Effects of Sodium Butyrate Supplementation in Milk on the Growth Performance and Intestinal Microbiota of Preweaning Holstein Calves
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Procedure
2.2. Feed Intake, Growth-Related Indices, and Sample Collection for GIM
2.3. DNA Extraction, PCR Amplification, and 16S rRNA Sequencing
2.4. Microbial Data Processing
2.5. Statistical Analysis
3. Results
3.1. Growth-Related Indices
3.2. Diversity of the Intestinal Microbiota
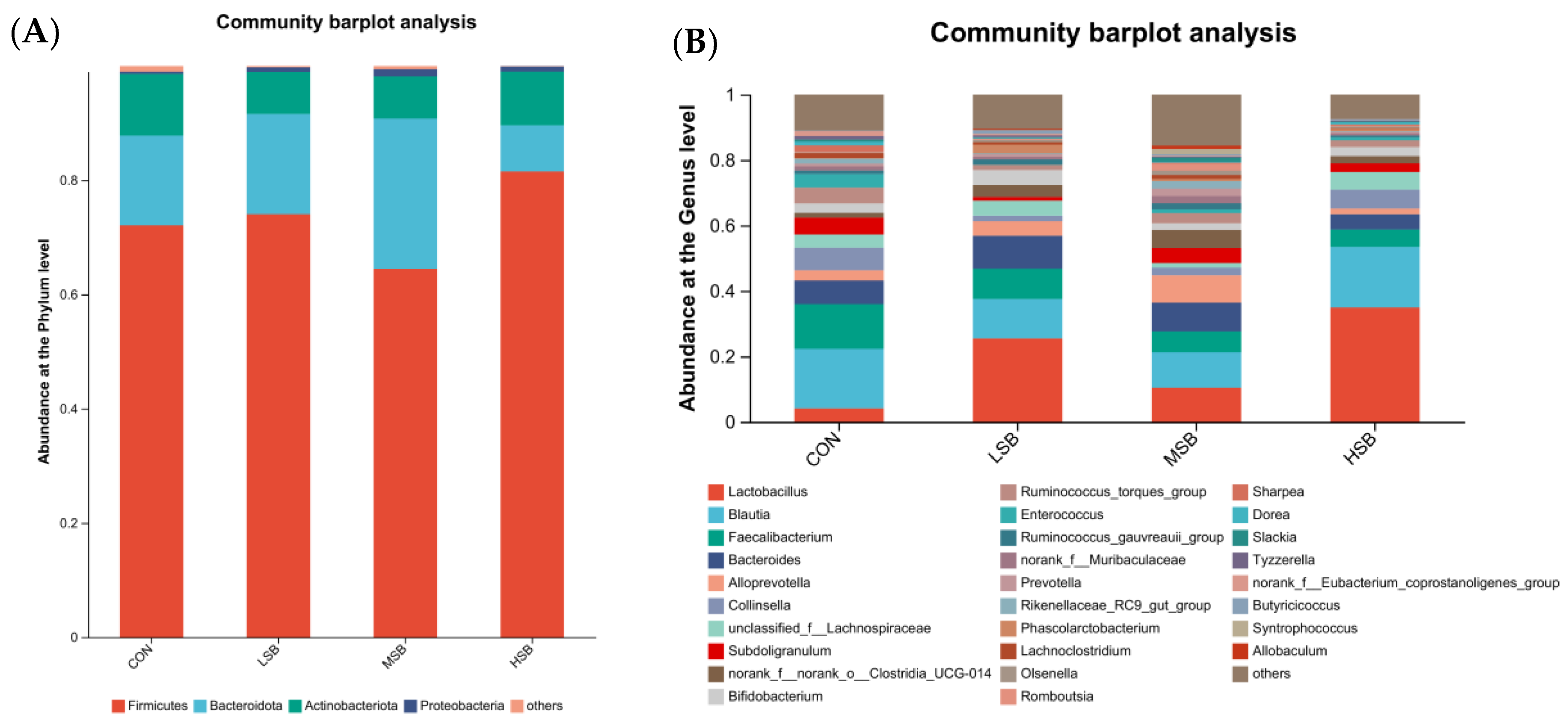
3.3. Composition of the Intestinal Microbiota
3.4. Function of the Intestinal Microbiota
3.5. Correlation of Growth-Related Indices and the Intestinal Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jorgensen, M.W.; Adams-Progar, A.; de Passillé, A.M.; Rushen, J.; Salfer, J.A.; Endres, M.I. Mortality and health treatment rates of dairy calves in automated milk feeding systems in the Upper Midwest of the United States. J. Dairy Sci. 2017, 100, 9186–9193. [Google Scholar] [CrossRef]
- Meale, S.J.; Chaucheyras-Durand, F.; Berends, H.; Guan, L.L.; Steele, M.A. From pre- to postweaning: Transformation of the young calf’s gastrointestinal tract. J. Dairy Sci. 2017, 100, 5984–5995. [Google Scholar] [CrossRef]
- Van De Stroet, D.L.; Calderón Díaz, J.A.; Stalder, K.J.; Heinrichs, A.J.; Dechow, C.D. Association of calf growth traits with production characteristics in dairy cattle. J. Dairy Sci. 2016, 99, 8347–8355. [Google Scholar] [CrossRef]
- Crannell, P.; Abuelo, A. Comparison of calf morbidity, mortality, and future performance across categories of passive immunity: A retrospective cohort study in a dairy herd. J. Dairy Sci. 2023, 106, 2729–2738. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Ye, Y.; Wang, F.; Chen, F.; Zheng, C. The Role of Microbiota in Infant Health: From Early Life to Adulthood. Front. Immunol. 2021, 12, 708472. [Google Scholar] [CrossRef]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef]
- Verbeke, K.A.; Boobis, A.R.; Chiodini, A.; Edwards, C.A.; Franck, A.; Kleerebezem, M.; Nauta, A.; Raes, J.; van Tol, E.A.; Tuohy, K.M. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr. Res. Rev. 2015, 28, 42–66. [Google Scholar] [CrossRef]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The Human Microbiome and Child Growth—First 1000 Days and Beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef]
- Górka, P.; Kowalski, Z.M.; Zabielski, R.; Guilloteau, P. Invited review: Use of butyrate to promote gastrointestinal tract development in calves. J. Dairy Sci. 2018, 101, 4785–4800. [Google Scholar] [CrossRef]
- Górka, P.; Kowalski, Z.M.; Pietrzak, P.; Kotunia, A.; Jagusiak, W.; Holst, J.J.; Guilloteau, P.; Zabielski, R. Effect of method of delivery of sodium butyrate on rumen development in newborn calves. J. Dairy Sci. 2011, 94, 5578–5588. [Google Scholar] [CrossRef]
