1. Introduction
The period of transition from suckling on sow’s milk to feeding on a dry vegetable-based diet in the early weaning phase is critical for the piglet’s growth performance, health status and intestinal development [
1,
2]. Because commercially produced piglets are weaned relatively early compared with those observed under semi-natural conditions [
3], and because the developmental stage of the gut, as a physiological feature, has been shown to depend more on age than on body weight [
4,
5,
6,
7], questions have been raised about pigs’ abilities to digest and utilize the more complex feed provided after weaning. More specifically, the activity of digestible enzymes, namely maltase, sucrase and lactase, have proved to be of great interest as indicators of a pig’s ability to adapt to different carbohydrate sources in the diet [
8,
9]. Furthermore, a pig’s ability to abruptly shift from milk with a dry matter (DM) content of ~18–20% [
10,
11] to a dry diet with an ~85% DM has also been questioned. The latter has been associated with the frequently observed growth check caused by feed refusal during the first few days after weaning [
12]. Feed refusal leads to the deterioration of villi in the small intestine (SI), which further impairs digestible capacity and prolongs growth check [
13]. Thus, it is imperative that piglets either adapt to other sources of nutrients besides sow’s milk or that the gut reaches a more mature state prior to weaning.
Several studies have investigated how the early provision of creep feed during the suckling period affects growth performance in the post-weaning period. Van Der Meulen et al. [
14] found that creep feeding familiarized pigs with solid feed and produced more eaters. Additionally, it was previously observed that the physical characteristics of feeding treatments, such as play feeders [
15] and larger sizes of pelleted feed [
16], positively affected feed intake and eased the weaning transition. The provision of liquid creep has scarcely been investigated; Byrgesen et al. [
17] showed greater feed disappearance when piglets were fed liquid feed compared with dry creep feed. However, this observation was not followed by an increase in disaccharidase activity around weaning nor an increase in growth performance during the post-weaning period. While some research has been carried out to investigate the impact of weaning age on growth performance [
18], no studies have reported on comparisons of four- and five-week weaning ages and the effect of the feeding strategy.
Therefore, the present study aimed to explore the effects of a one-week increase in weaning age, the impact of liquid form feeding treatment compared to dry feed and the interaction between weaning age and feed treatment on growth performance and intestinal development after weaning. It was hypothesized that providing liquid creep feed would increase pre-weaning feed intake, thereby accelerating maturational changes in the small intestine and increasing the digestive and absorptive capacity of pigs. Additionally, it was hypothesized that an increased weaning age would promote maturational changes in the small intestine through both increased feed intake and age-related development.
2. Materials and Methods
2.1. Ethical Approval
All procedures involving animals were conducted in accordance with the guidelines of the Danish Ministry of Justice with respect to animal experimentation and the care of animals under study (The Danish Ministry of Justice, 1995) and were approved under the following AEIRB approval number: 2021-10-PNH-018A.
2.2. Animals and Exprimental Design
To elucidate the effects of weaning age and liquid versus dry creep feed on the pre- and post-weaning performance of commercially reared pigs, a large-scale trial was conducted in a functioning Danish sow herd producing (Landrace × Yorkshire) × Duroc crossbred piglets. The large-scale trial included 12,923 piglets at weaning age and was conducted from March 2020 to February 2021. The experimental period lasted from post-partum day three until the pigs reached nine weeks of age.
To determine whether age and creep feeding promoted maturational changes in the digestive system, two subpopulations of pigs were selected and euthanized for organ and tissue sampling according to weaning age and the type of creep feed provided during their stay in the farrowing unit. Sixty pre-weaning piglets were sacrificed three days prior to being weaned, and the results of the pre-weaning subpopulation are presented in Lyderik et al. [
19]. The subpopulation described in the present study consisted of 60 post-weaning pigs euthanized in the immediate post-weaning period. The sampled pigs were born from 54 different sows ranging from 2nd to 8th parity.
