Effects of Glycine Supplementation in Drinking Water on the Growth Performance, Intestinal Development, and Genes Expression in the Jejunum of Chicks
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Performance Measurement and Sampling
2.3. Intestinal Morphology Determination
2.4. Quantitative Real-Time PCR
2.5. Statistical Analysis
3. Results
3.1. Body Weight and Average Daily Gain
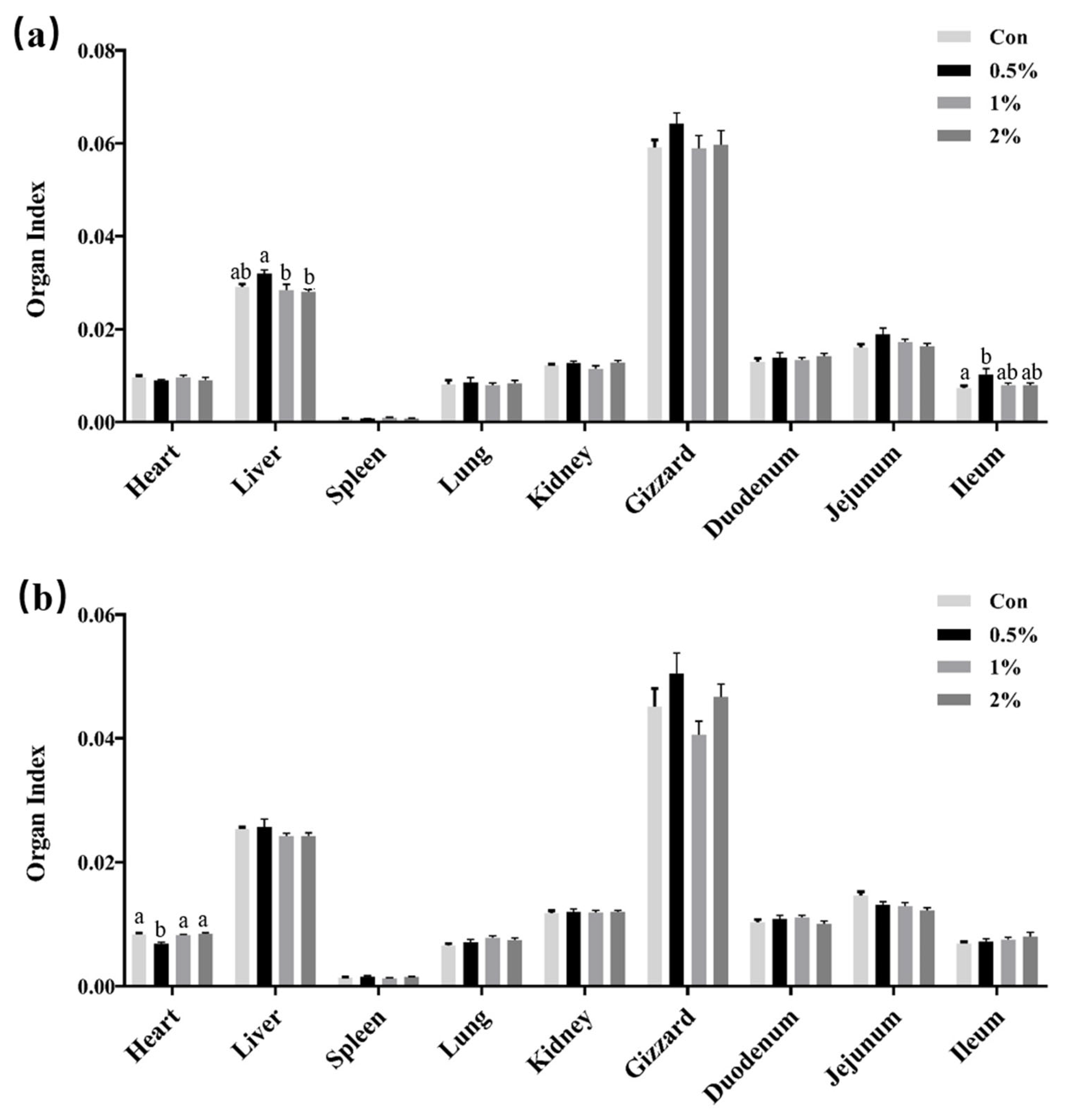
3.2. Organ Development
3.3. Intestinal Morphology
3.4. Expression of Tight Junction Protein-Related, Keap1/Nrf2 Signaling Pathway-Related, and Amino Acid Transporter Genes in Jejunum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibson, N.R.; Jahoor, F.; Ware, L.; Jackson, A.A. Endogenous glycine and tyrosine production is maintained in adults consuming a marginal-protein diet. Am. J. Clin. Nutr. 2002, 75, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Rojas, I.C.; Murakami, A.E.; Eyng, C.; Nunes, R.V.; Duarte, C.R.; Vargas, M.D. Commercially available amino acid supplementation of low-protein diets for broiler chickens with different ratios of digestible glycine+serine: Lysine. Poult. Sci. 2012, 91, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Petrat, F.; Boengler, K.; Schulz, R.; de Groot, H. Glycine, a simple physiological compound protecting by yet puzzling mechanism(s) against ischaemia-reperfusion injury: Current knowledge. Br. J. Pharmacol. 2012, 165, 2059–2072. [Google Scholar] [CrossRef] [PubMed]
- Paolini, C.L.; Marconi, A.M.; Ronzoni, S.; Di Noio, M.; Fennessey, P.V.; Pardi, G.; Battaglia, F.C. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J. Clin. Endocrinol. Metab. 2001, 86, 5427–5432. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Zhong, Z.; Wheeler, M.D.; Li, X.; Froh, M.; Schemmer, P.; Yin, M.; Bunzendaul, H.; Bradford, B.; Lemasters, J.J. L-Glycine: A novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 229–240. [Google Scholar] [CrossRef]
- Carmans, S.; Hendriks, J.J.; Thewissen, K.; Van den Eynden, J.; Stinissen, P.; Rigo, J.M.; Hellings, N. The inhibitory neurotransmitter glycine modulates macrophage activity by activation of neutral amino acid transporters. J. Neurosci. Res. 2010, 88, 2420–2430. [Google Scholar] [CrossRef]
- Jin-Xiu, H.; Xu-Gang, L.; Lin, L.; Bin, L. Study on dynamics and hormonal regulation of yolk sac utilization in different breeds of newly-hatched broilers. Chin. J. Anim. Vet. Sci. 2008, 39, 233–239. [Google Scholar]
- Su, Y.; Zhang, X.; Xin, H.; Li, S.; Li, J.; Zhang, R.; Li, X.; Li, J.; Bao, J. Effects of prior cold stimulation on inflammatory and immune regulation in ileum of cold-stressed broilers. Poult. Sci. 2018, 97, 4228–4237. [Google Scholar] [CrossRef]
