cytochrome P450-mediated pulmonary bioactivation of phytotoxins bypassing hepatic metabolism is possible. Indeed, besides horses, pulmonary-selective toxicities mediated through pulmonary P450 bioactivation after toxicant oral ingestion have been documented in several animal species [95,96,97]. Previous works in the literature have demonstrated the potential of in vitro models (e.g., lung microsomes, pneumocyte type II, and hepatocyte cultured cells) to investigate the role of pulmonary and hepatic biotransformative enzymes after exposure to lung-specific toxicants [98,99]. Indeed, mechanistic studies using equine-derived in vitro models could provide new information on the mechanisms of equine Crofton weed pneumotoxicity.
Crofton weed induces interstitial pneumonia in horses, characterized by multinodular pulmonary fibrosis. Though uncommon, this multinodular pulmonary fibrosis and interstitial pneumonia in horses is histologically distinctive and might be induced by only a handful of infectious agents and toxins [19,20,21,22,23]. The most morphologically similar being is Equine Herpesvirus 5 (EHV 5) [21,100], which was only discovered in the late 2000s, and is a possible differential diagnosis for Crofton weed intoxication. The complex pathogenesis and severe pulmonary fibrosis associated with the interstitial pneumonia caused by Crofton weed have limited the development of therapeutic strategies [19,20,21,22,23]. Further studies investigating the complex molecular mechanisms behind pulmonary fibrosis in horses are necessary to identify specific targets mediating tissue remodeling, such as fibroblast activation, the epithelial–mesenchymal transition, and the excessive accumulation of an extracellular matrix. Interestingly, feral herbivores and migratory goats sporadically consuming the plant do not develop toxicity leading to the postulation of possible mechanisms behind toxicity resistance [14,30,38,101]. Beneficial ruminal bacteria isolated from animals exhibiting resistance to Crofton weed toxicity might play a role, as possible prophylactic or therapeutic strategy [7].
5. Conclusions
This review has integrated cross-disciplinary research findings to identify the key areas for future research: (1) to identify the specific toxin/s causing pneumotoxicity in horses; (2) to establish the palatability, toxic dose, disease progression parameters, prevalence, and geographic distribution of Crofton weed cases; (3) to identify the suitable screening methods for the detection of suspected toxins in affected animals’ blood and tissues; (4) to identify the possible phytotoxins across different parts and growth stages of the plant; (5) to characterize relevant mechanistic processes and biotransformative enzyme concentrations using in vitro models and equine tissue cultures; and (6) to investigate the potential prophylactic and therapeutic strategies to ameliorate the impacts of toxicity in horses, including the use of intestinal bacteria that are capable of degrading toxins.
Crofton weed and Mist weed are easily recognizable, highly invasive global weeds. In geographic regions where Crofton weed and Mist weed are established, education of veterinarians and their clients about the risks posed, how to recognize the plants in situ, and control measures is critical [14]. Once the plants are identified, horse owners must prevent any grazing of infiltrated pastures [31,32]. Ingestion results in pulmonary fibrosis, which is ultimately fatal. Horses present with exercise intolerance, increased respiratory rates, and progressive dyspnea. Although not well documented, pulmonary changes should be identifiable ultrasonographically and radiographically. Fibrosis observed on histopathology of lung samples collected antemortem by percutaneous biopsy or at necropsy without the identification of etiologic agents such as fungal hyphae or EHV5 by PCR should make Crofton weed pneumotoxicity a likely differential in horses grazing in paddocks with access to Crofton weed or Mist weed.