The present study included 4 treatment groups of 15 weaned pigs in a 2 × 2 factorial design. Each treatment consisted of a combination of a given weaning age (WA) and creep feeding strategy (FS), where pigs were weaned in the 4th or 5th postnatal week (W4 or W5) and received either dry or liquid creep feed (DF or LF). Therefore, the 4 treatment groups were W4DF (n = 15), W5DF (n = 15), W4LF (n = 15) and W5LF (n = 15). Extending the lactation period by one week and providing liquid creep feed are realistic and implementable interventions which might ease the weaning transition by allowing the gut to ontogenetically mature through a potential increase in piglet feed intake. Another important consideration was to include cost-effective treatments so as to ensure greater implementation with potential to reduce antimicrobial use.
The mean weaning age was 24.5 ± 0.8 days for pigs in the W4 group and 31.6 ± 0.9 days for pigs in the W5 group. The pigs were euthanized 5 days post-weaning; thus, the W4 pigs had a mean age of 29.5 ± 0.8 days at euthanasia, whereas the W5 pigs were 36.6 ± 0.9 days of age. All pigs in the large-scale trial, including those in the sampled post-weaning subpopulation, were weighed at post-partum day three and again at weaning, and the feed allowance per double pen was monitored daily.
2.3. Housing and Management
The commercial herd had an average production size of 1170 year-sows and weaned approximately 800 pigs per week. In the farrowing unit, sows and litters were kept in traditional farrowing pens with crates and partially slatted floors.
To stimulate the ingestion of creep feed through hunger, a sow nursed one additional piglet more than her number of functional teats. Therefore, the litter size was determined by the number of functional teats on day 3, when the litters were locked. The litter size on day 3 varied from 9–17 piglets per sow. Shortly after birth, all piglets received Vetrimoxin treatment (Ceva Aninal Health, Libourne, France), and two days post-partum, the piglets were treated with Dozuril (Dopharma Research, Raamsdonksveer, The Nederlands), and male piglets were castrated.
In the weaner unit, pigs from the same treatment group were reared in connected double pens and littermates were kept together; thus, the stocking density varied from 31 to 49 pigs per pen. One-third of the pen floor was solid and served as a covered creep area. The rest of the floor was slatted concrete.
2.4. Diet and Feeding Regime
To ensure that the piglets’ nutrient and energy requirements were met, all litters had access to milk supplement (Danmilk™ Supreme 1.0, Agilia™, Videbæk, Danmark) during the first 8 postnatal days (MS1). Litters receiving the LF diet had access to a second milk supplement (MS2; Danmilk™ Gain Agilia™, Videbæk, Denmark) from day 9 to 17. The compositions and nutrient contents of MS1 and MS2 can be seen in
Table 1.
Regarding pre-weaning creep feeding, from post-partum day 9 to the time of weaning, the W4DF and W5DF piglets had access to a dry zinc-free weaner diet as a creep feed (weaner diet 1). Piglets in groups W4LF and W5LF received MS2 from post-partum days 9 to 17, and from post-partum day 18 to weaning, they received weaner diet 1 but as a liquid feed. Here, the dry feed was mixed with water so that the DM constituted 20%. The diet compositions can be seen in
Table 2.
Computerized Mini Wet Feed Systems (BoPil A/S, Sønderborg, Denmark) were installed in the farrowing pens where sows reared LF litters. Feeding troughs were placed above the slatted floor and in the separating wall between two mirrored and connected farrowing pens. Thus, two litters shared one feeder. To ensure continuous access to creep feed, the troughs were equipped with sensors registering when they were empty. When this occurred, 20% of the daily ration would be allocated. Both MS2 and LF were allocated in this manner. The computer registrations of the allocated feed amounts were used to estimate the average feed allowance. In the DF pens, piglets received their feed through separate computerized feeding pipes, and the allocated amounts were therefore registered separately. For the DF piglets, the dry feed was served in the same trough as MS1, and both were provided 5–6 times per day, ensuring constant availability.
As for the post-weaning diet and feeding regime, all weaned pigs continued to receive the same feed that they had received as creep feed during the first eight post-weaning days. From post-weaning days 9 to 14, the DF pigs were gradually transferred to LF feed. All pigs then received the same liquid weaner diet 1 until they reached 18 kg in body weight (BW). From 18kg and onwards, all pigs received a second weaner diet (weaner diet 2). All weaner diets were formulated based on the national nutritional standards set by SEGES Innovation P/S [
20], and their compositions can be seen in
Table 2. In the weaner unit, connected double pens shared a feeder, and therefore, the estimated feed allocation is based on the registrations per double pen.
