Effects of Autologous Microfragmented Adipose Tissue on Healing of Tibial Plateau Levelling Osteotomies in Dogs: A Prospective Clinical Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Surgical Procedures
2.4. Isolation, Preparation and Inoculation of MFAT
2.5. Radiographic Evaluations
2.6. Statistical Analysis
3. Results
3.1. Enrolled Patients
3.2. Surgical Variables
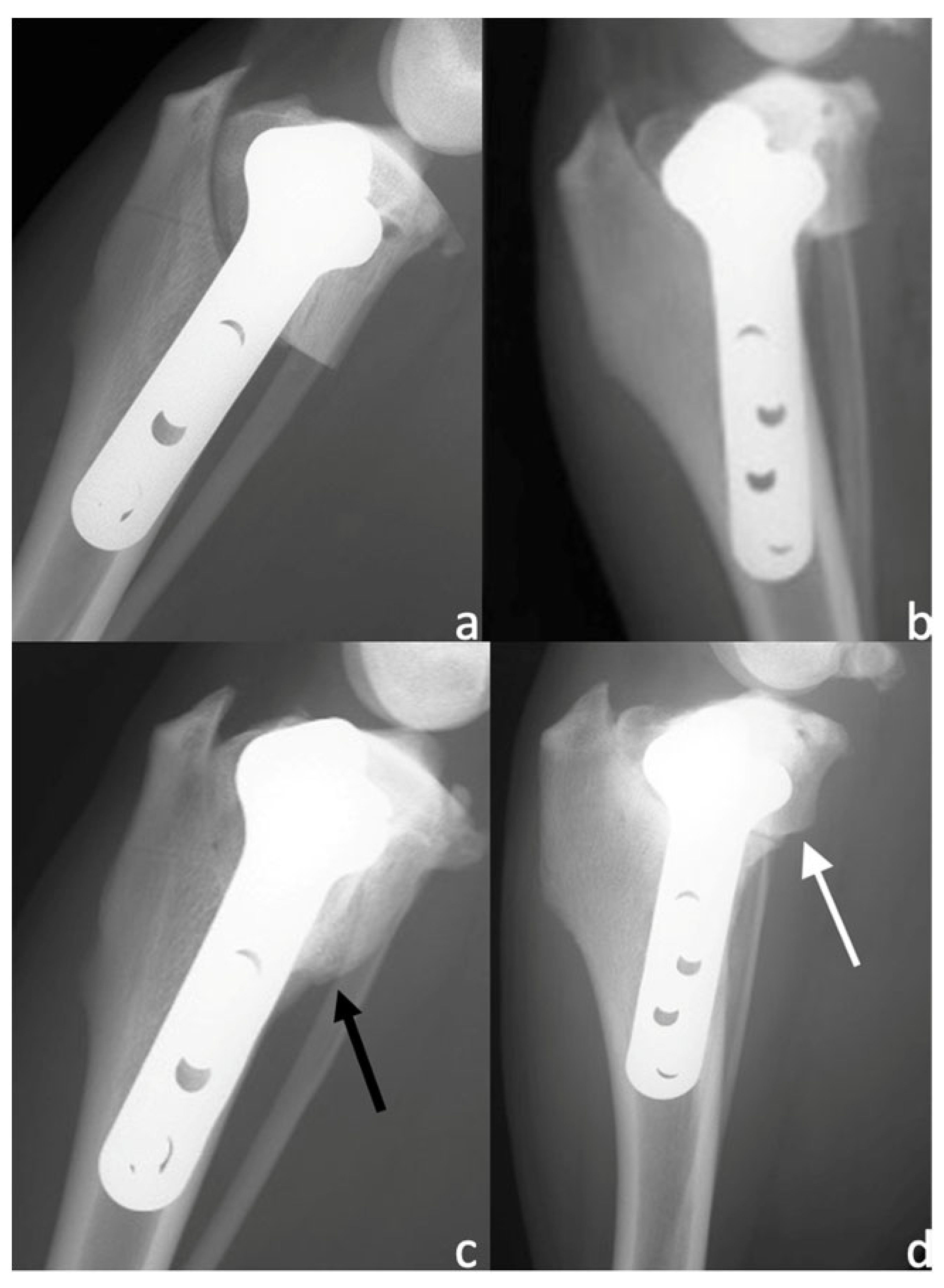
3.3. Radiographic Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Witsberger, T.H.; Villamil, J.A.; Schultz, L.G.; Hahn, A.W.; Cook, J.L. Prevalence of and risk factors for hip dysplasia and cranial cruciate ligament deficiency in dogs. J. Am. Vet. Med. Assoc. 2008, 232, 1818–1824. [Google Scholar] [PubMed]
- Sellon, D.C.; Marcellin-Little, D.J. Risk factors for cranial cruciate ligament rupture in dogs participating in canine agility. BMC Vet. Res. 2022, 18, 39. [Google Scholar]
- Griffon, D.J. A review of the pathogenesis of canine cranial cruciate ligament disease as a basis for future preventive strategies. Vet. Surg. 2010, 39, 399–409. [Google Scholar] [PubMed]
- Comerford, E.J.; Smith, K.; Hayashi, K. Update on the aetiopathogenesis of canine cranial cruciate ligament disease. Vet. Comp. Orthop. Traumatol. 2011, 24, 91–98. [Google Scholar] [PubMed] [Green Version]
- Terhaar, H.M.; Muir, P.; Baker, L.A.; Binversie, E.E.; Chi, J.; Sample, S.J. Contribution of habitual activity to cruciate ligament rupture in Labrador retrievers. Vet. Comp. Orthop. Traumatol. 2020, 33, 82–88. [Google Scholar] [CrossRef]
- Kyllar, M.; Čížek, P. Cranial cruciate ligament structure in relation to the tibial plateau slope and intercondylar notch width in dogs. J. Vet. Sci. 2018, 19, 699–707. [Google Scholar]
- Bergh, M.S.; Sullivan, C.; Ferrell, C.L.; Troy, J.; Budsberg, S.C. Systematic review of surgical treatments for cranial cruciate ligament disease in dogs. J. Am. Anim. Hosp. Assoc. 2014, 50, 315–321. [Google Scholar]
- Moore, K.W.; Read, R.A. Cranial cruciate ligament rupture in the dog—A retrospective study comparing surgical techniques. Aust. Vet. J. 1995, 72, 281–285. [Google Scholar]
- Arnoczky, S.P. The over-the-top procedure: A technique for anterior cruciate ligament substitution in the dog. J. Am. Anim. Hosp. Assoc. 1979, 15, 283–290. [Google Scholar]
- Geels, J.J.; Roush, J.K.; Hoskinson, J.J.; McLaughlin, R.M. Evaluation of an intracapsular technique for the treatment of cranial cruciate ligament rupture. Vet. Comp. Orthop. Traumatol. 2000, 13, 197–203. [Google Scholar]
- Kim, S.E.; Pozzi, A.; Kowaleski, M.P.; Lewis, D.D. Tibial osteotomies for cranial cruciate ligament insufficiency in dogs. Vet. Surg. 2008, 37, 111–125. [Google Scholar]