- Yulan, L.; Jingjing, H.; Yongqing, H.; Huiling, Z.; Shengjun, Z.; Binying, D.; Yulong, Y.; Ganfeng, Y.; Junxia, S.; Wei, F. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br. J. Nutr. 2008, 100, 552–560. [Google Scholar]
- Zechner, E.L. Inflammatory disease caused by intestinal pathobionts. Curr. Opin. Microbiol. 2017, 35, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Li, S.; Pi, D.; Zhu, H.; Hou, Y.; Shi, H.; Leng, W. Asparagine attenuates intestinal injury, improves energy status and inhibits AMP-activated protein kinase signalling pathways in weaned piglets challenged with Escherichia coli lipopolysaccharide. Br. J. Nutr. 2015, 114, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zheng, J.; Zhou, H.; You, J.; Li, G. Dietary glycine supplementation prevents heat stress-induced impairment of antioxidant status and intestinal barrier function in broilers. Poult. Sci. 2023, 102, 102408. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, X.; Wu, H.; Zhu, H.; Liu, C.; Hou, Y.; Dai, B.; Liu, X.; Liu, Y. Glycine relieves intestinal injury by maintaining mtor signaling and suppressing AMPK, TLR4, and NOD signaling in weaned piglets after lipopolysaccharide challenge. Int. J. Mol. Sci. 2018, 19, 1980. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Z.; Lin, G.; Hu, S.; Wang, B.; Dai, Z.; Wu, G. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J. Nutr. 2014, 144, 1540–1548. [Google Scholar] [CrossRef]
- Fan, X.; Li, S.; Wu, Z.; Dai, Z.; Li, J.; Wang, X.; Wu, G. Glycine supplementation to breast-fed piglets attenuates post-weaning jejunal epithelial apoptosis: A functional role of CHOP signaling. Amino Acids 2019, 51, 463–473. [Google Scholar] [CrossRef]
- Li, W.; Sun, K.; Ji, Y.; Wu, Z.; Wang, W.; Dai, Z.; Wu, G. Glycine regulates expression and distribution of Claudin-7 and ZO-3 proteins in intestinal porcine epithelial cells. J. Nutr. 2016, 146, 964–969. [Google Scholar] [CrossRef]
- Kasai, K.; Suzuki, H.; Nakamura, T.; Shiina, H.; Shimoda, S.I. Glycine stimulated growth hormone release in man. Acta Endocrinol. 1980, 93, 283–286. [Google Scholar] [CrossRef]
- De Aguiar, P.E.; Lopes-Paulo, F.; Marques, R.G.; Diestel, C.F.; Caetano, C.E.; de Souza, M.V.; Moscoso, G.M.; Pazos, H.M. L-arginine and glycine supplementation in the repair of the irradiated colonic wall of rats. Int. J. Color. Dis. 2011, 26, 561–568. [Google Scholar] [CrossRef]
- Dean, D.W.; Bidner, T.D.; Southern, L.L. Glycine supplementation to low protein, amino acid-supplemented diets supports optimal performance of broiler chicks. Poult. Sci. 2006, 85, 288–296. [Google Scholar] [CrossRef]
- Siegert, W.; Rodehutscord, M. The relevance of glycine and serine in poultry nutrition: A review. Br. Poult. Sci. 2019, 60, 579–588. [Google Scholar] [CrossRef]