2.5. Organ and Tissue Sampling
Pigs were euthanized 5 days post-weaning on the same weekday for four consecutive weeks. For each of the first three weeks, 16 pigs were sacrificed, and for the last week, 12 pigs were sacrificed in one day. Thus, a total of 60 pigs were sacrificed.
Sex and litter origin were included as selection criteria. Thirty males and thirty females were included, having been chosen evenly across treatment groups. The aim was to include 1 random pig from 60 different litters. Unfortunately, this was not possible, and therefore 2 pigs were selected from 6 randomly chosen litters of the 54 litters available. Otherwise, pigs were randomly chosen. Stocking density was not included in the selection criteria, as the present study aimed to reflect general on-farm conditions in which stocking density varies greatly from batch to batch.
Before euthanasia, the BW was registered. Pigs were euthanized using a bolt pistol and were exsanguinated before the removal of the entire gastrointestinal tract. The stomach, SI and colon were emptied and weighed, and the length of the SI was measured.
Tissue samples of the proximal SI were collected from each of the post-weaning pigs for a later analysis of enzyme activity and gene expression. This sampling site was chosen based on earlier findings by Amdi et al. [
8,
9], where the inclusion of complex carbohydrates in a pre-weaning milk replacer affected the proximal part of the SI but not the medial or distal part. The proximal sampling site was located by dividing the entire SI into 3 sections of equal length. The section cut near the stomach was considered as the proximal part. This section was then folded to find the middle, and from this area, two 0.5 cm samples were collected.
Tissue samples intended for enzyme activity analysis were rinsed with water and immediately placed in cryotubes on dry ice and later stored at −80 °C, while samples for the analysis of gene expression were placed in cryotubes with RNAlater™ (Invitrogen, Thermo Fischer Scientific, Vilnius, Lithuania) and stored at −20 °C.
2.6. Analysis of Enzyme Activity
The activities of maltase, sucrase and lactase were determined for each SI tissue sample homogenized in 1% Triton X-100. The tissue to Triton X-100 ratio was 0.20 mg tissue per 1 mL solution. Using the method described by Dahlqvist (1968) [
21] to determine disaccharidase activity, the concentration of generated glucose was measured via the absorbance at 340 nm using an EL × 808 microplate reader (Bio-tek, Winooski, VT, USA), and the generated quantity of glucose was determined based on a nicotinamide adenine dinucleotide phosphate (NADP) chain reaction:
For each sample, enzyme activity measured in Units per liter (U/L) was calculated as:
In this calculation, ∆c/t denotes the change in glucose concentration over the reaction time, and ∆E is the change in absorption measured per minute. The a signifies the total volume in a well, d is the dilution factor for the specific sample, and the extinction coefficient of NADPH (6300 M−1 cm−1) is denoted as b. The well depth is denoted as c, and e is the sample volume. The number of glucose molecules generated per molecule of hydrolyzed disaccharide is denoted as n. For maltose, n = 2, while n = 1 for sucrose and lactose. Before performing any statistical analysis of the results, the unit U/L was converted to U/mg tissue by multiplying ∆c/t with 0.2 mg tissue per mL Triton X-100 solution.
A single sample from a pig in the W5LF treatment group contained an insufficient amount of tissue to be analyzed for all three enzymes, and therefore, maltase activity was not determined for this sample. Thus, maltase activity was determined for 59 pigs, while sucrase and lactase were determined for all 60 pigs.
2.7. Analysis of Nutrient Transporter Gene Expression
Using relative quantification and real-time polymerase chain reaction (RT qPCR), the gene expression of sodium-glucose linked transporter 1 (SGLT-1), glucose transporter 2 (GLUT-2) and peptide transporter 1 (PepT-1) was analyzed.