- Gordon-Evans, W.J.; Griffon, D.J.; Bubb, C.; Knap, K.M.; Sullivan, M.; Evans, R.B. Comparison of lateral fabellar suture and tibial plateau leveling osteotomy techniques for treatment of dogs with cranial cruciate ligament disease. J. Am. Vet. Med. Assoc. 2013, 243, 675–680. [Google Scholar]
- Slocum, B.; Slocum, T.D. Tibial plateau leveling osteotomy for repair of cranial cruciate ligament rupture in the canine. Vet. Clin. N. Am. Small Anim. Pract. 1993, 23, 777–795. [Google Scholar]
- Hans, E.C.; Barnhart, M.D.; Kennedy, S.C.; Naber, S.J. Comparison of complications following tibial tuberosity advancement and tibial plateau levelling osteotomy in very large and giant dogs 50 kg or more in body weight. Vet. Comp. Orthop. Traumatol. 2017, 30, 299–305. [Google Scholar]
- Krotscheck, U.; Nelson, S.A.; Todhunter, R.J.; Stone, M.; Zhang, Z. Long term functional outcome of tibial tuberosity advancement vs. tibial plateau leveling osteotomy and extracapsular repair in a heterogeneous population of dogs. Vet. Surg. 2016, 45, 261–268. [Google Scholar]
- Corr, S.A.; Brown, C. A comparison of outcomes following tibial plateau levelling osteotomy and cranial tibial wedge osteotomy procedures. Vet. Comp. Orthop. Traumatol. 2007, 20, 312–319. [Google Scholar]
- Fitzpatrick, N.; Solano, M.A. Predictive variables for complications after TPLO with stifle inspection by arthrotomy in 1000 consecutive dogs. Vet. Surg. 2010, 39, 460–474. [Google Scholar]
- Conkling, A.L.; Bennett Fagin, R.; Daye, M. Comparison of tibial plateau angle changes after tibial plateau leveling osteotomy fixation with conventional or locking screw technology. Vet. Surg. 2010, 39, 475–481. [Google Scholar]
- Franklin, S.P.; Burke, E.E.; Holmes, S.P. The effect of platelet-rich plasma on osseous healing in dogs undergoing high tibial osteotomy. PLoS ONE 2017, 12, e0177597. [Google Scholar]
- Bergh, M.S.; Peirone, B. Complications of tibial plateau levelling osteotomy in dogs. Vet. Comp. Orthop. Traumatol. 2012, 25, 349–358. [Google Scholar]
- Bhan, K.; Patel, R.; Hasan, K.; Pimplé, M.; Sharma, S.; Nandwana, V.; Basta, M. Fracture nonunions and delayed unions treated with low-intensity pulsed ultrasound therapy: A clinical series. Cureus 2021, 13, e17067. [Google Scholar] [PubMed]
- Kieves, N.R.; MacKay, C.S.; Adducci, K.; Rao, S.; Goh, C.; Palmer, R.H.; Duerr, F.M. High energy focused shock wave therapy accelerates bone healing. Vet. Comp. Orthop. Traumatol. 2015, 28, 425–432. [Google Scholar] [PubMed]
- Kieves, N.R.; Canapp, S.O.; Lotsikas, P.J.; Christopher, S.A.; Leasure, C.S.; Canapp, D.; Gavin, P.R. Effects of low-intensity pulsed ultrasound on radiographic healing of tibial plateau leveling osteotomies in dogs: A prospective, randomized, double-blinded study. Vet. Surg. 2018, 47, 614–622. [Google Scholar] [PubMed]
- Stewart, S.K. Fracture non-union: A review of clinical challenges and future research needs. Malays. Orthop. J. 2019, 13, 1. [Google Scholar]