- Górka, P.; Pietrzak, P.; Kotunia, A.; Zabielski, R.; Kowalski, Z.M. Effect of method of delivery of sodium butyrate on maturation of the small intestine in newborn calves. J. Dairy Sci. 2014, 97, 1026–1035. [Google Scholar] [CrossRef]
- Wu, D.L.; Meng, Q.S.; Wang, Y.D.; Wang, M.Y.; Xu, E.H.; Xiao, L.; Xu, M. Dietary supplementation of free or two fat-coated sodium butyrate with varying release times on gastrointestinal development and tight junctions in preweaning Holstein calves. Anim. Feed. Sci. Technol. 2022, 285, 115224. [Google Scholar] [CrossRef]
- O’Hara, E.; Kelly, A.; McCabe, M.S.; Kenny, D.A.; Guan, L.L.; Waters, S.M. Effect of a butyrate-fortified milk replacer on gastrointestinal microbiota and products of fermentation in artificially reared dairy calves at weaning. Sci. Rep. 2018, 8, 14901. [Google Scholar] [CrossRef]
- Liu, W.; La, A.T.Z.; Evans, A.; Gao, S.; Yu, Z.; Bu, D.; Ma, L. Supplementation with sodium butyrate improves growth and antioxidant function in dairy calves before weaning. J. Anim. Sci. Biotechnol. 2021, 12, 2. [Google Scholar] [CrossRef]
- Liu, S.; Ma, J.Y.; Zhou, J.; Wu, J.D.; Li, J.H.; Alugongo, G.M.; Xiao, J.X.; Wang, J.J.; Wang, Y.J.; Wang, W.; et al. Tributyrin supplementation in pasteurized waste milk: Effects on growth performance, health, and blood parameters of dairy calves. J. Dairy Sci. 2021, 104, 12496–12507. [Google Scholar] [CrossRef]
- Liu, S.; Wu, J.; Wu, Z.; Alugongo, G.M.; Zahoor Khan, M.; Li, J.; Xiao, J.; He, Z.; Ma, Y.; Li, S.; et al. Tributyrin administration improves intestinal development and health in pre-weaned dairy calves fed milk replacer. Anim. Nutr. 2022, 10, 399–411. [Google Scholar] [CrossRef]
- Drackley, J.K. Calf nutrition from birth to breeding. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 55–86. [Google Scholar] [CrossRef]
- Grosskopf, J.F. Studies on salivary lipase in young ruminants. Onderstepoort J. Vet. Res. 1965, 32, 153–180. [Google Scholar]
- Zhang, R.; Zhang, W.B.; Bi, Y.L.; Tu, Y.; Beckers, Y.; Du, H.C.; Diao, Q.Y. Early Feeding Regime of Waste Milk, Milk, and Milk Replacer for Calves Has Different Effects on Rumen Fermentation and the Bacterial Community. Animals 2019, 9, 443. [Google Scholar] [CrossRef]
- Wang, S.; Hu, F.; Diao, Q.; Li, S.; Tu, Y.; Bi, Y. Comparison of Growth Performance, Immunity, Antioxidant Capacity, and Liver Transcriptome of Calves between Whole Milk and Plant Protein-Based Milk Replacer under the Same Energy and Protein Levels. Antioxidants 2022, 11, 270. [Google Scholar] [CrossRef]