Author Contributions
Conceptualization: F.M.S.; Writing: J.L.G.-S., A.J.S., R.A. and F.R.B.; Review: J.L.G.-S., A.J.S., R.A. and F.R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors wish to acknowledge the technical expertise of Maxine Dawes in undertaking the Scanning Electron Microscopy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jones, N. Numinbah horse sickness. Institute of Inspectors of Stock. In New South Wales Year Book; Government Printer Sydney: Sydney, Australia, 1954; pp. 80–84. [Google Scholar]
- Nikaido, R. A Chemical Study of Some Hawaiian Plants; University of Hawai’i at Manoa: Honolulu, HI, USA, 1934. [Google Scholar]
- Wan, F.; Liu, W.; Guo, J.; Qiang, S.; Li, B.; Wang, J.; Yang, G.; Niu, H.; Gui, F.; Huang, W.; et al. Invasive mechanism and control strategy of Ageratina adenophora (Sprengel). Sci. China Life Sci. 2010, 53, 1291–1298. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.-Z. Invasion dynamics and potential spread of the invasive alien plant species Ageratina adenophora (Asteraceae) in China. Divers. Distrib. 2006, 12, 397–408. [Google Scholar] [CrossRef]
- Shi, W.; Luo, S.; Li, S. Defensive Sesquiterpenoids from Leaves of Eupatorium adenophorum. Chin. J. Chem. 2012, 30, 1331–1334. [Google Scholar] [CrossRef]
- Cronk, C.B.; Fuller, J.L. Plant Invaders the Threat to Natural Ecosystems; Routledge: London, UK, 2001. [Google Scholar]
- Ren, Z.; Okyere, S.K.; Wen, J.; Xie, L.; Cui, Y.; Wang, S.; Wang, J.; Cao, S.; Shen, L.; Ma, X.; et al. An Overview: The Toxicity of Ageratina adenophora on Animals and Its Possible Interventions. Int. J. Mol. Sci. 2021, 22, 11581. [Google Scholar] [CrossRef]
- Kundu, A.; Saha, S.; Walia, S.; Shakil, N.A.; Kumar, J.; Annapurna, K. Cadinene sesquiterpenes from Eupatorium adenophorum and their antifungal activity. J. Environ. Sci. Health Part B 2013, 48, 516–522. [Google Scholar] [CrossRef]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; National Institute of Science Communication and Information Resources: New Delhi, India, 2002. [Google Scholar]
- Jiangsu New Medical, C. Dictionary of Traditional Chinese Medicine; Shanghai Science and Technology Publishing House: Shanghai, China, 1977; Volume 1, pp. 318–320. [Google Scholar]
- Heras, B.D.L.; Slowing, K.; Benedí, J.; Carretero, E.; Ortega, T.; Toledo, C.; Bermejo, P.; Iglesias, I.; Abad, M.; Gómez-Serranillos, P.; et al. Antiinflammatory and antioxidant activity of plants used in traditional medicine in Ecuador. J. Ethnopharmacol. 1998, 61, 161–166. [Google Scholar] [CrossRef]
- El-Seedi, H.; Ohara, T.; Sata, N.; Nishiyama, S. Antimicrobial diterpenoids from Eupatorium glutinosum (Asteraceae). J. Ethnopharmacol. 2002, 81, 293–296. [Google Scholar] [CrossRef]
- Neupane, N.P.; Karn, A.K.; Mukeri, I.H.; Pathak, P.; Kumar, P.; Singh, S.; Qureshi, I.A.; Jha, T.; Verma, A. Molecular dynamics analysis of phytochemicals from Ageratina adenophora against COVID-19 main protease (Mpro) and human angiotensin-converting enzyme 2 (ACE2). Biocatal. Agric. Biotechnol. 2021, 32, 101924. [Google Scholar] [CrossRef]
- Poudel, R.; Neupane, N.P.; Mukeri, I.H.; Alok, S.; Verma, A. An updated review on invasive nature, phytochemical evaluation, & pharmacological activity of Ageratina adenophora. Int. J. Pharm. Sci. Res. 2020, 11, 2510–2520. [Google Scholar]
- Sharma, O.P.; Dawra, R.K.; Kurade, N.P.; Sharma, P.D. A review of the toxicosis and biological properties of the genus Eupatorium. Nat. Toxins 1998, 6, 1–14. [Google Scholar] [CrossRef]