- Taroni, M.; Cabon, Q.; Fèbre, M.; Cachon, T.; Saulnier, N.; Carozzo, C.; Maddens, S.; Labadie, F.; Robert, C.; Viguier, E. Evaluation of the effect of a single intra-articular injection of allogeneic neonatal mesenchymal stromal cells compared to oral non-steroidal anti-inflammatory treatment on the postoperative musculoskeletal status and gait of dogs over a 6-month period after tibial plateau leveling osteotomy: A pilot study. Front. Vet. Sci. 2017, 4, 83. [Google Scholar]
- Akpancar, S.; Tatar, O.; Turgut, H.; Akyildiz, F.; Ekinci, S. The current perspectives of stem cell therapy in orthopedic surgery. Arch. Trauma Res. 2016, 5, e37976. [Google Scholar]
- Yoshida, Y.; Matsubara, H.; Fang, X.; Hayashi, K.; Nomura, I.; Ugaji, S.; Hamada, T.; Tsuchiya, H. Adipose-derived stem cell sheets accelerate bone healing in rat femoral defects. PLoS ONE 2019, 14, e0214488. [Google Scholar]
- Rada, T.; Reis, R.L.; Gomes, M.L. Adipose tissue-derived stem cells and their application in bone and cartilage tissue engineering. Tissue Eng. 2009, 15, 113–125. [Google Scholar]
- Tapp, H.; Hanley, E.N., Jr.; Patt, J.C.; Gruber, H.E. Adipose-derived stem cells: Characterization and current application in orthopaedic tissue repair. Exp. Biol. Med. 2009, 234, 1–9. [Google Scholar]
- Qomi, R.T.; Sheykhhasan, M. Adipose-derived stromal cell in regenerative medicine: A review. World J. Stem Cells. 2017, 9, 107. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Gou, W.; Lu, Q.; Peng, J.; Lu, S. Role of mesenchymal stem cells in bone regeneration and fracture repair: A review. Int. Orthop. 2013, 37, 2491–2498. [Google Scholar]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar]
- Zhu, H.; Liu, Y.L.; Chen, J.D.; Li, H.; Liu, Y.X.; Xu, F.F.; Jang, X.X.; Zhang, Y.; Mao, N. Effect of osteogenically and adipogenically differentiated bone mesenchymal stem cells from mouse on osteoclast formation. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2012, 20, 1187–1190. [Google Scholar]
- Han, S.; Sun, H.M.; Hwang, K.C.; Kim, S.W. Adipose-derived stromal vascular fraction cells: Update on clinical utility and efficacy. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 145–152. [Google Scholar]
- Gentile, P.; Sterodimas, A.; Pizzicannella, J.; Dionisi, L.; De Fazio, D.; Calabrese, C.; Garcovich, S. Systematic review: Allogenic use of stromal vascular fraction (SVF) and decellularized extracellular matrices (ECM) as advanced therapy medicinal products (ATMP) in tissue regeneration. Int. J. Mol. Sci. 2020, 21, 4982. [Google Scholar]
- Mehranfar, S.; Rad, I.A.; Mostafav, E.; Akbarzadeh, A. The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: An overview of clinical trials. Artif. Cells Nanomed. Biotechnol. 2019, 47, 882–890. [Google Scholar]
