Anatomical, Histological and Histochemical Observations of the Eyelids and Orbital Glands in the Lowland Tapir (Tapirus terrestris Linnaeus, 1785) (Perissodactyla: Ceratomorpha)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Macroscopic Morphology of the Eyelids and Orbital Glands
3.2. Histological and Histochemical Analysis of the Eyelids and Orbital Glands
4. Discussion
5. Conclusions
- -
- presence of eyelashes in the anterior palpebral margin of both eyelids characterized by tapirs and Rhinocerotidae;
- -
- presence of simple alveolar glands that produced a mucous secretion, characterized by Tapirus terrestris;
- -
- presence of poor developed tarsal glands in the both eyelids;
- -
- presence of the upper eyelid palpebral part of the lacrimal gland in only Tapirus terrestris;
- -
- in the histological image in the conjunctival part of the third eyelid, presence of characteristic conjunctival folds with numerous diffuse lymphocytes, while in equine there are macroscopically visible conjunctival crypts;
- -
- the presence of structures of the CALT organized form of lymphoid follicles, diffuse lymphocytes and high endothelial venules only in lower eyelids;
- -
- the upper and lower branches of the third eyelid appeared in the shape of a curved “caudal fin”, while in the equine they could be T-shaped or in hook form;
- -
- the third eyelid composed of hyaline cartilage in Tapirus terrestris and in horse elastic cartilage;
- -
- the presence of CALT in the conjunctival part of the third eyelid in the lowland tapir as well as in equine;
- -
- the superficial gland of the third eyelid in the lowland tapir is a branched complex alveolar gland that produces mucus, while in equine these glands have tubuloacinar structure and produce seromucus secretion;
- -
- presence of only a deep gland of the third eyelid in the Tapirus terrestris;
- -
- the lacrimal gland in the lowland tapir and equine is an acinar gland of mucoserous nature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hibert, F.; Sabatier, D.; Andrivot, J.; Scotti-Saintagne, C.; Gonzalez, S.; Pre´vost, M.-F.; Grenand, P.; Chave, J.; Caron, H.; Richard-Hansen, C. Botany, Genetics and Ethnobotany: A Crossed. Investigation on the Elusive Tapir’s Diet in French Guiana. PLoS ONE 2011, 6, e25850. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, J.L.P.; Fragoso, J.M.V.; Crawshaw, D.; Oliveira, L.F.B. Lowland tapir distribution and habitat loss in South America. PeerJ 2016, 4, e2456. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.S.; Tassy, P. The interrelation between Proboscidea, Sirenia, Hyracoidea, and Mesaxonia: The morphological evidence. In Mammal Phylogeny: Placentals; Szalay, F.S., Novacek, M.J., McKenna, M.C., Eds.; Springer: New York, NY, USA, 1993; pp. 217–234. [Google Scholar]