- O’Sullivan, B.M. Crofton Weed (Eupatorium adenophorum) Toxicity in Horses. Aust. Vet. J. 1979, 55, 19–21. [Google Scholar] [CrossRef]
- O’Sullivan, B.M. Investigations into Crofton weed (Eupatorium adenophorum) toxicity in horses. Aust. Vet. J. 1985, 62, 30–32. [Google Scholar] [CrossRef]
- Stewart, A.J. Large Animal Internal Medicine, 6th ed.; Smith, B.P., Van Metre, D.C., Pusterla, N., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 550–559. [Google Scholar]
- Buergelt, C.D.; Hines, S.A.; Cantor, G.; Stirk, A.; Wilson, J.H. A Retrospective Study of Proliferative Interstitial Lung Disease of Horses in Florida. Vet. Pathol. 1986, 23, 750–756. [Google Scholar] [CrossRef]
- Nout, Y.S.; Hinchcliff, K.W.; Samii, V.F.; Kohn, C.W.; Jose-Cunilleras, E.; Reed, S.M. Chronic pulmonary disease with radiographic interstitial opacity (interstitial pneumonia) in foals. Equine Vet. J. 2010, 34, 542–548. [Google Scholar] [CrossRef]
- Spelta, C.; Axon, J.; Begg, A.; Diallo, I.; Carrick, J.; Russell, C.; Collins, N. Equine multinodular pulmonary fibrosis in three horses in Australia. Aust. Vet. J. 2013, 91, 274–280. [Google Scholar] [CrossRef]
- Winder, C.; Ehrensperger, F.; Hermann, M.; Howald, B.; Fellenberg, R. Interstitial pneumonia in the horse: Two unusual cases. Equine Vet. J. 1988, 20, 298–301. [Google Scholar] [CrossRef]
- Buergelt, C.D. Interstitial pneumonia in the horse: A fledgling morphological entity with mysterious causes. Equine Vet. J. 1995, 27, 4–5. [Google Scholar] [CrossRef]
- Katoch, R.; Sharma, O.P.; Dawra, R.K.; Kurade, N.P. Hepatotoxicity of Eupatorium adenophorum to rats. Toxicon 2000, 38, 309–314. [Google Scholar] [CrossRef]
- Oelrichs, P.B.; Calanasan, C.A.; Macleod, J.K.; Seawright, A.A.; Ng, J.C. Isolation of a compound from Eupatorium adenophorum (Spreng.) [Ageratina adenophora (Spreng.)] causing hepatotoxicity in mice. Nat. Toxins 1995, 3, 350–354. [Google Scholar] [CrossRef]
- Sun, W.; Zeng, C.; Yue, D.; Liu, S.; Ren, Z.; Zuo, Z.; Deng, J.; Peng, G.; Hu, Y. Ageratina adenophora causes spleen toxicity by inducing oxidative stress and pyroptosis in mice. R. Soc. Open Sci. 2019, 6, 190127. [Google Scholar] [CrossRef]
- He, Y.; Chen, W.; Hu, Y.; Luo, B.; Wu, L.; Qiao, Y.; Mo, Q.; Xu, R.; Zhou, Y.; Ren, Z.; et al. E. adenophorum Induces Cell Cycle and Apoptosis of Renal Cells through Mitochondrial Pathway and Caspase Activation in Saanen Goat. PLoS ONE 2015, 10, e0138504. [Google Scholar] [CrossRef]
- He, Y.; Mo, Q.; Hu, Y.; Chen, W.; Luo, B.; Wu, L.; Qiao, Y.; Xu, R.; Zhou, Y.; Zuo, Z.; et al. E. adenophorum induces Cell Cycle Arrest and Apoptosis of Splenocytes through the Mitochondrial Pathway and Caspase Activation in Saanen Goats. Sci. Rep. 2015, 5, 15967. [Google Scholar] [CrossRef]
- He, Y.; Mo, Q.; Luo, B.; Qiao, Y.; Xu, R.; Zuo, Z.; Deng, J.; Nong, X.; Peng, G.; He, W.; et al. Induction of apoptosis and autophagy via mitochondria- and PI3K/Akt/mTOR-mediated pathways by E. adenophorum in hepatocytes of Saanen goat. Oncotarget 2016, 7, 54537–54548. [Google Scholar] [CrossRef]
- Sharma, D.; Mal, G.; Kannan, A.; Bhar, R.; Sharma, R.; Singh, B. Degradation of euptox A by tannase-producing rumen bacteria from migratory goats. J. Appl. Microbiol. 2017, 123, 1194–1202. [Google Scholar] [CrossRef]
- Crofton Weed. 2022. Available online: https://www.daf.qld.gov.au/__data/assets/pdf_file/0005/74966/crofton-weed.pdf (accessed on 10 June 2023).