- Arshad, M.A.; Hassan, F.U.; Rehman, M.S.; Huws, S.A.; Cheng, Y.; Din, A.U. Gut microbiome colonization and development in neonatal ruminants: Strategies, prospects, and opportunities. Anim. Nutr. 2021, 7, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.T.; AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Artington, VA, USA, 2015. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Jones, C.; Guo, Q.; Lewis, L.; Stark, C.R.; Alavi, S. An evaluation of total starch and starch gelatinization methodologies in pelleted animal feed. J. Anim. Sci. 2016, 94, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Prentice, P.M.; Schoemaker, M.H.; Vervoort, J.; Hettinga, K.; Lambers, T.T.; van Tol, E.A.F.; Acerini, C.L.; Olga, L.; Petry, C.J.; Hughes, I.A.; et al. Human Milk Short-Chain Fatty Acid Composition is Associated with Adiposity Outcomes in Infants. J. Nutr. 2019, 149, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Perdijk, O.; van Baarlen, P.; Fernandez-Gutierrez, M.M.; van den Brink, E.; Schuren, F.H.J.; Brugman, S.; Savelkoul, H.F.J.; Kleerebezem, M.; van Neerven, R.J.J. Sialyllactose and Galactooligosaccharides Promote Epithelial Barrier Functioning and Distinctly Modulate Microbiota Composition and Short Chain Fatty Acid Production In Vitro. Front. Immunol. 2019, 10, 94. [Google Scholar] [CrossRef]
- Free, A.; McDonald, M.A.; Pagaling, E. Diversity-Function Relationships in Natural, Applied, and Engineered Microbial Ecosystems. Adv. Appl. Microbiol. 2018, 105, 131–189. [Google Scholar] [CrossRef]
- Ford, S.L.; Lohmann, P.; Preidis, G.A.; Gordon, P.S.; O’Donnell, A.; Hagan, J.; Venkatachalam, A.; Balderas, M.; Luna, R.A.; Hair, A.B. Improved feeding tolerance and growth are linked to increased gut microbial community diversity in very-low-birth-weight infants fed mother’s own milk compared with donor breast milk. Am. J. Clin. Nutr. 2019, 109, 1088–1097. [Google Scholar] [CrossRef]
- Gibiino, G.; Lopetuso, L.R.; Scaldaferri, F.; Rizzatti, G.; Binda, C.; Gasbarrini, A. Exploring Bacteroidetes: Metabolic key points and immunological tricks of our gut commensals. Dig. Liver Dis. 2018, 50, 635–639. [Google Scholar] [CrossRef]
- Duerkop, B.A.; Vaishnava, S.; Hooper, L.V. Immune responses to the microbiota at the intestinal mucosal surface. Immunity 2009, 31, 368–376. [Google Scholar] [CrossRef]
- Hooper, L.V.; Midtvedt, T.; Gordon, J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 2002, 22, 283–307. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Wei, G.Y.; Zhang, R.P.; Zhu, Y.; Wang, Z.; Wang, S.M.; Du, G.H. Cryptotanshinone alleviates chemotherapy-induced colitis in mice with colon cancer via regulating fecal-bacteria-related lipid metabolism. Pharmacol. Res. 2021, 163, 105232. [Google Scholar] [CrossRef]
- Sun, Z.; Yu, Z.; Wang, B. Perilla frutescens Leaf Alters the Rumen Microbial Community of Lactating Dairy Cows. Microorganisms 2019, 7, 562. [Google Scholar] [CrossRef]