- Noy, Y.; Sklan, D. Nutrient use in chicks during the first week posthatch. Poult. Sci. 2002, 81, 391–399. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Wang, W.; Dai, Z.; Wu, Z.; Lin, G.; Jia, S.; Hu, S.; Dahanayaka, S.; Wu, G. Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 2014, 46, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Hasanpur, K.; Hosseinzadeh, S.; Mirzaaghayi, A.; Alijani, S. Investigation of chicken housekeeping genes using next-generation sequencing data. Front. Genet. 2022, 13, 827538. [Google Scholar] [CrossRef] [PubMed]
- Corzo, A.; Kidd, M.T.; Burnham, D.J.; Kerr, B.J. Dietary glycine needs of broiler chicks. Poult. Sci. 2004, 83, 1382–1384. [Google Scholar] [CrossRef] [PubMed]
- Coon, C.N.; Grossie, V.J.; Couch, J.R. Glycine-serine requirement for chicks. Poult. Sci. 1974, 53, 1709–1713. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, S.; Lynch, J.W.; Schofield, P.R. The glycine receptor. Pharmacol. Ther. 1997, 73, 121–146. [Google Scholar] [CrossRef]
- El, H.M.; Perez, I.; Zamora, J.; Soto, V.; Carvajal-Sandoval, G.; Banos, G. Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose-fed rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, R1387–R1393. [Google Scholar] [CrossRef]
- Li, X.G.; Chen, X.L.; Wang, X.Q. Changes in relative organ weights and intestinal transporter gene expression in embryos from White Plymouth Rock and WENS Yellow Feather Chickens. Comp. Biochem. Physiol. Part A. Mol. Integr. Physiol. 2013, 164, 368–375. [Google Scholar] [CrossRef]
- Noy, Y.; Geyra, A.; Sklan, D. The effect of early feeding on growth and small intestinal development in the posthatch poult. Poult. Sci. 2001, 80, 912–919. [Google Scholar] [CrossRef]
- Wijtten, P.J.A.; Langhout, D.J.; Verstegen, M.W.A. Small intestine development in chicks after hatch and in pigs around the time of weaning and its relation with nutrition: A review. Acta Agric. Scand. Section A—Anim. Sci. 2012, 62, 1–12. [Google Scholar] [CrossRef]
- Ziegler, T.R.; Evans, M.E.; Fernandez-Estivariz, C.; Jones, D.P. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu. Rev. Nutr. 2003, 23, 229–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Xu, M.; Wang, F.N.; Yu, Z.P.; Yao, J.H.; Zan, L.S.; Yang, F.X. Effect of dietary starch on rumen and small intestine morphology and digesta pH in goats. Livest. Sci. 2009, 122, 48–52. [Google Scholar] [CrossRef]
- Caspary, W.F. Physiology and pathophysiology of intestinal absorption. Am. J. Clin. Nutr. 1992, 55, 299S–308S. [Google Scholar] [CrossRef]
- Anderson, R.C.; Dalziel, J.E.; Gopal, P.K.; Bassett, S.; Ellis, A.; Roy, N.C. The Role of Intestinal Barrier Function in Early Life in the Development of Colitis. In Colitis; InTechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol.-Cell Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef]