RNA was extracted from the SI tissue samples using a lysis reagent and RNeasy mini kits (Qiagen, Hilden, Germany) following the accompanying protocol from the manufacturer. For each of the 60 samples, 500 ng of RNA was used to synthesize cDNA with a Reverse Transcription kit (Qiagen, Hilden, Germany). An Aria MX PCR system (Agilent Technologies, Santa Clara, CA, USA) was used to perform the qPCR analysis using the following cycling program: 2 min hot start at 95 °C, forty amplification cycles of 5 s at 95 °C and 20 s at 60 °C, and a melt cycle of 3 × 30 s at 95 °C, 65 °C and 95 °C, respectively. The primer sequences can be seen in
Table 3.
The primer efficiency was evaluated through the construction of linear standard curves for each primer pair. Porcine Beta-2-Microglobulin (
B2M) served as the reference gene due to its stable expression in the intestinal tissue samples, according to which the normalized relative genetic expression of nutrient transporter genes was calculated. The ∆Ct value of each gene was calculated for each of the original tissue samples and was chosen as a parameter because of its ability to be analyzed using linear modelling and regression [
22]. When interpreting ∆Ct results, it is important to consider the inverted relationship between ∆Ct and the gene expression level, where a lower ∆Ct value signifies a higher level of gene expression.
Despite repeated attempts, three samples could not be analyzed for gene expression. Additionally, another tissue sample did not contain enough tissue to be analyzed for all three genes and was therefore not analyzed for PepT1 gene expression. Hence, 57 samples were analyzed for SGLT-1 and GLUT-2 gene expression, while the expression of PepT-1 was determined for 56 samples.
2.8. Calculations and Statistical Analysis
The data on feed allowance and growth performance generated in the large-scale study were analyzed and evaluated with SEGES Innovation P/S. The growth performance data were statistically analyzed using a Proc Mixed model in SAS (SAS inst. Inc., Cary, NC, USA). Data on the creep feed allowance were not statistically analyzed, as they were based on estimates per double pen. The data were averaged and are presented as simple mean values of allocated dry matter per pig within the treatment groups.
The statistical analysis of BW at weaning and nine weeks of age, average daily gain (ADG) and accumulated gain was based on individual BW measurements taken on the day of weaning and again 4 or 5 weeks after weaning for treatment groups W5 and W4, respectively. The analysis was performed using the following model, where a single pig is the experimental unit:
Yijk denotes the response variables of BW at weaning, BW at nine weeks of age, ADG and accumulated gain, and μ is the independent variable mean. The systematic effects of WA and FS are denoted as αi (i = W4, W5) and βj (j = DF, LF), respectively. The systematic effect of sow parity is denoted as ρk (k = 2, …, 8), and the model includes (αβ)ij as the interaction between WA and FS. The number of pigs in a single weaner pen is included as φij, and θ denotes the random effect of the sow and reflects sow-dependent parameters such as the milk yield and genetics. Litter size is included in the model as δθ, and pig body weight at day three is denoted as ωijk and included as a covariate. εijk is the normally distributed random residual of a model. The data and model residuals were assessed and confirmed to be normally distributed. The least square means for BW at weaning and nine weeks, ADG and accumulated gain were calculated for each treatment group.
The sub-population dataset, including BW at euthanasia, the weight of digestive organs, SI length, organ weight relative to BW, disaccharidase activity and the gene expression of SGLT-1, GLUT-2 and PepT-1, contained individual measurements and values for the 60 sacrificed pigs, taking a single pig as the experimental unit for these parameters. The data were analyzed using R 4.1.1 and R studio version 1.4.1717 (R Development Core Team, 2020; The R Foundation, 2021, Indianapolis, IN, USA). The sub-population dataset was analyzed using linear modelling. The models were reduced using AIC-stepwise reduction, determining the significance level of the included systematic effects and their interactions.
The residuals of each model were evaluated using qqnorm to ensure a normal distribution. The Emmeans function was used to obtain least square means, which are presented with the pooled standard error of the mean (SEM). The data analysis was based on the following model:
where Y
ijk denotes the result of an individual response variable, and μ is the mean of that variable. The systematic effect of WA (i = W4, W5) is denoted as α
i, while β
j is the systematic effect of FS (j = DF, LF). Here, δ
k is the systematic effect of sex (k = female, male). Second-order interactions between the main factors are denoted as (αβ)
ij, (αδ)
ik and (βδ)
jk, and ε
ijk is the normally distributed random residual of a model.