- Steiner, C.C.; Ryder, O.A. Molecular phylogeny and evolution of the Perissodactyla. Zool. J. Linnean. Soc. 2011, 163, 1289–1303. [Google Scholar] [CrossRef]
- Witmer, L.M.; Sampson, S.D.; Solounias, N. The proboscis of tapirs (Mammalia: Perissodactyla): A case study in novel narial anatomy. J. Zool. 1999, 249, 249–267. [Google Scholar] [CrossRef]
- García, M.J.; Medici, E.P.; Naranjo, E.J.; Novarino, W.; Leonardo, R.S. Distribution, habitat and adaptability of the genus Tapirus. Integr. Zool. 2012, 7, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Prothero, D.R.; Schoch, R.M. The Evolution of Perissodactyls; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Radinsky, L.B. Evolution of the tapiroid skeleton from Heptodon to Tapirus. Bull. Mus. Comp. Zool. 1965, 134, 69–106. [Google Scholar]
- Brooks, D.M.; Bodmer, R.E.; Matola, S. Tapirs: Status survey and conservation action plan. In IUCN/SSC Tapir Specialist Group IUCN; IUCN: Gland, Switzerland; Cambridge, UK, 1997; p. 164. [Google Scholar]
- Fragoso, J.M.V.; Huffman, J.M. Seed-dispersal and seedling recruitment patterns by the last Neotropical megafaunal element in Amazonia, the tapir. J. Trop. Ecol. 2000, 16, 369–385. [Google Scholar] [CrossRef]
- Nowak, R.M. Walker’s Mammals of the World, 5th ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 1991. [Google Scholar]
- Tobler, M.W. The ecology of the Lowland Tapir in Madre de Dios, Peru: Using new technologies to study large rainforest mammals. Ph.D. Thesis, Texas A/&M University, College Station, TX, USA, 2008. Volume 2. p. 132. [Google Scholar]
- Fradrich, H.; Thenius, E. Tapirs. In Animal Life Encyclopedia; Grzimek, B., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1972; Volume 13, p. 566. [Google Scholar]
- Husson, A.M. The mammals of Suriname. In Zoologische Monographieen, Rijksmuseum van Natuurlijke Historie; EJ Brill: Leiden, The Netherlands, 1978; Volume 2, pp. 1–569. [Google Scholar]
- Miller, F.W. Notes on some mammals of southern Mato Grosso, Brazil. J. Mammal. 1930, 11, 10–22. [Google Scholar] [CrossRef]
- Padilla, M.; Dowler, R.C. Tapirus terrestris. Mamm. Species 1994, 481, 1–8. [Google Scholar] [CrossRef]
- Padilla, M.; Dowler, R.C.; Dowler, C.G. Tapirus pinchaque (Perissodactyla: Tapiridae). Mamm. Species 2010, 42, 166–182. [Google Scholar] [CrossRef]
- Da Silva, M.-A.O.; Hermoza, C.; Rojas, G.; Freundt, J.M. Identifying an Effective Treatment for Corneal Ulceration in Captive Tapirs. Tapir Conserv. 2013, 22, 12–14. [Google Scholar]