- Ormerod, K.L.; Wood, D.L.; Lachner, N.; Gellatly, S.L.; Daly, J.N.; Parsons, J.D.; Dal’Molin, C.G.; Palfreyman, R.W.; Nielsen, L.K.; Cooper, M.A.; et al. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 2016, 4, 36. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef]
- Zened, A.; Combes, S.; Cauquil, L.; Mariette, J.; Klopp, C.; Bouchez, O.; Troegeler-Meynadier, A.; Enjalbert, F. Microbial ecology of the rumen evaluated by 454 GS FLX pyrosequencing is affected by starch and oil supplementation of diets. FEMS Microbiol. Ecol. 2013, 83, 504–514. [Google Scholar] [CrossRef]
- Daghio, M.; Ciucci, F.; Buccioni, A.; Cappucci, A.; Casarosa, L.; Serra, A.; Conte, G.; Viti, C.; McAmmond, B.M.; Van Hamme, J.D.; et al. Correlation of Breed, Growth Performance, and Rumen Microbiota in Two Rustic Cattle Breeds Reared Under Different Conditions. Front. Microbiol. 2021, 12, 652031. [Google Scholar] [CrossRef]
- Holman, D.B.; Gzyl, K.E. A meta-analysis of the bovine gastrointestinal tract microbiota. FEMS Microbiol. Ecol. 2019, 95, fiz072. [Google Scholar] [CrossRef]
- Dai, Q.; Ma, J.; Cao, G.; Hu, R.; Zhu, Y.; Li, G.; Zou, H.; Wang, Z.; Peng, Q.; Xue, B.; et al. Comparative study of growth performance, nutrient digestibility, and ruminal and fecal bacterial community between yaks and cattle-yaks raised by stall-feeding. AMB Express 2021, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Hu, J.; Wu, X.; Pan, J.A.; Jiao, N.; Li, Y.; Huang, Y.; Lin, X.; Zou, Y.; Chen, Y.; et al. Altered gut microbiome in FUT2 loss-of-function mutants in support of personalized medicine for inflammatory bowel diseases. J. Genet. Genom. Yi Chuan Xue Bao 2021, 48, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jing, H.; Wang, J.; Zhang, S.; Chang, Q.; Li, Z.; Wu, X.; Zhang, Z. Disordered Gut Microbiota Correlates With Altered Fecal Bile Acid Metabolism and Post-cholecystectomy Diarrhea. Front. Microbiol. 2022, 13, 800604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Elolimy, A.A.; Akbar, H.; Thanh, L.P.; Yang, Z.; Loor, J.J. Association of residual feed intake with peripartal ruminal microbiome and milk fatty acid composition during early lactation in Holstein dairy cows. J. Dairy Sci. 2022, 105, 4971–4986. [Google Scholar] [CrossRef] [PubMed]
- Freier, T.A.; Beitz, D.C.; Li, L.; Hartman, P.A. Characterization of Eubacterium coprostanoligenes sp. nov., a cholesterol-reducing anaerobe. Int. J. Syst. Bacteriol. 1994, 44, 137–142. [Google Scholar] [CrossRef]
- Chen, Y.T.; Hsu, A.H.; Chiou, S.Y.; Lin, Y.C.; Lin, J.S. AB-Kefir Reduced Body Weight and Ameliorated Inflammation in Adipose Tissue of Obese Mice Fed a High-Fat Diet, but Not a High-Sucrose Diet. Nutrients 2021, 13, 2182. [Google Scholar] [CrossRef]