- Palumbo Piccionello, A.; Riccio, V.; Senesi, L.; Volta, A.; Pennasilico, L.; Botto, R.; Rossi, G.; Tambella, A.M.; Galosi, L.; Marini, C.; et al. Adipose micro-grafts enhance tendinopathy healing in ovine model: An in vivo experimental perspective study. Stem Cells Transl. Med. 2021, 10, 1544–1560. [Google Scholar]
- Rhee, S.C.; Ji, Y.H.; Gharibjanian, N.A.; Dhong, E.S.; Park, S.H.; Yoon, E.S. In vivo evaluation of mixtures of uncultured freshly isolated adipose-derived stem cells and demineralized bone matrix for bone regeneration in a rat critically sized calvarial defect model. Stem Cells Dev. 2011, 20, 233–242. [Google Scholar]
- Saxer, F.; Scherberich, A.; Todorov, A.; Studer, P.; Miot, S.; Schreiner, S.; Guven, S.; Tchang, L.A.H.; Haugh, M.; Heberer, M.; et al. Implantation of stromal vascular fraction progenitors at bone fracture sites: From a rat model to a first-in-man study. Stem Cells 2016, 34, 2956–2966. [Google Scholar]
- Kim, A.; Kim, D.H.; Song, H.R.; Kang, W.H.; Kim, H.J.; Lim, H.C.; Cho, D.G.; Bae, J.H. Repair of rabbit ulna segmental bone defect using freshly isolated adipose-derived stromal vascular fraction. Cytotherapy 2012, 14, 296–305. [Google Scholar]
- François, P.; Rusconi, G.; Arnaud, L.; Mariotta, L.; Giraudo, L.; Minonzio, G.; Veran, J.; Bertrand, B.; Dumoulin, C.; Grimaud, F.; et al. Inter-center comparison of good manufacturing practices-compliant stromal vascular fraction and proposal for release acceptance criteria: A review of 364 productions. Stem Cell Res. Ther. 2021, 12, 373. [Google Scholar] [PubMed]
- De Francesco, F.; Mannucci, S.; Conti, G.; Dai Prè, E.; Sbarbati, A.; Riccio, M. A non-enzymatic method to obtain a fat tissue derivative highly enriched in adipose stem cells (ASCs) from human lipoaspirates: Preliminary results. Int. J. Mol. Sci. 2018, 19, 2061. [Google Scholar] [PubMed] [Green Version]
- De Francesco, F.; Riccio, V.; Biswas, R.; Busato, A.; Di Bella, C.; Serri, E.; Sbarbati, A.; Zavan, B.; Riccio, M.; Palumbo Piccionello, A. In Vitro characterization of canine microfragmented adipose tissue non-enzymatically extracted from the thigh and lumbar regions. Animals 2021, 11, 3231. [Google Scholar] [PubMed]
- Tremolada, C. Microfractured Adipose Tissue Graft (lipogems) and Regenerative Surgery. J. Orthop. Res. Ther. 2022, 7, 1210. [Google Scholar]
- Vezzani, B.; Shaw, I.; Lesme, H.; Yong, L.; Khan, N.; Tremolada, C.; Péault, B. Higher pericyte content and secretory activity of microfragmented human adipose tissue compared to enzymatically derived stromal vascular fraction. Stem Cells Transl. Med. 2018, 7, 876–886. [Google Scholar]
- Copcu, H.E. A new classification for adipose-derived stromal-cell systems. Plast. Reconstr. Surg. 2022, 10, e4712. [Google Scholar]