- Gilger, B.C.; Matthews, A.G. Ophthalmology of Perissodactyla: Zebras, Tapirs, Rhinoceroses, and Relatives. In Wild and Exotic Animal Ophthalmology; Springer: Cham, Switzerland, 2022; Volume 2, pp. 145–154. [Google Scholar]
- Montiani-Ferreira, F. Ophthalmology. In Biology, Medicine and Surgery of South American Wild Animals; Fowler, M.E., Cubas, Z.S., Eds.; Iowa State University Press: Ames, IA, USA, 2001; pp. 437–456. [Google Scholar]
- Ramsay, E.C. Infectious diseases of the rhinoceros and tapir. In Zoo and Wild Animal Medicine; Fowler, M.E., Ed.; WB Saunders Co: Philadelphia, FL, USA, 1993; pp. 459–466. [Google Scholar]
- Karpinski, L.G.; Miller, C.L. Fluorouracil Treatment for Corneal Papilloma Fluorouracil as a treatment for corneal papilloma in a Malayan tapir. Vet. Ophthalmol. 2002, 5, 241–243. [Google Scholar] [CrossRef]
- Miller, C.L.; Templeton, R.; Karpinski, L.G. Successful treatment of oral squamous cell carcinoma with intralesional fluorouracil in a Malayan tapir (Tapirus indicus). J. Zoo Wildlife Med. 2000, 31, 262–264. [Google Scholar]
- Jorge, A.T.; de Oliveira, L.T.; Gomes Soares, R.; Magalhãesm, G.M.; Rocham, T.G.; dos Santos Honshm, C. Primary Corneal Hemangiosarcoma in a Tapir (Tapirus terrestris). Acta Sci. Vet. 2022, 50, 812. [Google Scholar]
- Barongi, R.A. Tapirs. AZA Minimum Husbandry Guidelines for Mammals. In Mammal Standards Task Force; American Zoo and Aquarium Association: Silver Spring, MD, USA, 1997. [Google Scholar]
- Abd-El-Tawab, M.; Abd-El-Aal, A.M.; Mekkaway, N.H. Bilateral entropion in male donkey (a clinical report). Egypt. J. Phytopathol. 1990, 17, 193–200. [Google Scholar]
- Barnett, K.C.; Crispin, S.M.; Lavach, J.D.; Matthew, A.G. Equine Ophthalmology, 2nd ed.; Saunders: London, UK, 2004. [Google Scholar]
- Brooks, D.E.; Wolf, E.D. Ocular trauma in the horse. Equine Vet. J. 1983, 15, 141–146. [Google Scholar] [CrossRef]
- Crispin, S.M. Tear-deficient and evaporative dry eye syndromes of the horse. Vet. Ophthalmol. 2000, 3, 87–92. [Google Scholar] [CrossRef]
- Esson, D.W.; Wellehan, J.F.X.; Lafortune, M.; Valverde, A.; Citino, S.B. Surgical management of a malacic corneal ulcer in a greater one-horned Asian rhinoceros (Rhinoceros unicornis) using a free island tarsoconjunctival graft. Vet. Ophthalmol. 2006, 9, 65–69. [Google Scholar] [CrossRef]
- Gandolf, A.R.; Willis, A.M.; Blumer, E.S.; Atkinson, M.W. Melting corneal ulcer management in a greater one-horned rhinoceros (Rhinoceros unicornis). J. Zoo Wildl. Med. 2000, 31, 112–117. [Google Scholar]
- Gearhart, P.M.; Steficek, B.A.; Peteresen-Jones, S.M. Hemangiosarcoma and squamous cell carcinoma in the third eyelid of a horse. Vet. Ophthalmol. 2007, 10, 121–126. [Google Scholar] [CrossRef]