- NSW Weedwise, Crofton Weed (Ageratina adenophora). Available online: https://weeds.dpi.nsw.gov.au/Weeds/Details/47 (accessed on 10 June 2023).
- Tripathi, R.S.; Yadav, A.S. Population dynamics of Eupatorium adenophorum Spreng. and Eupatorium riparium Regel in relation to burning. Weed Res. 1987, 27, 229–236. [Google Scholar] [CrossRef]
- Tripathi, R.S.; Kushwaha, S.P.S.; Yadav, A.S. Ecology of three invasive species of Eupatorium: A review. Int. J. Ecol. Environ. Sci. 2006, 32, 301–326. [Google Scholar]
- Tripathi, R.S.; Yadav, A.S. Population dynamics of invasive alien species of Eupatorium. In Invasive Alien Plants: An Ecological Appraisal for the Indian Subcontinent; CABI: Wallingford, UK, 2012; pp. 257–270. [Google Scholar] [CrossRef]
- Bess, H.A.; Haramoto, F.H. Biological Control of Pamakani, Eupatorium adenophorum, in Hawaii by a Tephritid Gall Fly, Procecidochares Utilis. 2. Population Studies of the Weed, the Fly, and the Parasites of the Fly. Ecology 1959, 40, 244–249. [Google Scholar] [CrossRef]
- Huffaker, C.B. Biological Control of Weeds with Insects. Annu. Rev. Èntomol. 1959, 4, 251–276. [Google Scholar] [CrossRef]
- Muniappan, R.; Raman, A.; Reddy, G.V.P. Biological Control of Tropical Weeds Using Arthropods; Muniappan, R., Reddy, G.V.P., Raman, A., Eds.; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Lu, H.; Shen, J.; Sang, W.; Zhang, X.; Lin, J. Pollen Viability, Pollination, Seed Set, and Seed Germination of Croftonweed (Eupatorium Adenophorum) in China. Weed Sci. 2008, 56, 42–51. [Google Scholar] [CrossRef]
- Sullivan, V.I. Pollen and pollination in the genus Eupatorium (Compositae). Can. J. Bot. 1975, 53, 582–589. [Google Scholar] [CrossRef]
- Grashoff, J.L.; Beaman, J.H. Studies in Eupatorium (Compositae), III. Apparent Wind Pollination. Brittonia 1970, 22, 77–84. [Google Scholar] [CrossRef]
- Hui, L. Biological replacement control of “Crofton weed”. Rangel. Arch. 1987, 9, 180. [Google Scholar]
- Mo, Q.; Hu, L.; Weng, J.; Zhang, Y.; Zhou, Y.; Xu, R.; Zuo, Z.; Deng, J.; Ren, Z.; Zhong, Z.; et al. Euptox A Induces G1 Arrest and Autophagy via p38 MAPK- and PI3K/Akt/mTOR-Mediated Pathways in Mouse Splenocytes. J. Histochem. Cytochem. 2017, 65, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Okyere, S.K.; Mo, Q.; Pei, G.; Ren, Z.; Deng, J.; Hu, Y. Euptox A Induces G0 /GI arrest and apoptosis of hepatocyte via ROS, mitochondrial dysfunction and caspases-dependent pathways in vivo. J. Toxicol. Sci. 2020, 45, 661–671. [Google Scholar] [CrossRef]
- Motooka, P.; Castro, L.; Nelson, D.; Nagai, G.; Ching, L. Weeds of Hawaii’s Pastures and Natural Areas: An Identification and Management Guide College of Tropical Agriculture and Human Resources; University of Hawai ‘i at Manoa: Manoa, HI, USA, 2003; Volume 316. [Google Scholar]
- Trujillo, E.V.I. International Symposia on Biological Control of Weeds; Delfosse, E.S., Ed.; Agric-Can: Vancouver, BC, Canada, 1985; pp. 661–671. [Google Scholar]
- Borges, A.S.; Mair, T.; Pasval, I.; Saulez, M.N.; Tennent-Brown, B.S.; van Eps, A.W. Emergency Diseases Outside the Continental United States. In Equine Emergencies; Elsevier Saunders: St Louis, MO, USA, 2014; pp. 656–686. [Google Scholar]
- Connor, H.E. The poisonous plants in New Zealand. Government Printer: Wellington, New Zealand, 1977. [Google Scholar]
- Government, Q. Crofton Weed. Available online: https://www.daf.qld.gov.au/__data/assets/pdf_file/74966/crofton-weed.pdf (accessed on 22 June 2023).