- Broom, L.J. Gut barrier function: Effects of (antibiotic) growth promoters on key barrier components and associations with growth performance. Poult. Sci. 2018, 97, 1572–1578. [Google Scholar] [CrossRef]
- Chang, C.H.; Teng, P.Y.; Lee, T.T.; Yu, B. Effects of multi-strain probiotic supplementation on intestinal microbiota, tight junctions, and inflammation in young broiler chickens challenged with Salmonella enterica subsp. enterica. Asian Australas. J. Anim. Sci. 2020, 33, 1797–1808. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; Ju, Z.; Wu, J.; Wang, L.; Lin, H.; Sun, S. Clostridium butyricum Ameliorates Salmonella Enteritis Induced Inflammation by Enhancing and Improving Immunity of the Intestinal Epithelial Barrier at the Intestinal Mucosal Level. Front. Microbiol. 2020, 11, 299. [Google Scholar] [CrossRef]
- Jia, G.; Yu, S.; Sun, W.; Yang, J.; Wang, Y.; Qi, Y.; Chen, Y. Hydrogen sulfide attenuates particulate matter-induced emphysema and airway inflammation through Nrf2-dependent manner. Front. Pharmacol. 2020, 11, 29. [Google Scholar] [CrossRef]
- Jo, H.S.; Kim, D.S.; Ahn, E.H.; Kim, D.W.; Shin, M.J.; Cho, S.B.; Park, J.H.; Lee, C.H.; Yeo, E.J.; Choi, Y.J.; et al. Protective effects of Tat-NQO1 against oxidative stress-induced HT-22 cell damage, and ischemic injury in animals. BMB Rep. 2016, 49, 617–622. [Google Scholar] [CrossRef]
- Jiang, L.; Li, H.; Zhao, N. Thymoquinone protects against cobalt chloride-induced neurotoxicity via Nrf2/GCL-regulated glutathione homeostasis. J. Biol. Regul. Homeost. Agents 2017, 31, 843–853. [Google Scholar]
- Ali, I.; Li, C.; Kuang, M.; Shah, A.U.; Shafiq, M.; Ahmad, M.A.; Abdalmegeed, D.; Li, L.; Wang, G. Nrf2 Activation and NF-Kb & caspase/bax signaling inhibition by sodium butyrate alleviates LPS-induced cell injury in bovine mammary epithelial cells. Mol. Immunol. 2022, 148, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.W.; Li, W.; Harrison, L.; Aw, T.Y. Activation of promoter activity of the catalytic subunit of gamma-glutamylcysteine ligase (GCL) in brain endothelial cells by insulin requires antioxidant response element 4 and altered glycemic status: Implication for GCL expression and GSH synthesis. Free Radic. Biol. Med. 2011, 51, 1749–1757. [Google Scholar] [CrossRef]
- Yang, H.; Magilnick, N.; Ou, X.; Lu, S.C. Tumour necrosis factor α induces co-ordinated activation of rat GSH synthetic enzymes via nuclear factor κB and activator protein-1. Biochem. J. 2005, 391, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.A.; Gibson, N.R.; Lu, Y.; Jahoor, F. Synthesis of erythrocyte glutathione in healthy adults consuming the safe amount of dietary protein. Am. J. Clin. Nutr. 2004, 80, 101–107. [Google Scholar] [CrossRef]