- Gionfriddo, J.R.; Severin, G.A.; Schou, E.; Woodard, S. Tattooing of the Equine Eyelid: A retrospective Study. J. Equine Vet. Sci. 2009, 29, 82–86. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Plummer, C.E.; Brooks, D.E.; Porter, M.; Farina, L.L.; Winter, M.D. Unilateral orbital lacrimal gland abscess in a horse. Vet. Ophthalmol. 2011, 14, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Hewes, C.A.; Keoughan, G.C.; Gutierrez–Nibeyro, S. Standing enucleation in the horse: A report of 5 cases. Can. Vet. J. 2007, 48, 512–514. [Google Scholar] [PubMed]
- Howland, H.C.; Rowland, M.; Murphy, C.J. Refractive state of the rhinoceros. Vis. Res. 1993, 33, 2649–2651. [Google Scholar] [CrossRef]
- Horowitz, I.H.; Dubielzig, R.R.; Botero-Anug, A.M.; Lucio-Forster, A.; Bowman, D.D.; Rosenzweig, A.B.; Frenkel, S.; Ofri, R. Conjunctival habronemiasis in a square-lipped rhinoceros (Ceratotherium simum). Vet. Ophthalmol. 2016, 19, 161–166. [Google Scholar] [CrossRef]
- Komáromy, A.M.; Andrew, S.E.; Brooks, D.E.; Detrisac, C.J.; Gelatt, K.N. Periocular sarcoid in a horse. Vet. Ophthalmol. 2004, 7, 141–146. [Google Scholar] [CrossRef]
- Kretzschmar, P.; Sipangkui, R.; Schaffer, N. Eye Disorders in Captive Sumatran Rhinoceros (Dicerorhinus sumatrensis harissoni) In Sabah, Malaysia. In Proceedings of the International Conference on Diseases of Zoo and Wild Animals, Beekse Bergen, The Netherlands, 20–24 May 2009; pp. 236–242. [Google Scholar]
- Murphy, C.J.; Lavoie, J.P.; Groff, J.; Hacker, D.; Pryor, P.; Bellhorn, R.W. Bilateral eyelid swelling attributable to lymphosarcoma in a horse. J. Am. Vet. Med. Assoc. 1989, 194, 939–942. [Google Scholar]
- Pigatto, J.A.T.; de Albuquer Lbuquerque, L.; Stieven Hünning, P.; da olina da Veiga, A.C.; de Almeida, R.; Nóbrega, F.; de Souza Leal, J.; de Souza Leal, J. Squamous cell carcinoma in the third eyelid of a horse. Acta Sci. Vet. 2011, 39, 952. [Google Scholar]
- Young, E. Lesions in the vicinity of the eye of the white rhinoceros, Diceros simus. Int. Zoo Yearb. 1965, 5, 194–195. [Google Scholar] [CrossRef]
- Adams, M.F.; Castro, J.R.; Morandi, F.; Reese, R.E.; Reed, R.B. The nasolacrimal duct of the mule: Anatomy and clinical considerations. Equine Vet. Educ. 2013, 25, 636–642. [Google Scholar] [CrossRef]
- Alsafy, M. Comparative Morphological Studies on the Lacrimal Apparatus of One Humped Camel, Goat and Donkey. J. Biol. Sci. 2010, 4, 49–53. [Google Scholar] [CrossRef]
- Ibrahim, A.; Ahmed, A.F. The Impact of Surgical Excision of the Orbital Lacrimal Gland on the Aqueous Tear Production and Ocular Surface Health in Donkeys (Equus asinus). J. Equine Vet. Sci. 2021, 97, 103344. [Google Scholar] [CrossRef] [PubMed]