- Inderjit; Van der Putten, W.H. Impacts of soil microbial communities on exotic plant invasions. Trends Ecol. Evol. 2010, 25, 512–519. [Google Scholar] [CrossRef]
- Kaushal, V.; Dawra, R.K.; Sharma, O.; Kurade, N. Biochemical alterations in the blood plasma of rats associated with hepatotoxicity induced by Eupatorium adenophorum. Vet. Res. Commun. 2001, 25, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.L.; Seawright, A.A.; Ng, J.; Hertle, A.T.; Thomson, J.A.; Bostock, P.D. Ptaquiloside in Bracken Ferns (Pteridium Spp) from Eastern Australia and from a Cultivated Collection of Bracken from World-Wide Sources. Plant-Assoc. Toxins 1994, 2, 347–353. [Google Scholar]
- Liu, Y.; Chen, P.; Zhou, M.; Wang, T.; Fang, S.; Shang, X.; Fu, X. Geographic Variation in the Chemical Composition and Antioxidant Properties of Phenolic Compounds from Cyclocarya paliurus (Batal) Iljinskaja Leaves. Molecules 2018, 23, 2440. [Google Scholar] [CrossRef]
- Inderjit Evans, H.; Crocoll, C.; Bajpai, D.; Kaur, R.; Feng, Y.L.; Silva, C.; Carreón, J.T.; Valiente-Banuet, A.; Gershenzon, J.; Callaway, R.M. Volatile chemicals from leaf litter are associated with invasiveness of a neotropical weed in Asia. Ecology 2011, 92, 316–324. [Google Scholar] [CrossRef]
- Inderjit; Simberloff, D.; Kaur, H.; Kalisz, S.; Bezemer, T.M. Novel chemicals engender myriad invasion mechanisms. New Phytol. 2021, 232, 1184–1200. [Google Scholar] [CrossRef]
- Okyere, S.K.; Wen, J.; Cui, Y.; Xie, L.; Gao, P.; Wang, J.; Wang, S.; Hu, Y. Toxic mechanisms and pharmacological properties of euptox A, a toxic monomer from A. adenophora. Fitoterapia 2021, 155, 105032. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, M.U.; Khazeo, P.; Puro, K.N.; Jyrwa, R.; Jamir, N.; Sailo, L. Qualitative and Quantitative Analysis of Phyto-Chemicals of Crude Extracts of Ageratina Adenophora Leaves; Atlantis Press: Amsterdam, The Netherlands, 2018; pp. 178–182. [Google Scholar]
- Subba, B.; Kandel, R.C. Chemical Composition and Bioactivity of Essential Oil of Ageratina adenophora from Bhaktapur District of Nepal. J. Nepal Chem. Soc. 2013, 30, 78–86. [Google Scholar] [CrossRef]
- Hu, Y.; Liao, F.; Hu, Y.; Luo, B.; He, Y.; Mo, Q.; Zuo, Z.; Ren, Z.; Deng, J.; Wei, Y. Clinical efficacy of 9-oxo-10, 11-dehydroageraphorone extracted from Eupatorium adenophorum against Psoroptes cuniculi in rabbits. BMC Vet. Res. 2014, 10, 970. [Google Scholar] [CrossRef]
- Liao, F.; Hu, Y.; Tan, H.; Wu, L.; Wang, Y.; Huang, Y.; Mo, Q.; Wei, Y. Acaricidal activity of 9-oxo-10,11-dehydroageraphorone extracted from Eupatorium adenophorum in vitro. Exp. Parasitol. 2014, 140, 8–11. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, B.; Yang, J.; Ma, X.; Deng, S.; Huang, Y.; Wen, Y.; Yuan, J.; Yang, X. Essential Oil Derived From Eupatorium adenophorum Spreng. Mediates Anticancer Effect by Inhibiting STAT3 and AKT Activation to Induce Apoptosis in Hepatocellular Carcinoma. Front. Pharmacol. 2018, 9, 483. [Google Scholar] [CrossRef]