- Trivisonno, A.; Alexander, R.W.; Baldari, S.; Cohen, S.R.; Di Rocco, G.; Gentile, P.; Magalon, G.; Magalon, J.; Miller, R.B.; Womack, H.; et al. Intraoperative strategies for minimal manipulation of autologous adipose tissue for cell-and tissue-based therapies: Concise review. Stem Cells Transl. Med. 2019, 8, 1265–1271. [Google Scholar]
- Raffaini, M.; Tremolada, C. Micro fractured and purified adipose tissue graft (Lipogems®) can improve the orthognathic surgery outcomes both aesthetically and in postoperative healing. CellR4 2014, 2, e1118. [Google Scholar]
- Striano, R.D.; Battista, V.; Bilboo, N. Non-responding knee pain with osteoarthritis, meniscus and ligament tears treated with ultrasound guided autologous, micro-fragmented and minimally manipulated adipose tissue. Open J. Regen. Med. 2017, 6, 17–26. [Google Scholar]
- Lonardi, R.; Leone, N.; Gennai, S.; Trevisi Borsari, G.; Covic, T.; Silingardi, R. Autologous micro-fragmented adipose tissue for the treatment of diabetic foot minor amputations: A randomized controlled single-center clinical trial (MiFrAADiF). Stem Cell Res. Ther. 2019, 10, 223. [Google Scholar]
- Cattaneo, G.; De Caro, A.; Napoli, F.; Chiapale, D.; Trada, P.; Camera, A. Micro-fragmented adipose tissue injection associated with arthroscopic procedures in patients with symptomatic knee osteoarthritis. BMC Musculoskelet. Disord. 2018, 19, 176. [Google Scholar]
- Giori, A.; Tremolada, C.; Vailati, R.; Navone, S.E.; Marfia, G.; Caplan, A.I. Recovery of function in anal incontinence after micro-fragmented fat graft (Lipogems®) injection: Two years follow up of the first 5 cases. CellR4 2015, 3, e1544. [Google Scholar]
- Botto, R.; Riccio, V.; Galosi, L.; Rossi, G.; Vincenzetti, S.; Tambella, A.M.; De Francesco, F.; Pennasilico, L.; Riccio, M.; Salvaggio, A.; et al. Effects of intra-articular autologous adipose micrograft for the treatment of osteoarthritis in dogs: A prospective, randomized, controlled study. Animals 2022, 12, 1844. [Google Scholar]
- Pennasilico, L.; Di Bella, C.; Botto, R.; Murgia, E.; Riccio, V.; Piccionello, A.P. Use of micro-grafts in a chronic infected open wound after limb amputation in a cat. Vet. Med. 2021, 66, 448–455. [Google Scholar]
- Zeira, O.; Scaccia, S.; Pettinari, L.; Ghezzi, E.; Asiag, N.; Martinelli, L.; Zahirpour, D.; Dumas, M.P.; Konar, M.; Lupi, D.V.; et al. Intra-articular administration of autologous micro-fragmented adipose tissue in dogs with spontaneous osteoarthritis: Safety, feasibility, and clinical outcomes. Stem Cells Transl. Med. 2018, 7, 819–828. [Google Scholar]
- Pavarotti, G.S.; Hivernaud, V.; Brincin, M.; Roche, R.; Barreau, P.; Festy, F.; Gauthier, O. Evaluation of a single intra-articular injection of autologous adipose tissue for the treatment of osteoarthritis: A prospective clinical study in dogs. Vet. Comp. Orthop. Traumatol. 2020, 33, 258–266. [Google Scholar]