- Pohlmeyer, K.; Wissdorf, H. Anatomical basis for the irrigation of the lacrimal ducts of donkeys, Equus africanus f. asinus Macedonian dwarf donkey. Dtsch. Tierarztl. Wochensch. 1975, 82, 314–316. [Google Scholar]
- Ollivier, F. The precorneal tear film in horses: Its importance and disorders. Vet. Clin N. Am. Equine Pract. 2004, 20, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Latimer, C.A.; Wyman, M.; Diesem, C.D.; Burt, J.K. Radiographic and gross anatomy of the nasolacrimal duct of the horse. Am. J. Vet. Res. 1984, 45, 451–458. [Google Scholar]
- Laus, F.; Paggi, E.; Faillace, V.; Marchegiani, A.; Spaziante, D.; Cerquetella, M.; Tesei, B. Ultrasonographic biometry and relationship with gender, age and weight in healthy donkeys. J. Anim. Vet. Adv. 2013, 12, 803–806. [Google Scholar]
- Laus, F.; Paggi, E.; Marchegiani, A.; Cerquetella, M.; Spaziante, D.; Faillace, V.; Tesei, B. Ultrasonographic biometry of the eyes of healthy adult donkeys. Vet. Rec. 2014, 174, 326. [Google Scholar] [CrossRef]
- Schlegel, T.; Brehm, H.; Amselgruber, W.M. The cartilage of the third eyelid: A comparative macroscopical and histological study in domestic animals. Ann. Anat. 2001, 183, 165–169. [Google Scholar] [CrossRef]
- Vallone, L.V.; Scott, E.S.; Dubielzig, R.R.; Irby, N.I.; Teixeira, L.B.C. The equine third eyelid contains a conjunctival pocket with lymphatic tissue. The 47th annual conference for the American College of Veterinary Ophthalmologists Monterey, CA. Vet. Ophthalmol. 2016, 19, 21–43. [Google Scholar]
- Vallone, L.E.; Scott, E.; Irby, N. The conjunctival crypt of the equine third eyelid. Equine Vet. Educ. 2019, 31, 491–495. [Google Scholar] [CrossRef]
- Bapodra, P.; Wolfe, B.A. Baseline assessment of ophthalmic parameters in the greater one-horned rhinoceros (Rhinoceros unicornis). J. Zoo Wildl. Med. 2014, 45, 859–865. [Google Scholar] [CrossRef]
- Cave, A.J.E.; Allbrook, D.B. The skin and nuchal eminence of the white rhinoceros. Proc. Zool. Soc. Lond. 1959, 132, 99–107. [Google Scholar] [CrossRef]
- Cave, J.E. Hairs and vibrissae in the Rhinocerotidae. J. Zool. Lond. 1969, 157, 247–257. [Google Scholar] [CrossRef]
- Cave, J.E.; Wingstrand, K.G. Palpebral vibrissae in the Sumatran rhinoceros (Didermocerus sumatrensis). J. Zool. Lond. 1972, 167, 351. [Google Scholar] [CrossRef]
- Coimbra, J.P.; Manger, P.R. Retinal ganglion cell topography and spatial resolving power in the white rhinoceros (Ceratotherium simum). J. Comp. Neurol. 2017, 525, 2484–2498. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, J.D.; Manger, R.P. Retinal ganglion cell density of the black rhinoceros Diceros bicornis: Calculating visual resolution. Vis. Neurosci. 2008, 25, 215–220. [Google Scholar] [CrossRef]