- Colegate, S.M.; Upton, R.; Gardner, D.R.; Panter, K.E.; Betz, J.M. Potentially toxic pyrrolizidine alkaloids in Eupatorium perfoliatum and three related species. Implications for herbal use as boneset. Phytochem. Anal. 2018, 29, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, Z.; Wong, L.; He, Y.; Zhao, Z.; Ye, Y.; Fu, P.P.; Lin, G. Contamination of hepatotoxic pyrrolizidine alkaloids in retail honey in China. Food Control 2018, 85, 484–494. [Google Scholar] [CrossRef]
- Kast, C.; Dübecke, A.; Kilchenmann, V.; Bieri, K.; Böhlen, M.; Zoller, O.; Beckh, G.; Lüllmann, C. Analysis of Swiss honeys for pyrrolizidine alkaloids. J. Apic. Res. 2014, 53, 75–83. [Google Scholar] [CrossRef]
- Kempf, M.; Heil, S.; Haßlauer, I.; Schmidt, L.; von der Ohe, K.; Theuring, C.; Reinhard, A.; Schreier, P.; Beuerle, T. Pyrrolizidine alkaloids in pollen and pollen products. Mol. Nutr. Food Res. 2009, 54, 292–300. [Google Scholar] [CrossRef]
- Gibson, J.A.; O’Sullivan, B.M. Lung lesions in horses fed mist flower (Eupatorium riparium). Aust. Vet. J. 1984, 61, 271. [Google Scholar] [CrossRef]
- Stewart, A.J.; Cuming, R.S. Update on Fungal Respiratory Disease in Horses. Vet. Clin. N. Am. Equine Pract. 2015, 31, 43–62. [Google Scholar] [CrossRef]
- Wang, X.; Wise, J.C.; Stewart, A.J. Hendra Virus: An Update on Diagnosis, Vaccination, and Biosecurity Protocols for Horses. Vet. Clin. N. Am. Equine Pract. 2023, 39, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.P.; Shrestha, N.P.; Gatenby, R.M.; Aryal, I.K. Performance of Goats Given Different Levels of Banmara (Eupatorium adenophorum) at Pakhribas Agricultural Centre; PAC Technical Paper; Pakhribas Agricultural Centre: Dhankuta, Nepal, 1992.
- Kaushal, V.; Dawra, R.; Sharma, O.; Kurade, N. Hepatotoxicity in rat induced by partially purified toxins from Eupatorium adenophorum (Ageratina adenophora). Toxicon 2000, 39, 615–619. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Singh, A.; Sharma, O.P.; Dawra, R.K.; Kurade, N.P.; Mahato, S.B. Hepatotoxicity and cholestasis in rats induced by the sesquiterpene, 9-oxo-10,11-dehydroageraphorone, isolated from Eupatorium adenophorum. J. Biochem. Mol. Toxicol. 2001, 15, 279–286. [Google Scholar] [CrossRef]
- Cui, Y.; Okyere, S.K.; Gao, P.; Wen, J.; Cao, S.; Wang, Y.; Deng, J.; Hu, Y. Ageratina adenophora Disrupts the Intestinal Structure and Immune Barrier Integrity in Rats. Toxins 2021, 13, 651. [Google Scholar] [CrossRef] [PubMed]
- Sani, Y.; Harper, P.A.W.; Cook, R.L.; Seawright, A.A.; Ng, J.C. The Toxicity of Eupatorium Adenophorum for the Liver of the Mouse; Iowa State University Press: Ames, IA, USA, 1992; pp. 626–629. [Google Scholar]
- Singh, Y.D.; Mukhopadhayay, S.K.; Shah, M.A.; Ali, M.A.; Tolenkhomba, T.C. Effects of Eupatorium adenophorum on Antioxidant Enzyme Status in a Mice Model. Int. J. Pharm. Pharm. Sci. 2012, 4, 436–439. [Google Scholar]
- Singh, D.; Mukhopadhayay, S.K.; Tolenkhomba, T.C.; Shah, A. Short-term toxicity studies of Eupatorium adenophorum in Swiss albino mice. Int. J. Res. Phytochem. Pharmacol. 2011, 1, 165–171. [Google Scholar]