- Fujino, H.; Honnami, M.; Mochizuki, M. Preoperative planning for tibial plateau leveling osteotomy based on proximal tibial width. J. Vet. Med. Sci. 2020, 82, 661–667. [Google Scholar] [CrossRef] [Green Version]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classification in btief: Kellgren-Lawrence classification of osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Bunchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar]
- Nguyen, A.; Guo, J.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Payadar, K.J.; Evans, G.R.D.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 170–179. [Google Scholar]
- Busato, A.; De Francesco, F.; Biswas, R.; Mannucci, S.; Conti, G.; Fracasso, G.; Conti, F.; Riccio, V.; Riccio, M.; Sbarbati, A. Simple and rapid non-enzymatic procedure allows the isolation of structurally preserved connective tissue micro-fragments enriched with SVF. Cells 2020, 10, 36. [Google Scholar] [PubMed]
- Trzyna, A.; Banaś-Ząbczyk, A. Adipose-derived stem cells secretome and its potential application in “stem cell-free therapy”. Biomolecules 2021, 11, 878. [Google Scholar] [PubMed]
- Toplu, G.; Ozcelik, D.; Serin, M.; Erdem, H.; Topacoglu, A.T. Adipose tissue-derived stromal vascular fraction increases osteogenesis in an experimental design zygomatic bone defect model. J. Craniofacial Surg. 2017, 28, 2179–2182. [Google Scholar]
- Poniatowski, Ł.A.; Wojdasiewicz, P.; Gasik, R.; Szukiewicz, D. Transforming growth factor Beta family: Insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Mediat. Inflamm. 2015, 2015, 137823. [Google Scholar]
- Sarahrudi, K.; Thomas, A.; Mousavi, M.; Kaiser, G.; Köttstorfer, J.; Kecht, M.; Hajdu, S.; Aharinejad, S. Elevated transforming growth factor-beta 1 (TGF-β1) levels in human fracture healing. Injury 2011, 42, 833–837. [Google Scholar]
- Sananta, P.; Dradjat, R.S.; Rosandi, R.D.; Siahaan, L.D. TGF-1 biomarker level evaluation on fracture healing in a murine model with a bone defect after stromal vascular fraction application. Med. Glas 2022, 19, 63–67. [Google Scholar]
- Bukowska, J.; Szóstek-Mioduchowska, A.Z.; Kopcewicz, M.; Walendzik, K.; Machcińska, S.; Gawrońska-Kozak, B. Adipose-derived stromal/stem cells from large animal models: From basic to applied science. Stem Cell Rev. Rep. 2021, 17, 719–738. [Google Scholar]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar]
- Tsuji, W.; Rubin, J.P.; Marra, K.G. Adipose-derived stem cells: Implications in tissue regeneration. World J. Stem Cells 2014, 6, 312. [Google Scholar]
- El-Badawy, A.; Amer, M.; Abdelbaset, R.; Sherif, S.N.; Abo-Elela, M.; Ghallab, Y.H.; Abdelhamid, H.; Ismail, Y.; El-Badri, N. Adipose stem cells display higher regenerative capacities and more adaptable electro-kinetic properties compared to bone marrow-derived mesenchymal stromal cells. Sci. Rep. 2016, 6, 37801. [Google Scholar]