- Van den Bergh, H.K. A note on eyelashes in an African black rhinoceros, Diceros bicornis. J. Zool. Lond. 1970, 161, 191. [Google Scholar]
- Burck, N.C. Technika Histologiczna; PZWL: Warszawa, Poland, 1975. [Google Scholar]
- Movat, H.Z. Demonstration of all connective tissue elements in a single section. A.M.A. Arch. Pathol. 1955, 60, 289. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 6th ed.; Churchill Livingstone Elsevier: Oxford, UK, 2008; pp. 173–174. [Google Scholar]
- Carson, F. Histotechnology a Self-Instructional Text, 1st ed.; ASCP: Baltimore, MD, USA, 1990; pp. 126–127. [Google Scholar]
- Munakata, H.; Isemura, M.; Yosizawa, Z. An application of the high-iron diamine staining for detection of sulfated glycoproteins (glycopeptides) in electrophoresis on cellulose acetate membrane. Tohoku J. Exp. Med. 1985, 145, 251–257. [Google Scholar] [CrossRef]
- Sheehan, D.C.; Hrapchak, B.B. Theory and practice of Histotechnology, 2nd ed.; CV Mosby: St. Louis, MO, USA, 1980; Volume 52, pp. 164–167. [Google Scholar]
- Spicer, S.C.; Henson, J.G. Methods for localizing mucosubstances in epithelial and connective tissue. In Series on Methods and Achievements in Experimental Pathology; Bajusz, E., Jamin, F., Eds.; S Karger Press: Basal, Switzerland, 1967; Volume 2, pp. 78–112. [Google Scholar]
- International Committee on Veterinary Gross Anatomical Nomenclature. Nomina Anatomica Veterinaria, 6th ed.; Editorial Committee: Hanover, Germany, 2017. [Google Scholar]
- International Committee on Veterinary Histological Nomenclature (ICVHN). Nomina Histologica Veterinaria; World Association Veterinary Anatomists: Montevideo, Uruguay, 2017. [Google Scholar]
- Nickel, R.; Schummer, A.; Seiferle, E. Lehrbuch der Anatomie der Haustiere; Parey: Berlin/Heidelberg, Hamburg, Germany, 2004; Volume 1. [Google Scholar]
- Dyce, K.M.; Sack, W.O.; Wensing, C.J.G. Textbook of Veterinary Anatomy, 4th ed.; W.B. Saunders Company: Philadelphia, PA, USA, 2010. [Google Scholar]
- Colbert, M.W. The Facial Skeleton of The Early Oligocene Colodon (Perissodactyla, Tapiroidea). Palaeontol. Electron. 2005, 8, 1–27. [Google Scholar]
- Pocock, R.I. On the facial vibrissae of the Mammalia. Proc. Zool. Soc. Lond. 1914, 889–912. [Google Scholar] [CrossRef]
- Cooley, P.L. Normal equine ocular anatomy and eye examination. Vet. Clin. N. Am. 1992, 8, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Carastro, S.M. Equine ocular anatomy and ophthalmic examination. Vet. Clin. N. Am. Equine Pract. 2004, 20, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Spodnik, J.H. Mianownictwo Anatomiczne Polsko-Angielsko-Łacińskie; Edra Urban & Partner: Wrocław, Poland, 2017. [Google Scholar]