- Dai, S.J.; Zhang, K.Y.; Ding, X.M.; Bai, S.P.; Luo, Y.H.; Wang, J.P.; Zeng, Q.F. Effect of Dietary Non-phytate Phosphorus Levels on the Diversity and Structure of Cecal Microbiota in Meat Duck from 1 to 21 d of age. Poult. Sci. 2018, 97, 2441–2450. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Yu, C.; Liu, S.; Chen, L.; Shen, J.; Niu, Y.; Wang, T.; Zhang, W.; Fu, L. Effect of exercise and butyrate supplementation on microbiota composition and lipid metabolism. J. Endocrinol. 2019, 243, 125–135. [Google Scholar] [CrossRef]
- Xu, Y.; Peng, S.; Cao, X.; Qian, S.; Shen, S.; Luo, J.; Zhang, X.; Sun, H.; Shen, W.L.; Jia, W.; et al. High doses of butyrate induce a reversible body temperature drop through transient proton leak in mitochondria of brain neurons. Life Sci. 2021, 278, 119614. [Google Scholar] [CrossRef]
- Mallo, J.J.; Balfagón, A.; Gracia, M.I.; Honrubia, P.; Puyalto, M. Evaluation of different protections of butyric acid aiming for release in the last part of the gastrointestinal tract of piglets. J. Anim. Sci. 2012, 90 (Suppl. 4), 227–229. [Google Scholar] [CrossRef]



| Items | Supplementation Level, g/d | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 4.4 | 8.8 | 17.6 | ANOVA | Linear | Quadratic | ||
| Initial BW, kg | 39.83 | 39.70 | 39.81 | 39.84 | 0.55 | 0.99 | 0.93 | 0.91 |
| Final BW, kg | 72.02 | 73.75 | 75.00 | 72.72 | 0.75 | 0.096 | 0.013 | 0.014 |
| DMI, g/d | 142.55 ab | 121.34 bc | 147.64 a | 115.03 c | 8.66 | 0.020 | 0.73 | 0.39 |
| ADG, g/d | ||||||||
| Week 1 | 554.33 b | 619.1 a | 673.54 a | 654.63 a | 15.83 | 0.002 | 0.001 | 0.011 |
| Week 2 | 655.84 b | 709.09 a | 727.45 a | 710.03 a | 13.16 | 0.039 | 0.008 | 0.025 |
| Week 3 | 910.18 | 941.98 | 945.86 | 888.57 | 14.67 | 0.17 | 0.099 | 0.045 |
| Week 4 | 890.6 | 907.98 | 915.00 | 847.31 | 14.47 | 0.079 | 0.15 | 0.044 |
| Week 5 | 927.82 | 950.42 | 946.83 | 889.08 | 13.23 | 0.067 | 0.16 | 0.045 |
| Week 6 | 931.93 | 935.71 | 932.54 | 915.79 | 9.01 | 0.62 | 0.73 | 0.49 |
| In the whole period | 775.03 b | 808.09 ab | 836.97 a | 770.37 b | 13.93 | 0.037 | 0.009 | 0.005 |
| FE | 0.162 | 0.138 | 0.150 | 0.135 | 0.009 | 0.12 | 0.31 | 0.60 |
| Gain of height at the withers, cm | 10.25 | 10.44 | 10.47 | 9.64 | 0.58 | 0.43 | 0.42 | 0.23 |
| Gain of body length, cm | 13.15 | 13.74 | 13.35 | 13.29 | 0.39 | 0.76 | 0.53 | 0.51 |
| Gain of hearth girth, cm | 18.72 | 18.87 | 19.56 | 18.50 | 0.36 | 0.20 | 0.093 | 0.064 |
| Items | Supplementation Level, g/d | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 4.4 | 8.8 | 17.6 | ANOVA | Linear | Quadratic | ||
| Phylum level | ||||||||
| Bacteroidota | 15.79 | 17.95 | 26.85 | 8.16 | 5.09 | 0.095 | 0.084 | 0.038 |
| Genus level | ||||||||
| Lactobacillus | 4.11 b | 25.16 a | 10.03 b | 34.95 a | 3.98 | <0.001 | 0.47 | 0.70 |