- Guo, J.; Nguyen, A.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.D.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 2: Mechanisms of regenerative action. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 180–188. [Google Scholar]
- Oberbauer, E.; Steffenhagen, C.; Wurzer, C.; Gabriel, C.; Redl, H.; Wolbank, S. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: Current state of the art. Cell Regen. 2015, 4, 7. [Google Scholar]
- Yoshimura, K.; Shigeura, T.; Matsumoto, D.; Sato, T.; Takaki, Y.; Aiba-Kojima, E.; Sato, K.; Inoue, K.; Koshima, I.; Gonda, K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell Phys. 2006, 208, 64–76. [Google Scholar]
- Dai Prè, E.; Busato, A.; Mannucci, S.; Vurro, F.; De Francesco, F.; Riccio, V.; Sbarbati, A. In vitro characterization of adipose stem cells non-enzymatically extracted from the thigh and abdomen. Int. J. Mol. Sci. 2020, 21, 3081. [Google Scholar]
- Neupane, M.; Chang, C.C.; Kiupel, M.; Yuzbasiyan-Gurkan, V. Isolation and characterization of canine adipose–derived mesenchymal stem cells. Tissue Eng. 2008, 14, 1007–1015. [Google Scholar] [CrossRef]
- Hendawy, H.; Uemura, A.; Ma, D.; Namiki, R.; Samir, H.; Ahmed, M.F.; El-Husseiny, H.M.; Chieh-Jen, C.; Tanaka, R. Tissue harvesting site effect on the canine adipose stromal vascular fraction quantity and quality. Animals 2021, 11, 460. [Google Scholar]
- Astor, D.E.; Hoelzler, M.G.; Harman, R.; Bastian, R.P. Patient factors influencing the concentration of stromal vascular fraction (SVF) for adipose-derived stromal cell (ASC) therapy in dogs. Can. J. Vet. Res. 2013, 77, 177–182. [Google Scholar]
- Yaylacı, S.; Kaçaroğlu, D.; Hürkal, Ö.; Ulaşlı, A.M. An enzyme-free technique enables the isolation of a large number of adipose-derived stem cells at the bedside. Sci. Rep. 2023, 13, 8005. [Google Scholar]
- Fontes, T.; Brandão, I.; Negrão, R.; Martins, M.J.; Monteiro, R. Autologous fat grafting: Harvesting techniques. Ann. Med. Surg. 2018, 36, 212–218. [Google Scholar]
- Gause, T.M.; Kling, R.E.; Sivak, W.N.; Marra, K.G.; Rubin, J.P.; Kokai, L.E. Particle size in fat graft retention: A review on the impact of harvesting technique in lipofilling surgical outcomes. Adipocyte 2014, 3, 273–279. [Google Scholar]
- Francis, A.; Wang, W.Z.; Goldman, J.J.; Fang, X.H.; Williams, S.J.; Baynosa, R.C. Enhancement of viable adipose-derived stem cells in lipoaspirate by buffering tumescent with sodium bicarbonate. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2138. [Google Scholar]
- Aronowitz, J.A.; Lockhart, R.A.; Cloe, S. Hakakian. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 2015, 4, 713. [Google Scholar] [PubMed] [Green Version]