Using AIC-stepwise reduction, the model was reduced to the following:
Only the interaction between WA and FS was included in the analysis, as the AIC-stepwise reduction revealed that the other second-order interactions and the third-order interaction were statistically non-significant (p > 0.10). Where relevant, Tukey–Kramer adjusted LS means were obtained and presented with the pooled SEM.
4. Discussion
The aim of the present study was to investigate the effects of weaning age and the strategy of creep feeding on the digestive and absorptive capacities of newly weaned pigs. It was hypothesized that an additional week of suckling and the allocation of liquid feed would accelerate intestinal maturation and increase the utilization of nutrients in post-weaning pigs. The potential increase in utilization was measured as BW gain and organ weight gain, whereas the brush border activity of disaccharidases and the level of gene expression of selected nutrient transporters were used as indicators of intestinal maturation. The digestion of carbohydrates (CHO) was prioritized above other nutrients, as CHO was the main nutrient in the allocated creep and weaner feeds. Furthermore, the CHO complexes in the feeds would have been distinctly different than those in sow milk. The activities of SI brush border maltase, lactase and sucrase were selected as parameters attesting to the pigs’ digestive capacity for CHO [
23,
24,
25]. Additionally, the
SGLT-1 and
GLUT-2 gene expression levels served as parameters for the absorptive capacity of monosaccharides, and the gene expression level of
PepT-1 was included as a parameter for peptide absorption.
Observing the main effect of the FS, LF increased the BW at nine weeks compared to dry feed by more than one kilo. This was the result of a continuous increase in the BW of LF pigs compared with DF pigs. At weaning, LF pigs were approximately 225 g heavier, while they were 640 g heavier at euthanasia and 1150 g heavier at nine weeks of age. At euthanasia, approximately 70 g of the difference in BW could be explained by an observed increase in the weight of the digestive organs, leaving approximately 570 g of BW unaccounted for. Therefore, this additional weight must have been retained in other organs, as fat, muscle or bone tissue or as water. In a study by Lynegaard et al. [
26], 24-day-old weaned pigs retained approximately 7% body fat and 55% muscle tissue. Madsen et al. [
27] found that the carcasses of 28-day-old weaned pigs contained 26% DM, and chemical analysis of the DM revealed 14% ash, 27% ether extract and 57% crude protein. Therefore, it seems reasonable to assume that the additional weight of the LF pigs could be explained largely by muscle growth and associated water retention.
As expected, WA affected BW, so that W5 pigs were heavier than W4 pigs at weaning, five days later at euthanasia and at nine weeks of age. At euthanasia, W5 pigs were 2.24 ± 0.19 kg heavier, and collectively, their weighed digestive organs were 170 g heavier than those of W4 pigs. At week nine, W5 pigs were approximately 0.55 ± 0.18 kg heavier than W4 pigs. These findings are partly in accordance with an earlier study by Leliveld et al. [
28], where pigs weaned at 5 rather than 4 weeks of age were 2.2 kg heavier at weaning and 3.9 kg heavier 2 weeks post-weaning. However, Leliveld et al. [
28] did not find a significant effect of later weaning on BW at 10 weeks of age, arguing that WA accounts for most of the effect on growth performance in the early post-weaning period. This could be a consequence of the growth rate decreasing with age; therefore, W4 pigs would have caught up with W5 pigs in terms of BW gain. However, the effect of WA observed in the later post-weaning period in this study is supported by the study of Faccin et al. [
29], who found that the increase in WA from day 19 to 28 linearly increased BW from weaning to 10 weeks of age.