| norank_f__Muribaculaceae | 1.284 ab | 0.996 b | 2.563 a | 0.667 b | 0.481 | 0.045 | 0.095 | 0.059 |
| Rikenellaceae_RC9_gut_group | 1.474 ab | 0.348 b | 2.301 a | 0.232 b | 0.494 | 0.015 | 0.38 | 0.223 |
| norank_f__Eubacterium_ coprostanoligenes_group | 1.497 a | 0.440 b | 0.240 b | 0.063 b | 0.323 | 0.015 | 0.016 | 0.082 |
| Lachnoclostridium | 1.511 a | 0.784 ab | 1.224 a | 0.221 b | 0.258 | 0.007 | 0.63 | 0.71 |
| Ruminococcus | 0.092 b | 0.446 ab | 0.644 a | 0.081 b | 0.062 | <0.001 | <0.001 | <0.001 |
| Olsenella | 0.241 | 0.733 | 1.361 | 0.522 | 0.198 | 0.23 | 0.048 | 0.054 |
| Romboutsia | 0.229 b | 0.248 b | 1.940 a | 0.292 b | 0.260 | 0.042 | 0.036 | 0.038 |
| Lachnospiraceae_NK4A136_group | 0.028 b | 0.178 ab | 0.491 a | 0.124 ab | 0.055 | 0.013 | 0.003 | 0.004 |
| Erysipelotrichaceae_UCG-003 | 0.110 b | 0.133 b | 0.089 b | 0.564 a | 0.058 | 0.005 | 0.39 | 0.067 |
| Items | Supplementation Level, g/d | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 4.4 | 8.8 | 17.6 | ANOVA | Linear | Quadratic | ||
| Cellular processes | 5.38 a | 5.02 ab | 5.30 a | 4.65 b | 0.09 | 0.007 | 0.96 | 0.42 |
| Environmental information processing | 14.21 | 13.37 | 14.01 | 13.72 | 0.13 | 0.098 | 0.35 | 0.43 |
| Genetic information processing | 5.85 a | 6.50 b | 5.95 a | 6.66 b | 0.09 | <0.001 | 0.65 | 0.72 |
| Human diseases | 2.52 b | 2.72 a | 2.63 ab | 2.68 a | 0.02 | 0.011 | 0.052 | 0.13 |
| Metabolism | 70.97 | 71.13 | 70.99 | 71.04 | 0.12 | 0.97 | 0.88 | 0.89 |
| Organismal systems | 1.070 b | 1.266 a | 1.131 b | 1.253 a | 0.020 | <0.001 | 0.16 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Zhang, Z.; Shao, K.; Wang, X.; Huang, F.; Qi, J.; Duan, Y.; Jia, Y.; Xu, M. Effects of Sodium Butyrate Supplementation in Milk on the Growth Performance and Intestinal Microbiota of Preweaning Holstein Calves. Animals 2023, 13, 2069. https://doi.org/10.3390/ani13132069
Wu D, Zhang Z, Shao K, Wang X, Huang F, Qi J, Duan Y, Jia Y, Xu M. Effects of Sodium Butyrate Supplementation in Milk on the Growth Performance and Intestinal Microbiota of Preweaning Holstein Calves. Animals. 2023; 13(13):2069. https://doi.org/10.3390/ani13132069
Chicago/Turabian StyleWu, Donglin, Zhanhe Zhang, Kai Shao, Xing Wang, Fudong Huang, Jingwei Qi, Yizong Duan, Yang Jia, and Ming Xu. 2023. "Effects of Sodium Butyrate Supplementation in Milk on the Growth Performance and Intestinal Microbiota of Preweaning Holstein Calves" Animals 13, no. 13: 2069. https://doi.org/10.3390/ani13132069
APA StyleWu, D., Zhang, Z., Shao, K., Wang, X., Huang, F., Qi, J., Duan, Y., Jia, Y., & Xu, M. (2023). Effects of Sodium Butyrate Supplementation in Milk on the Growth Performance and Intestinal Microbiota of Preweaning Holstein Calves. Animals, 13(13), 2069. https://doi.org/10.3390/ani13132069