- Ouyang, C.-B.; Liu, X.-M.; Liu, Q.; Bai, J.; Li, H.-Y.; Li, Y.; Wang, Q.-X.; Yan, D.-D.; Mao, L.-G.; Cao, A.; et al. Toxicity Assessment of Cadinene Sesquiterpenes from Eupatorium adenophorum in Mice. Nat. Prod. Bioprospect. 2014, 5, 29–36. [Google Scholar] [CrossRef]
- Verma, A.; Yadav, B.P.S.; Sampath, K.T. Possible use of Spreng (Eupatorium adenophorum) in Animal Feeding. Indian J. Anim. Nutr. 1987, 4, 189. [Google Scholar]
- Botha, C.J.; Lewis, A.; du Plessis, E.C.; Clift, S.; Williams, M.C. Crotalariosis equorum (“jaagsiekte”) in horses in southern Mozambique, a rare form of pyrrolizidine alkaloid poisoning. J. Vet. Diagn. Investig. 2012, 24, 1099–1104. [Google Scholar] [CrossRef]
- Moreira, R.; Pereira, D.M.; Valentão, P.; Andrade, P.B. Pyrrolizidine Alkaloids: Chemistry, Pharmacology, Toxicology and Food Safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; He, Y.; Ma, J.; Fu, P.P.; Lin, G. Pulmonary toxicity is a common phenomenon of toxic pyrrolizidine alkaloids. J. Environ. Sci. Health Part C 2020, 38, 124–140. [Google Scholar] [CrossRef]
- He, Y.; Lian, W.; Ding, L.; Fan, X.; Ma, J.; Zhang, Q.-Y.; Ding, X.; Lin, G. Lung injury induced by pyrrolizidine alkaloids depends on metabolism by hepatic cytochrome P450s and blood transport of reactive metabolites. Arch. Toxicol. 2020, 95, 103–116. [Google Scholar] [CrossRef]
- Fink-Gremmels, J. Implications of hepatic cytochrome P450-related biotransformation processes in veterinary sciences. Eur. J. Pharmacol. 2008, 585, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.; Persson, E.; Tydén, E.; Tjälve, H. Cell-specific activation of aflatoxin B1 correlates with presence of some cytochrome P450 enzymes in olfactory and respiratory tissues in horse. Res. Vet. Sci. 2003, 74, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Chauret, N.; Gauthier, A.; Martin, J.; A Nicoll-Griffith, D. In vitro comparison of cytochrome P450-mediated metabolic activities in human, dog, cat, and horse. Drug Metab. Dispos. 1997, 25, 89. [Google Scholar]
- Baillie, T.A.; Rettie, A.E. Role of Biotransformation in Drug-Induced Toxicity: Influence of Intra- and Inter-Species Differences in Drug Metabolism. Drug Metab. Pharmacokinet. 2011, 26, 15–29. [Google Scholar] [CrossRef]
- Lakritz, J.; Winder, B.S.; Noorouz-Zadeh, J.; Huang, T.L.; Buckpitt, A.R.; Hammock, B.D.; Plopper, C.G. Hepatic and pulmonary enzyme activities in horses. Am. J. Vet. Res. 2000, 61, 152–157. [Google Scholar] [CrossRef]
- Becerra Jimenez, J. Phytochemical and analytical studies of feed and medicinal plants in relation to the presence of toxic pyrrolizidine alkaloids. Ph.D. Thesis, Universitäts-und Landesbibliothek Bonn, Bonn, Germany, 2013. [Google Scholar]
- Stewart, A.J. Coccidiomycosis: Evidence from human medicine to diagnose and treat equids. Equine Vet. Educ. 2021, 34, 352–354. [Google Scholar] [CrossRef]
- Yuen, K.Y.; Fraser, N.S.; Henning, J.; Halpin, K.; Gibson, J.S.; Betzien, L.; Stewart, A.J. Hendra virus: Epidemiology dynamics in relation to climate change, diagnostic tests and control measures. One Health 2020, 12, 100207. [Google Scholar] [CrossRef]