As previously mentioned, the weight of the digestive system differed between pigs weaned in week 4 and 5 and in pigs fed DF compared to those fed LF. As expected, older pigs had heavier stomachs, SIs and colons. Similarly, LF pigs had heavier organs than DF pigs. However, only the relative organ weight of the SI was affected by WA and FS, with older and LF-fed pigs having an increased SI weight in relation to BW. Having a larger and heavier SI is only beneficial when accompanied by increased digestive and absorptive capacities, meaning a lower organ weight relative to BW. In another context, this was also exemplified by high inclusion rates of dietary fiber, leading to increased intestinal weight but lower carcass weight [
30]. These conflicting results emphasize the need to link selected biomarkers of gut maturation and the absorptive and digestive capacities of the SI, such as brush border disaccharidase activity and nutrient transporter gene expression, to the more apparent digestibility of carbohydrates.
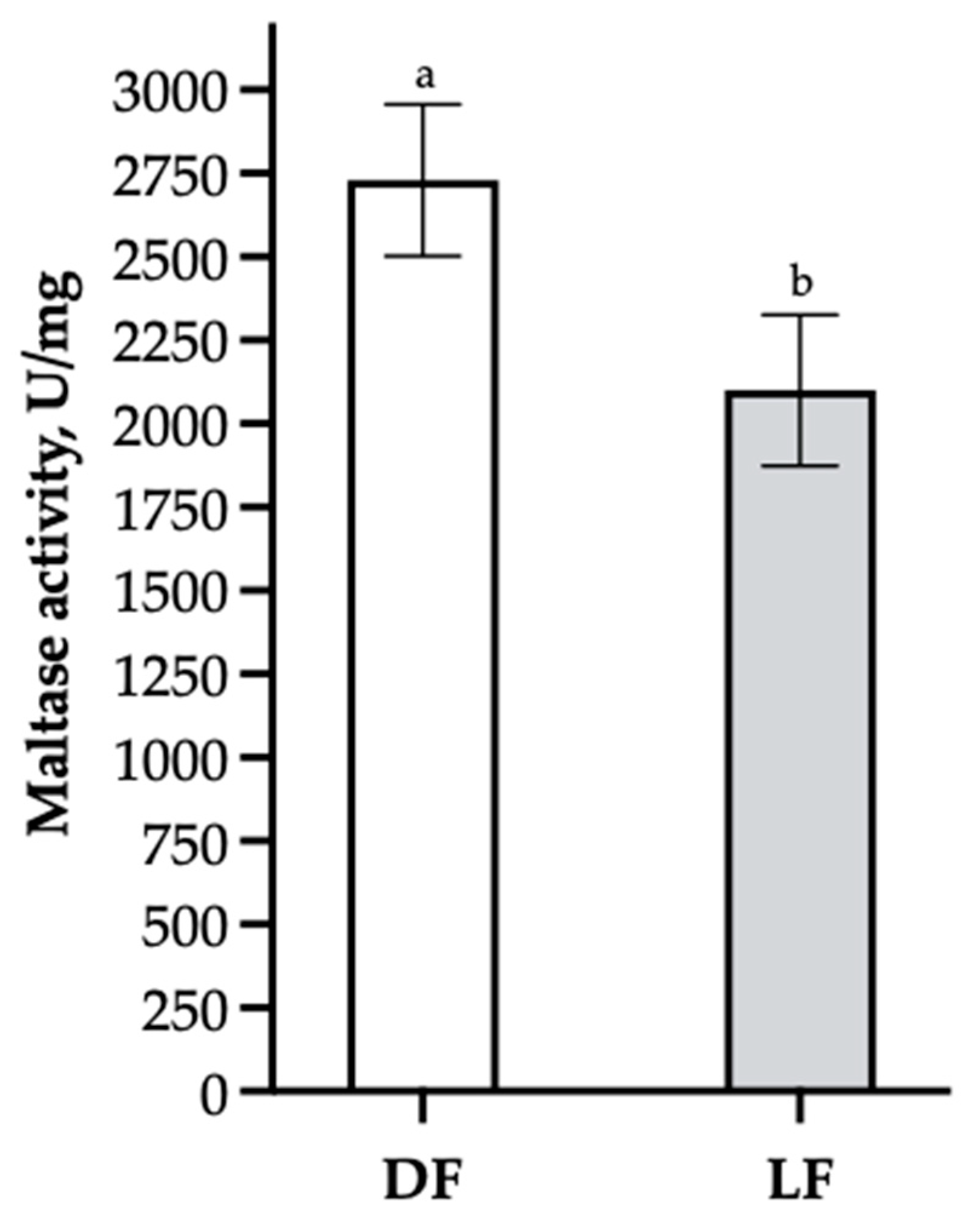
Contradicting the hypothesis, the present study did not find that increasing WA or feeding on LF increased disaccharidase activity in the proximal SI 5 days post-weaning. In fact, DF significantly increased maltase activity but showed a downregulated expression of SGLT-1 genes compared to LF-fed pigs. It could be expected that if FS affected maltase activity and SGLT-1 gene expression, the two parameters would show a positive relation, as increased maltase activity should lead to an increased luminal glucose concentration and might therefore stimulate an increase in SGLT-1 transporters in the brush border, thus optimizing feed utilization. However, the present results do not indicate such a relation.