- Howard, A.; Hirst, B.H. The glycine transporter GLYT1 in human intestine: Expression and function. Biol. Pharm. Bull. 2011, 34, 784–788. [Google Scholar] [CrossRef]
- Jando, J.; Camargo, S.; Herzog, B.; Verrey, F. Expression and regulation of the neutral amino acid transporter B0AT1 in rat small intestine. PLoS ONE 2017, 12, e184845. [Google Scholar] [CrossRef]
- Coady, M.J.; Chen, X.Z.; Lapointe, J.Y. rBAT is an amino acid exchanger with variable stoichiometry. J. Membr. Biol. 1996, 149, 1–8. [Google Scholar] [CrossRef]
- Spanier, B. Transcriptional and functional regulation of the intestinal peptide transporter PEPT1. J. Physiol. 2014, 592, 871–879. [Google Scholar] [CrossRef]

| Primer Names | Primer Sequence (5′→3′) | GenBank No |
|---|---|---|
| Keap1-F | GCCCTCAACAACTGCAT | MN416132.1 |
| Keap1-R | CGGGTCGTAACACTCCA | |
| NQO1-F | TCAATGCCGTGCTCTCA | NM_001277620.2 |
| NQO1-R | CAGCCGCTTCAATCTTC | |
| GCLM-F | TTCGGTCATTATTGCCC | NM_001007953.2 |
| GCLM-R | ACCTGATTGCTGCTTGG | |
| SOD1-F | ATGTGACTGCAAAGGGAGGA | NM_205064.2 |
| SOD1-R | AGCTAAACGAGGTCCAGCAT | |
| GSR-F | AGTGGCTTGCTGGAGGT | XM_040671422.1 |
| GSR-R | GGGTCAGGAGGGCTTTG | |
| GPX-F | GACCAACCCGCAGTACATCA | NM_001277853.3 |
| GPX-R | GAGGTGCGGGCTTTCCTTTA | |
| GLYT1-F | CGCCACTCTTCTTCCAG | NM_001031279.2 |
| GLYT1-R | CTCCAGTAGCGTGTCCC | |
| PepT1-F | CTTGGCAGATCCCTCAGTATTT | XM_034074354.1 |
| PepT1-R | GTTGGGCTTCAACCTCATTTG | |
| B0AT1-F | CATGATCGGACACAAGCCCA | XM_419056.6 |
| B0AT1-R | AGCATAGACCCAGCCAGGATA | |
| rBAT-F | GGAGAGGCACGAAGTGAAAT | XM_010727793.3 |
| rBAT-R | CGAGGGTAGACCTGGTAGATAG | |
| Occludin-F | AGCCCTCAATACCAGGATGTG | NM_205128.1 |
| Occludin-R | CGCTTGATGTGGAAGAGCTTG | |
| ZO-1-F | AAGAGGAAGCTGTGGGTAACTC | XM_040680632.1 |
| ZO-1-R | TGAAGAGTCACCGTGTGTTGT | |
| ZO-2-F | CCTACATTGGTTCAAGCATCGTGA | NM_001277622.1 |
| ZO⁃2-R | GATGTCGGGAGGCAGGTTGA | |
| Claudin1-F | AGAAGATGCGGATGGCT | NM_001013611.2 |
| Claudin1-R | AACGGGTGTGAAAGGGT | |
| Claudin2-F | GATACGTGTAGCAGCAGCAG | NM_001277622.1 |
| Claudin2-R | AGCTGGGATTTCTGAGCAGT | |
| Claudin3-F | GTCTATGGGGCTGGAGATCG | NM_204202.2 |
| Claudin3-R | ATCCACAGCCCTTCCCAGA | |
| POLR2B-F | TTACAAGCAGAAGCCCAGC | NM_001006448.2 |
| POLR2B-R | TCACAAAGGTCACGGTCAGT | |
| AP2M1-F | AAAAACAGGGCAAAGGGACT | NM_001079494.2 |
| AP2M1-R | CACGGAAGGGAAGGATGATG |
| Items | Treatments | p-Value | |||
|---|---|---|---|---|---|
| Control | 0.5% | 1% | 2% | ||
| Body weight, g | |||||
| 0 d | 38.80 ± 1.07 | 40.47 ± 0.70 | 39.05 ± 0.72 | 39.75 ± 0.84 | 0.51 |
| 7 d | 91.59 ± 1.34 | 93.83 ± 2.03 | 93.34 ± 1.42 | 95.31 ± 1.80 | 0.48 |
| 14 d | 164.12 ± 2.66 | 166.59 ± 4.87 | 174.00 ± 3.46 | 175.72 ± 4.16 | 0.46 |
| ADG, g | |||||