- Knop, N.; Knop, E. Conjunctiva-associated lymphoid tissue in the human eye. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1270–1279. [Google Scholar]
- Knop, E.; Knop, N. The role of eye-associated lymphoid tissue in corneal immune protection. J. Anat. 2005, 206, 271–285. [Google Scholar] [CrossRef]
- Wenzel-Hora, B.I.; Seifert, H.M.; Grüntzig, J. Animal experimental studies of indirect lymphography of the eye, face, and neck regions using Iotasul. Lymphology 1982, 15, 32–35. [Google Scholar]
- Mastropasqua, R.; Agnifili, L.; Fasanella, V.; Nubile, M.; Gnama, A.A.; Falconio, G.; Perri, P.; Di Staso, S.; Mariotti, C. The Conjunctiva-Associated Lymphoid Tissue in chronic ocular surface diseases. Mic. Microanaly 2017, 23, 697–707. [Google Scholar] [CrossRef]
- Brooks, D.E. Calcium degeneration and ocular surface failure in the horse. Equine Vet. Educat. 2012, 24, 8–11. [Google Scholar] [CrossRef]
- Rauz, S.; Saw, V.P. Serum eye drops, amniotic membrane and limbal epithelial stem cells-tools in the treatment of ocular surface disease. Cell Tissue Bank 2010, 11, 13–27. [Google Scholar] [CrossRef]
- Samuelson, D.A. Ophthalmic anatomy. In Veterinary Ophthalmology; Gelatt, K.N., Gilger, B.C., Kern, T.J., Eds.; Wiley-Blackwell: Ames, IA, USA, 2013; pp. 39–170. [Google Scholar]
- El-Naseery, N.I.; El-Behery, E.I.; El-Ghazali, H.M.; El-Hady, E. The structural characterization of the lacrimal gland in the adult dog (Canis familiaris). Benha Vet. Med. J. 2016, 31, 106–116. [Google Scholar] [CrossRef]
- Lantyer-Araujo, N.L.; Silva, D.N.; Estrela-Lima, A.; Muramoto, C.; Libo´rio, F.A.; SilvaE´, A.D.; Oriá, A.P. Anatomical, histological and computed tomography comparision of the eye and adnexa of crab-eating fox (Cerdocyon thous) to domestic dogs. PLoS ONE 2019, 14, e0224245. [Google Scholar] [CrossRef] [PubMed]
- Davidson, H.J.; Kuonen, V.J. The tear film and ocular mucins. Vet. Ophthalmol. 2004, 7, 71–77. [Google Scholar] [CrossRef]
- Abdelbaset-Ismail, A.; Aref, M.; Ezzeldein, S.; Eisa, E.; Gugjoo, M.B.; Abdelaal, A.; Emam, H.; Syaad, K.A.; Ahmed, A.E.; Alshati, A.; et al. Ultrasound, Dacryocystorhinography and Morphological Examination of Normal Eye and Lacrimal Apparatus of the Donkey (Equus asinus). Animals 2022, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Budras, K.; Sack, W.; Rock, S. Anatomy of the Horse an Illustrated Text, 3rd ed.; Verlag und Druckerei Hans-Böckler-Allee: Hannover, Germany, 2009. [Google Scholar]
- Diesem, C. Equine sense organs and common integument. Ruminant sense organs and common integument. Ruminant osteology. In Sisson and Grossman’s: The Anatomy of the Domestic Animals, 5th ed.; Getty, R., Ed.; W.B. Saunders Co.: Philadelphia, PA, USA, 1975. [Google Scholar]