- Rocha dos Santos, C.; da Rocha Filgueiras, R.; Furtado Malard, P.; Rodrigues da Cunha Barreto-Vianna, A.; Nogueira, K.; da Silva Leite, C.; Maurício Mendes de Lima, E. Mesenchymal stem cells in osteotomy repair after tibial tuberosity advancement in dogs with cranial cruciate ligament injury. J. Exp. Orthop. 2018, 5, 17. [Google Scholar] [PubMed] [Green Version]
- Franco, G.G.; Minto, B.W.; Dreibi, R.M.; Costa Junior, J.S.; Dias, L.G.G.G. Percutaneous application of allogeneic adipose-derived mesenchymal stem cell in dogs submitted to minimally invasive plate osteosynthesis of the tibia. Acta Cirúrgica Bras. 2021, 36, e360206. [Google Scholar]
- Hoffer, M.J.; Griffon, D.J.; Schaeffer, D.J.; Johnson, A.L.; Thomas, M.W. Clinical applications of demineralized bone matrix: A retrospective and case-matched study of seventy-five dogs. Vet. Surg. 2008, 37, 639–647. [Google Scholar]





| 12-Point Scoring System | |
|---|---|
| Cortical continuity (0–4) | 0 = no cortical continuity |
| 1 = one continuous cortex | |
| 2 = two continuous cortices | |
| 3 = three continuous cortices | |
| 4 = four continuous cortices | |
| Osteotomy line visibility (0–2) | 0 = osteotomy line visibility |
| 1 = osteotomy line barely visible | |
| 2 = no osteotomy line visible | |
| Subjective degree of callus formation or remodelling (0–4) | 0 = none |
| 1 = minimal | |
| 2 = moderate | |
| 3 = remodelled | |
| 4 = healed | |
| Degree of rounding of the distal step at the osteotomy site (0–2) | 0 = none |
| 1 = mild | |
| 2 = significant |
| 5-Point Scoring System | |
|---|---|
| 0 | No healing |
| 1 | 1–25% healing |
| 2 | 26–50% healing |
| 3 | 51–75% healing |
| 4 | 76–100% healing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennasilico, L.; Di Bella, C.; Sassaroli, S.; Salvaggio, A.; Roggiolani, F.; Piccionello, A.P. Effects of Autologous Microfragmented Adipose Tissue on Healing of Tibial Plateau Levelling Osteotomies in Dogs: A Prospective Clinical Trial. Animals 2023, 13, 2084. https://doi.org/10.3390/ani13132084
Pennasilico L, Di Bella C, Sassaroli S, Salvaggio A, Roggiolani F, Piccionello AP. Effects of Autologous Microfragmented Adipose Tissue on Healing of Tibial Plateau Levelling Osteotomies in Dogs: A Prospective Clinical Trial. Animals. 2023; 13(13):2084. https://doi.org/10.3390/ani13132084
Chicago/Turabian StylePennasilico, Luca, Caterina Di Bella, Sara Sassaroli, Alberto Salvaggio, Francesco Roggiolani, and Angela Palumbo Piccionello. 2023. "Effects of Autologous Microfragmented Adipose Tissue on Healing of Tibial Plateau Levelling Osteotomies in Dogs: A Prospective Clinical Trial" Animals 13, no. 13: 2084. https://doi.org/10.3390/ani13132084
APA StylePennasilico, L., Di Bella, C., Sassaroli, S., Salvaggio, A., Roggiolani, F., & Piccionello, A. P. (2023). Effects of Autologous Microfragmented Adipose Tissue on Healing of Tibial Plateau Levelling Osteotomies in Dogs: A Prospective Clinical Trial. Animals, 13(13), 2084. https://doi.org/10.3390/ani13132084