- Masetla, N.; Maila, Y.; Shadung, K. Accumulation of phytochemicals at different growth stages of Cleome gynandra grown under greenhouse and microplot conditions. Res. Crops 2022, 23, 657–665. [Google Scholar] [CrossRef]
- Richins, R.D.; Rodriguez-Uribe, L.; Lowe, K.; Ferral, R.; O’connell, M.A. Accumulation of bioactive metabolites in cultivated medical Cannabis. PLoS ONE 2018, 13, e0201119. [Google Scholar] [CrossRef]
- Hazrati, S.; Hosseini, S.J.; Ebadi, M.-T.; Nicola, S. Evolution of Phytochemical Variation in Myrtle (Myrtus communis L.) Organs during Different Phenological Stages. Horticulturae 2022, 8, 757. [Google Scholar] [CrossRef]
- Rivest, S.; Forrest, J.R.K. Defence compounds in pollen: Why do they occur and how do they affect the ecology and evolution of bees? New Phytol. 2019, 225, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Carlier, J.; Guitton, J.; Romeuf, L.; Bévalot, F.; Boyer, B.; Fanton, L.; Gaillard, Y. Screening approach by ultra-high performance liquid chromatography–tandem mass spectrometry for the blood quantification of thirty-four toxic principles of plant origin. Application to forensic toxicology. J. Chromatogr. B 2015, 975, 65–76. [Google Scholar] [CrossRef]
- Williams, J.H.; Whitehead, Z.; Van Wilpe, E. Paraquat intoxication and associated pathological findings in three dogs in South Africa. J. South Afr. Vet. Assoc. 2016, 87, e1–e9. [Google Scholar] [CrossRef]
- Boyd, M.R.; Wilson, B.J. Isolation and characterization of 4-ipomeanol, a lung-toxic furanoterpenoid produced by sweet potatoes (Ipomoea batatas). J. Agric. Food Chem. 1972, 20, 428–430. [Google Scholar] [CrossRef]
- Garst, J.E.; Wilson, W.C.; Kristensen, N.C.; Harrison, P.C.; Corbin, J.E.; Simon, J.; Philpot, R.M.; Szabo, R.R. Species Susceptibility to the Pulmonary Toxicity of 3-Furyl Isoamyl Ketone (Perilla Ketone): In Vivo Support for Involvement of the Lung Monooxygenase System. J. Anim. Sci. 1985, 60, 248–257. [Google Scholar] [CrossRef]
- Merrill, J.; Bray, T. Effects of species, MFO inducers and conjugation agents on the in vitro covalent binding of 14C-3-methylindole metabolite in liver and lung tissues. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1983, 75, 395–398. [Google Scholar] [CrossRef]
- Huijzer, J.C.; Adams, J.D.; Jaw, J.Y.; Yost, G.S. Inhibition of 3-methylindole bioactivation by the cytochrome P-450 suicide substrates 1-aminobenzotriazole and alpha-methylbenzylaminobenzotriazole. Drug Metab. Dispos. 1989, 17, 37–42. [Google Scholar]
- El-Hage, C.; Mekuria, Z.; Dynon, K.; Hartley, C.; McBride, K.; Gilkerson, J. Association of Equine Herpesvirus 5 with Mild Respiratory Disease in a Survey of EHV1, -2, -4 and -5 in 407 Australian Horses. Animals 2021, 11, 3418. Available online: https://mdpi-res.com/d_attachment/animals/animals-11-03418/article_deploy/animals-11-03418-v3.pdf?version=1639460331 (accessed on 10 May 2023). [CrossRef] [PubMed]
- Liao, F.; Wang, Y.; Huang, Y.; Mo, Q.; Tan, H.; Wei, Y.; Hu, Y. Isolation and identification of bacteria capable of degrading euptox A from Eupatorium adenophorum Spreng. Toxicon 2014, 77, 87–92. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