- Schneider, M.; Gerhards, H. The examination of the equine lacrimal gland. Pferdeheilkunde 2011, 27, 31–38. [Google Scholar] [CrossRef]
- Orlandini, G.E.; Bacchi, A.B. Sulla ultrastruttura dclla ghiandola lacrimale negli Equini. Arch. Ital. Anal. Embriol. 1977, LXXXII, 1–13. [Google Scholar]

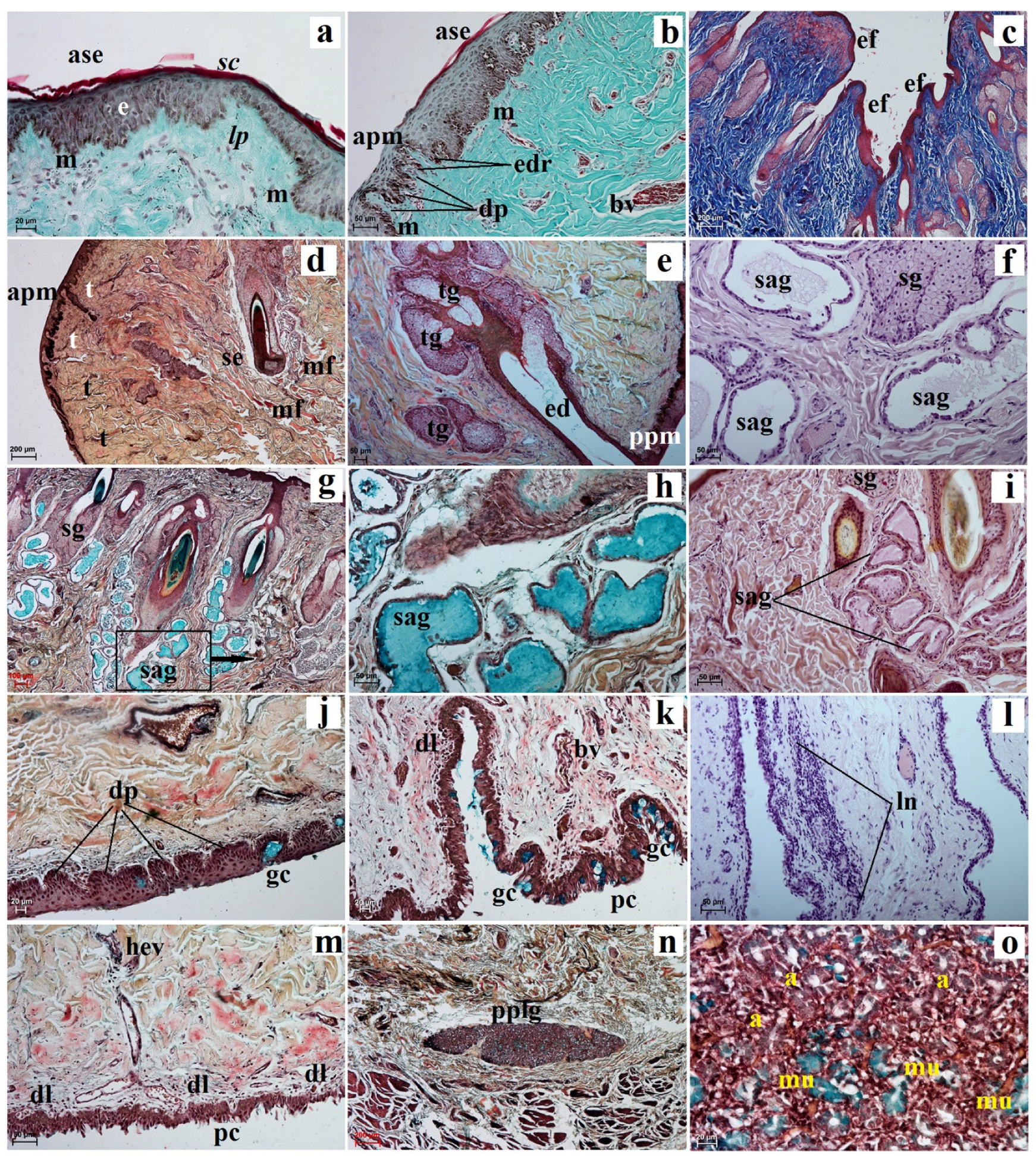
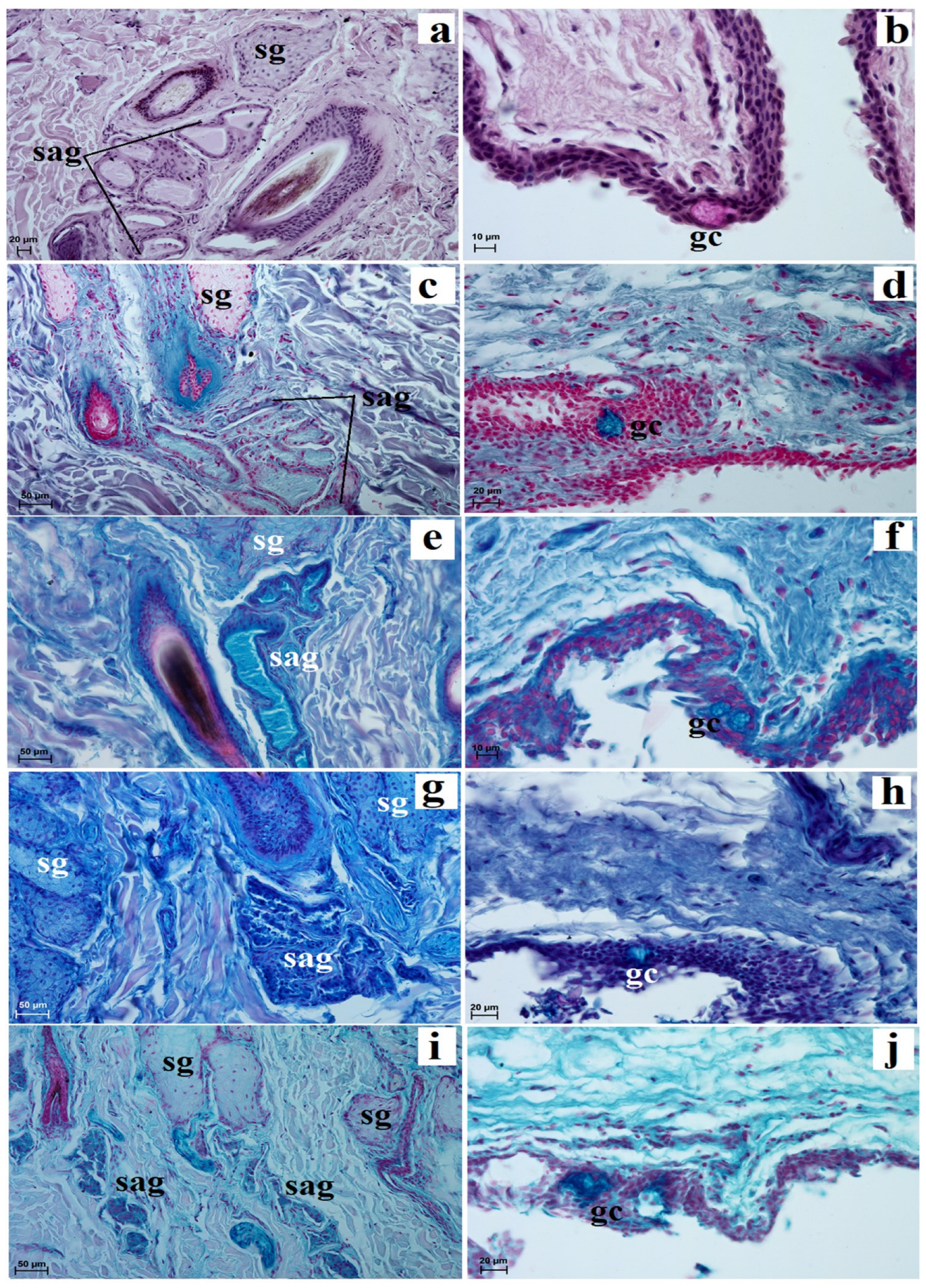
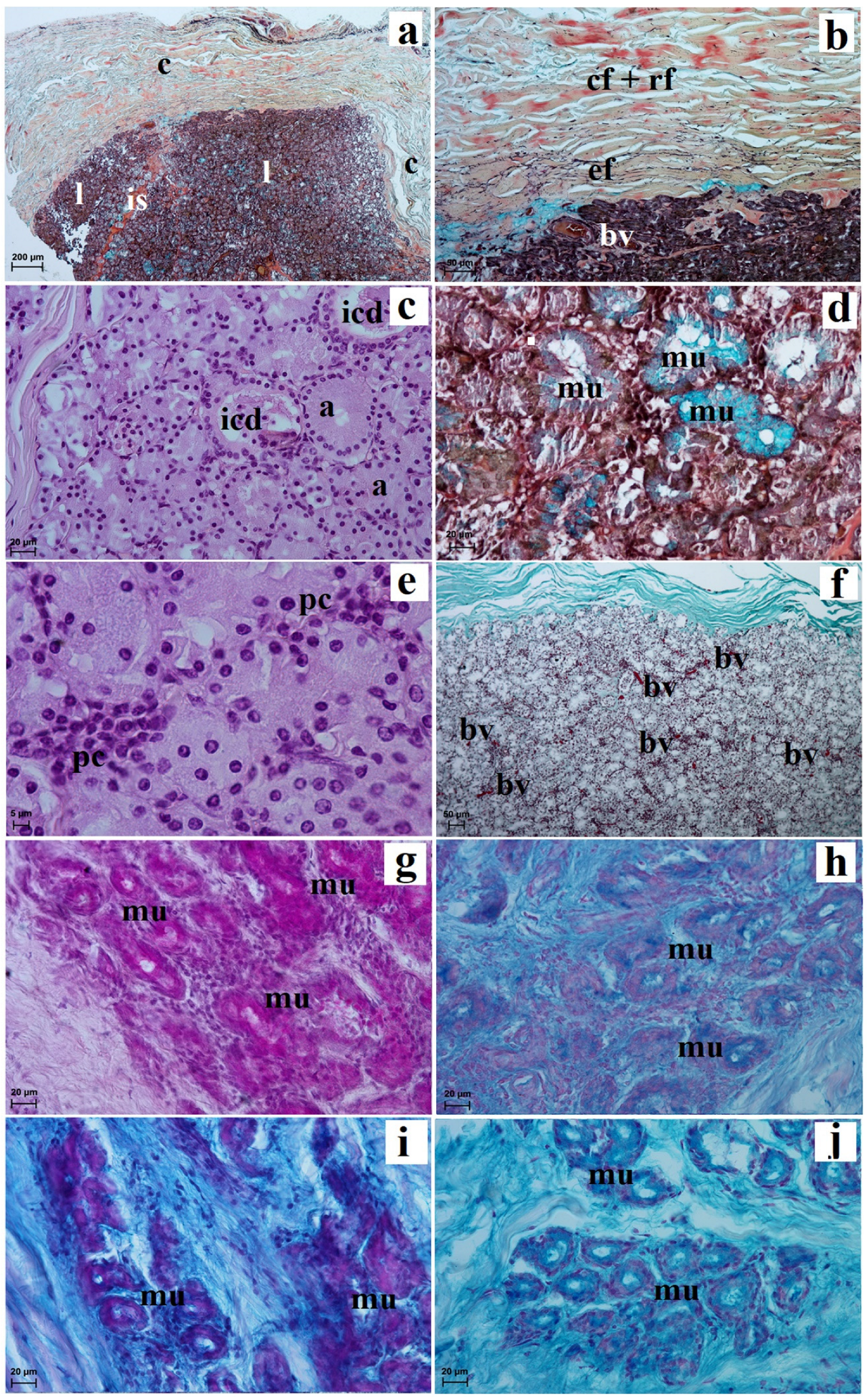
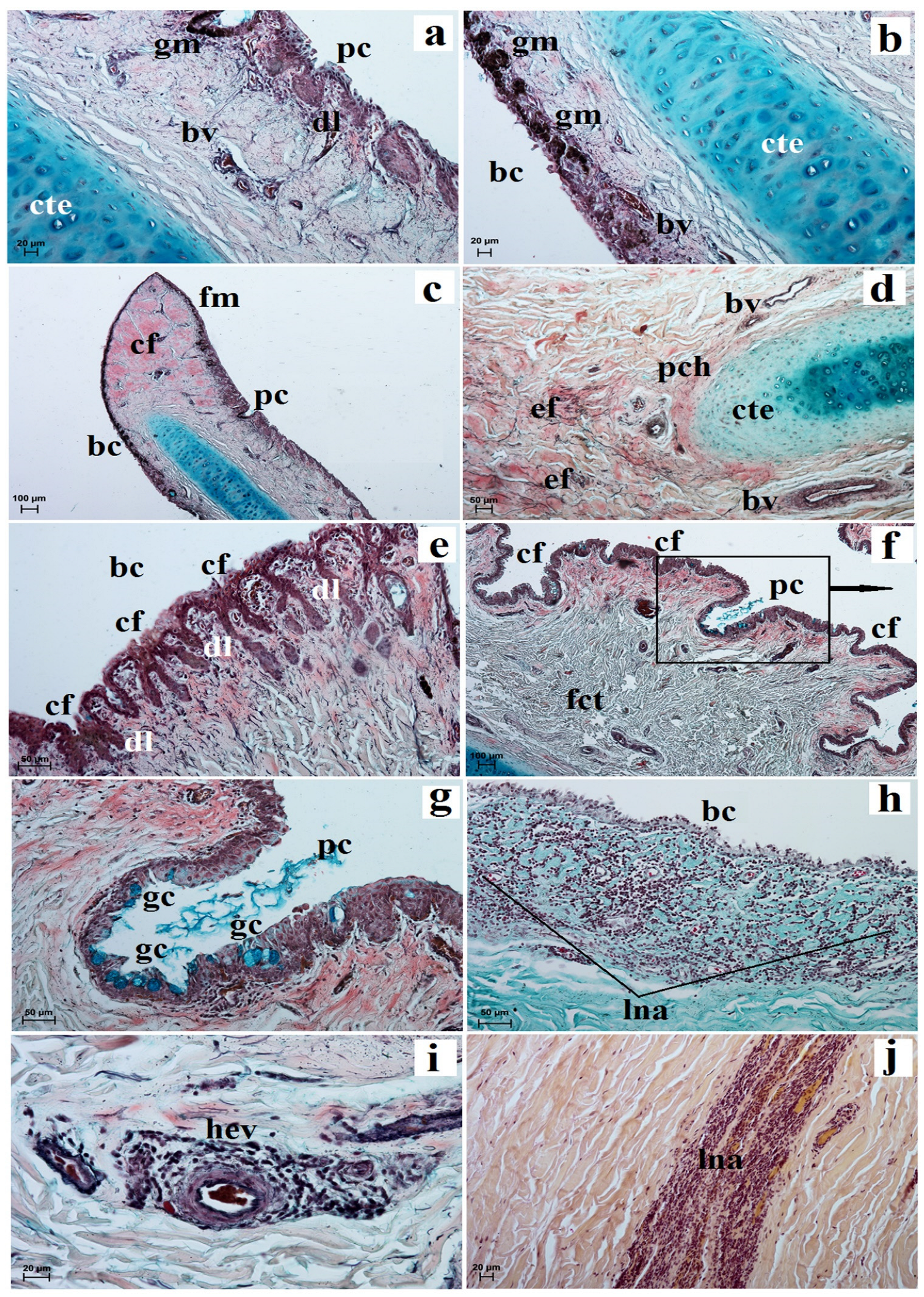
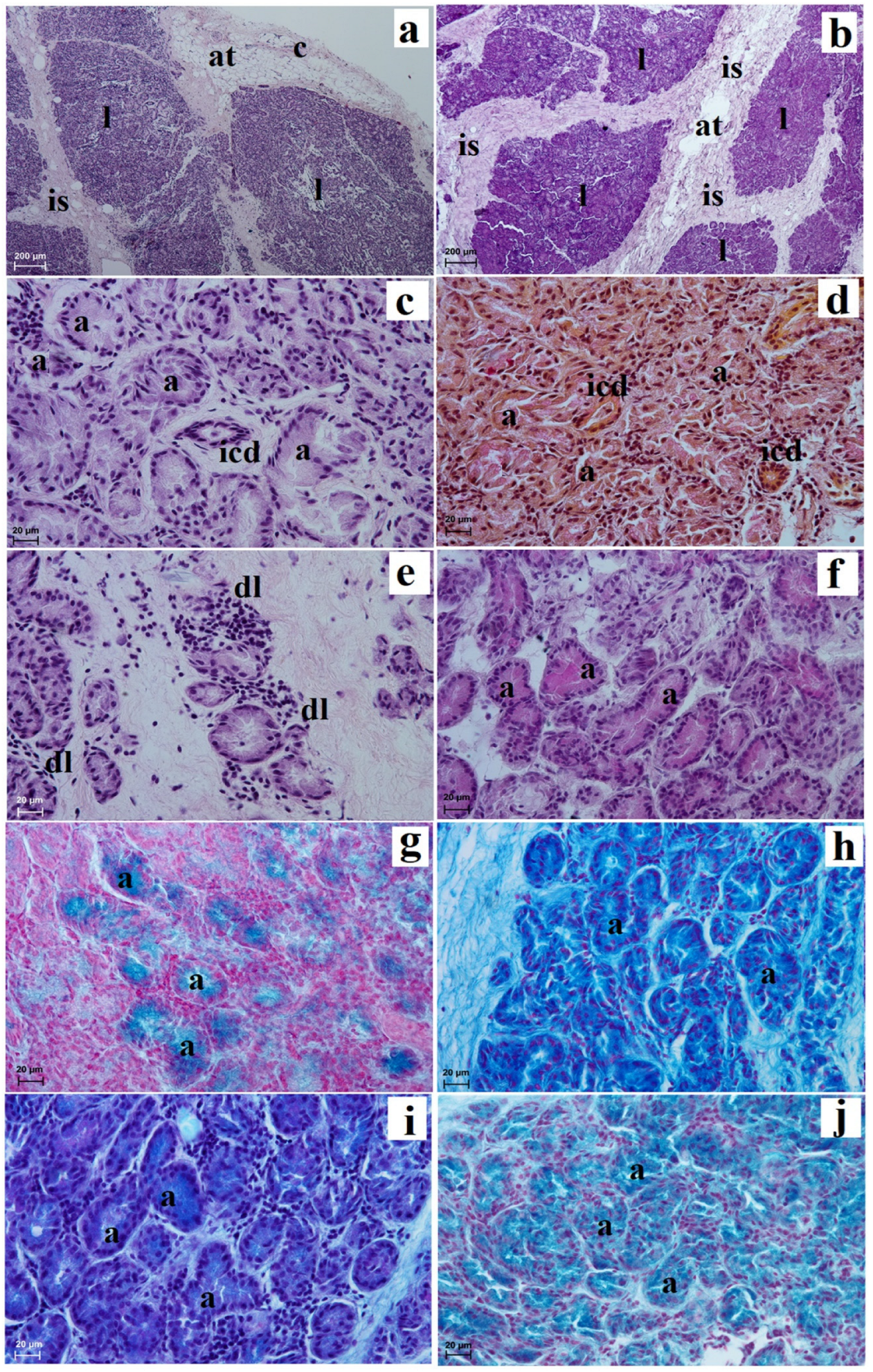
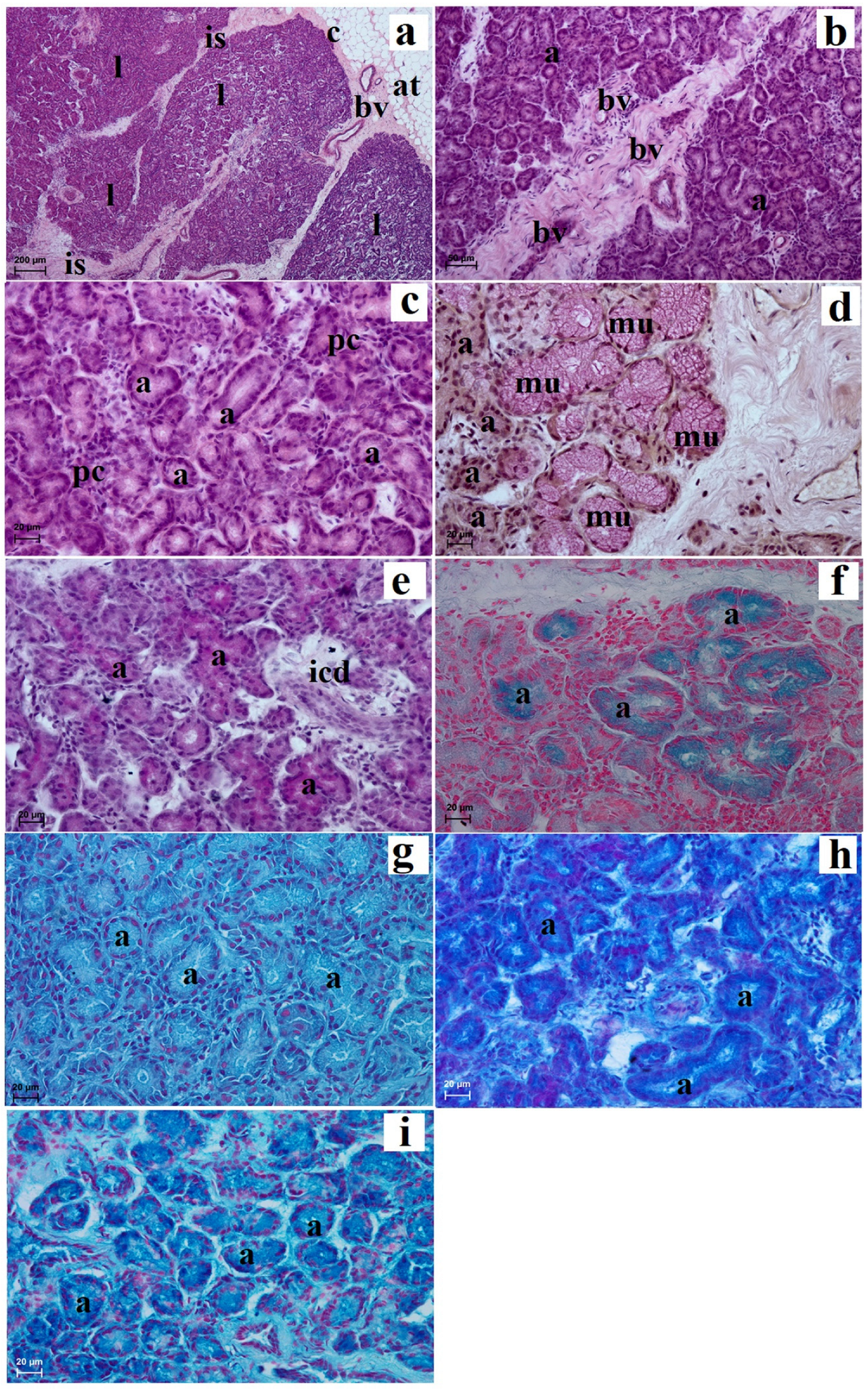
| Simple Alveolar Glands | Sebaceous Glands | Tarsal Glands | Goblet Cells in The Upper and Lower Eyelids | Superficial Gland of The Third Eyelid | Deep Gland of The Third Eyelid | Lacrimal Gland | |
|---|---|---|---|---|---|---|---|
| PAS | − | − | − | +++ | ++/+++ | −/+ | ++ |
| AB pH 1.0 | ++ | − | − | +++ | − | +++ | dominant + and sparse ++/+++ |
| AB pH 2.5 | +++ | − | − | +++ | ++/+++ | +++ | +++ |
| AB pH 2.5 PAS | +++ (blue color) | − | − | +++ (blue color) | ++ (dominant magenta color) | +++ (blue color) | dominant +++ (blue color) and sparse +++ (magenta color) |
| HDI | +++ | − | − | +++ | + | +++ | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klećkowska-Nawrot, J.; Goździewska-Harłajczuk, K.; Kupczyńska, M.; Kaleta-Kuratewicz, K.; Kuropka, P.; Barszcz, K. Anatomical, Histological and Histochemical Observations of the Eyelids and Orbital Glands in the Lowland Tapir (Tapirus terrestris Linnaeus, 1785) (Perissodactyla: Ceratomorpha). Animals 2023, 13, 2081. https://doi.org/10.3390/ani13132081
Klećkowska-Nawrot J, Goździewska-Harłajczuk K, Kupczyńska M, Kaleta-Kuratewicz K, Kuropka P, Barszcz K. Anatomical, Histological and Histochemical Observations of the Eyelids and Orbital Glands in the Lowland Tapir (Tapirus terrestris Linnaeus, 1785) (Perissodactyla: Ceratomorpha). Animals. 2023; 13(13):2081. https://doi.org/10.3390/ani13132081
Chicago/Turabian StyleKlećkowska-Nawrot, Joanna, Karolina Goździewska-Harłajczuk, Marta Kupczyńska, Katarzyna Kaleta-Kuratewicz, Piotr Kuropka, and Karolina Barszcz. 2023. "Anatomical, Histological and Histochemical Observations of the Eyelids and Orbital Glands in the Lowland Tapir (Tapirus terrestris Linnaeus, 1785) (Perissodactyla: Ceratomorpha)" Animals 13, no. 13: 2081. https://doi.org/10.3390/ani13132081
APA StyleKlećkowska-Nawrot, J., Goździewska-Harłajczuk, K., Kupczyńska, M., Kaleta-Kuratewicz, K., Kuropka, P., & Barszcz, K. (2023). Anatomical, Histological and Histochemical Observations of the Eyelids and Orbital Glands in the Lowland Tapir (Tapirus terrestris Linnaeus, 1785) (Perissodactyla: Ceratomorpha). Animals, 13(13), 2081. https://doi.org/10.3390/ani13132081