It can be speculated that LF might stimulate additional
SGLT-1 transporters to be formed in the intestinal brush border [
31]. As the DF and LF groups had numerically similar DM allocations, it was assumed that they consumed similar amounts of DM, and therefore, the heavier LF pigs had greater feed utilization, as reflected in the results for feed efficiency. Consequently, the present study cannot confirm that an elevated level of enzyme activity results in the increased utilization of nutrients, though the results may indicate that feed utilization depends more on the absorptive capacity of the SI than on the digestive capacity.
Though other studies have found that the level of brush border enzyme activity and nutrient transporter gene expression are indicative of gut maturation in piglets [
23,
24,
25], it is questionable whether enzyme activity and the nutrient transporter gene expression level are suitable parameters when applied to gut tissues from post-weaned pigs reared under commercial conditions. Another parameter of interest when examining gut maturation is gut integrity, assessed by measuring villus height, crypt depth and digestibility. Higher villi increase the surface area available for nutrient absorption and are thus considered as an indirect indicator of intestinal digestive capacity. Jijang et al. [
32] found that 24-day-old weaned pigs receiving LF had the same levels of maltase and sucrase activity as pigs given the same diet as DF; however, the LF pigs had a significantly increased jejunal villus height and a higher apparent digestibility of dietary fat and ash. This underlines the fact that there is not necessarily a direct link between the activity of disaccharidases and gut morphology, and even more curiously, there is no immediate link between the activity of disaccharidases and CHO digestibility. Furthermore, villus height and crypt depth are dynamic markers, in which significant changes can occur within a few days, even within the same treatment group [
33]. Again, this all emphasizes the complexity of the digestive process and stresses the difficulties in selecting suitable markers for gut maturation as well as digestive capacity.
Discussing the increased maltase activity seen in the DF pigs, it might be argued that DF strains the gut more than LF, which could result in a decrease in
SGLT-1 expression but an upregulation in maltase activity to compensate. Indeed, this could attest to the adaptability of the SI, but it is questionable whether this is actually a compensating mechanism or a symptom of an exhausted organ in energy deficit. A previous study by Byrgesen et al. [
17] showed results similar to those of the present study, where maltase, sucrase and lactase activities were elevated in dry-creep-fed pigs at the age of 25 days, just prior to being weaned. The authors argued that the amount of DM substrate itself affects brush border disaccharidase activity through a downstream relationship with salivary amylase [
17]. In the current study, LF pigs were heavier and had upregulated
SGLT-1 function, but DF pigs had increased maltase activity; thus, it is questionable whether enzyme activity and nutrient transporter gene expression are suitable measures of the digestive utilization of feed, as these measures do not correspond with the growth performance results. It should also be noted that the increase in maltase activity could be due to differences in nutrient composition between the two groups, although this was only the case for a short period during pre-weaning. Future research should consider the possible correlations between enzyme activity, nutrient digestibility and gut morphology.
In the pre-weaning study presented in Lyderik et al. [
19], very few differences were observed between the groups, indicating that it is perhaps necessary for weaning to take place in order to observe differences in gut absorption and function. This seems to be the case, as FS had WA-independent effects on BW at weaning, euthanasia and nine weeks of age, as well as organ weight, the relative weight of the SI, maltase activity and the expression of
SGLT-1 genes.