| 0–7 d | 7.54 ± 0.09 | 7.62 ± 0.22 | 7.76 ± 0.12 | 7.94 ± 0.17 | 0.33 |
| 7–14 d | 10.36 ± 0.18 a | 10.39 ± 0.41 ab | 11.52 ± 0.29 b | 11.49 ± 0.40 ab | 0.01 |
| Items | Treatments | p-Value | |||
|---|---|---|---|---|---|
| Control | 0.5% | 1% | 2% | ||
| Occludin | 1.00 ± 0.16 | 1.06 ± 0.15 | 0.90 ± 0.16 | 0.67 ± 0.13 | 0.41 |
| ZO-1 | 1.00 ± 0.22 a | 2.12 ± 0.39 ab | 1.81 ± 0.25 ab | 2.55 ± 0.50 b | <0.05 |
| ZO-2 | 1.00 ± 0.21 | 0.52 ± 0.08 | 0.74 ± 0.07 | 0.59 ± 0.05 | 0.07 |
| Claudin1 | 1.00 ± 0.18 | 1.03 ± 0.16 | 0.60 ± 0.09 | 0.62 ± 0.10 | 0.08 |
| Claudin2 | 1.00 ± 0.15 | 1.22 ± 0.22 | 0.68 ± 0.06 | 0.87 ± 0.15 | 0.14 |
| Claudin3 | 1.00 ± 0.55 | 0.33 ± 0.04 | 0.44 ± 0.08 | 0.56 ± 0.25 | 0.47 |
| Items | Treatments | p-Value | |||
|---|---|---|---|---|---|
| Control | 0.5% | 1% | 2% | ||
| Keap1 | 1.00 ± 0.41 | 1.53 ± 0.25 | 1.92 ± 0.48 | 1.42 ± 0.28 | 0.40 |
| NQO1 | 1.00 ± 0.17 | 1.72 ± 0.38 | 1.28 ± 0.16 | 0.84 ± 0.21 | 0.19 |
| GCLM | 1.00 ± 0.16 a | 0.59 ± 0.08 ab | 0.78 ± 0.17 ab | 0.41 ± 0.09 b | 0.04 |
| SOD1 | 1.00 ± 0.12 | 0.78 ± 0.07 | 0.69 ± 0.06 | 0.89 ± 0.16 | 0.26 |
| GSR | 1.00 ± 0.24 | 0.71 ± 0.05 | 0.57 ± 0.11 | 0.58 ± 0.08 | 0.16 |
| GPX | 1.00 ± 0.04 | 0.84 ± 0.17 | 0.61 ± 0.05 | 0.77 ± 0.09 | 0.11 |
| Items | Treatments | p-Value | |||
|---|---|---|---|---|---|
| Control | 0.5% | 1% | 2% | ||
| GLYT1 | 1.00 ± 0.18 | 1.90 ± 0.34 | 0.90 ± 0.15 | 0.67 ± 0.18 | 0.20 |
| PepT1 | 1.00 ± 0.18 | 0.83 ± 0.18 | 0.76 ± 0.13 | 0.66 ± 0.14 | 0.52 |
| B0AT1 | 1.00 ± 0.18 | 0.71 ± 0.12 | 0.78 ± 0.10 | 0.70 ± 0.06 | 0.34 |
| rBAT | 1.00 ± 0.04 a | 0.80 ± 0.10 a | 0.49 ± 0.06 b | 0.49 ± 0.08 b | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Xie, Y.; Chen, Z.; He, J.; Chen, J. Effects of Glycine Supplementation in Drinking Water on the Growth Performance, Intestinal Development, and Genes Expression in the Jejunum of Chicks. Animals 2023, 13, 3109. https://doi.org/10.3390/ani13193109
Zheng X, Xie Y, Chen Z, He J, Chen J. Effects of Glycine Supplementation in Drinking Water on the Growth Performance, Intestinal Development, and Genes Expression in the Jejunum of Chicks. Animals. 2023; 13(19):3109. https://doi.org/10.3390/ani13193109
Chicago/Turabian StyleZheng, Xiaotong, Yinku Xie, Ziwei Chen, Jiaheng He, and Jianfei Chen. 2023. "Effects of Glycine Supplementation in Drinking Water on the Growth Performance, Intestinal Development, and Genes Expression in the Jejunum of Chicks" Animals 13, no. 19: 3109. https://doi.org/10.3390/ani13193109
APA StyleZheng, X., Xie, Y., Chen, Z., He, J., & Chen, J. (2023). Effects of Glycine Supplementation in Drinking Water on the Growth Performance, Intestinal Development, and Genes Expression in the Jejunum of Chicks. Animals, 13(19), 3109. https://doi.org/10.3390/ani13193109





