Changes in Dynamic Thiol/Disulfide Homeostasis, and Substance P, B-Endorphin and α-Tocopherol Concentrations in the Spinal Cord of Chronically Lame Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Lameness Assessment
2.3. Spinal Cord Processing, Protein Extraction, and Quantification
2.4. Spinal Dynamic Thiol/Disulfide Homeostasis Assay
2.5. Spinal Substance P and β-Endorphin Immunoassay
2.6. Determination of α-Tocopherol Concentration by High-Performance Liquid Chromatography (HPLC)
2.7. Statistical Analysis
3. Results
3.1. Substance P Spinal Cord Concentrations
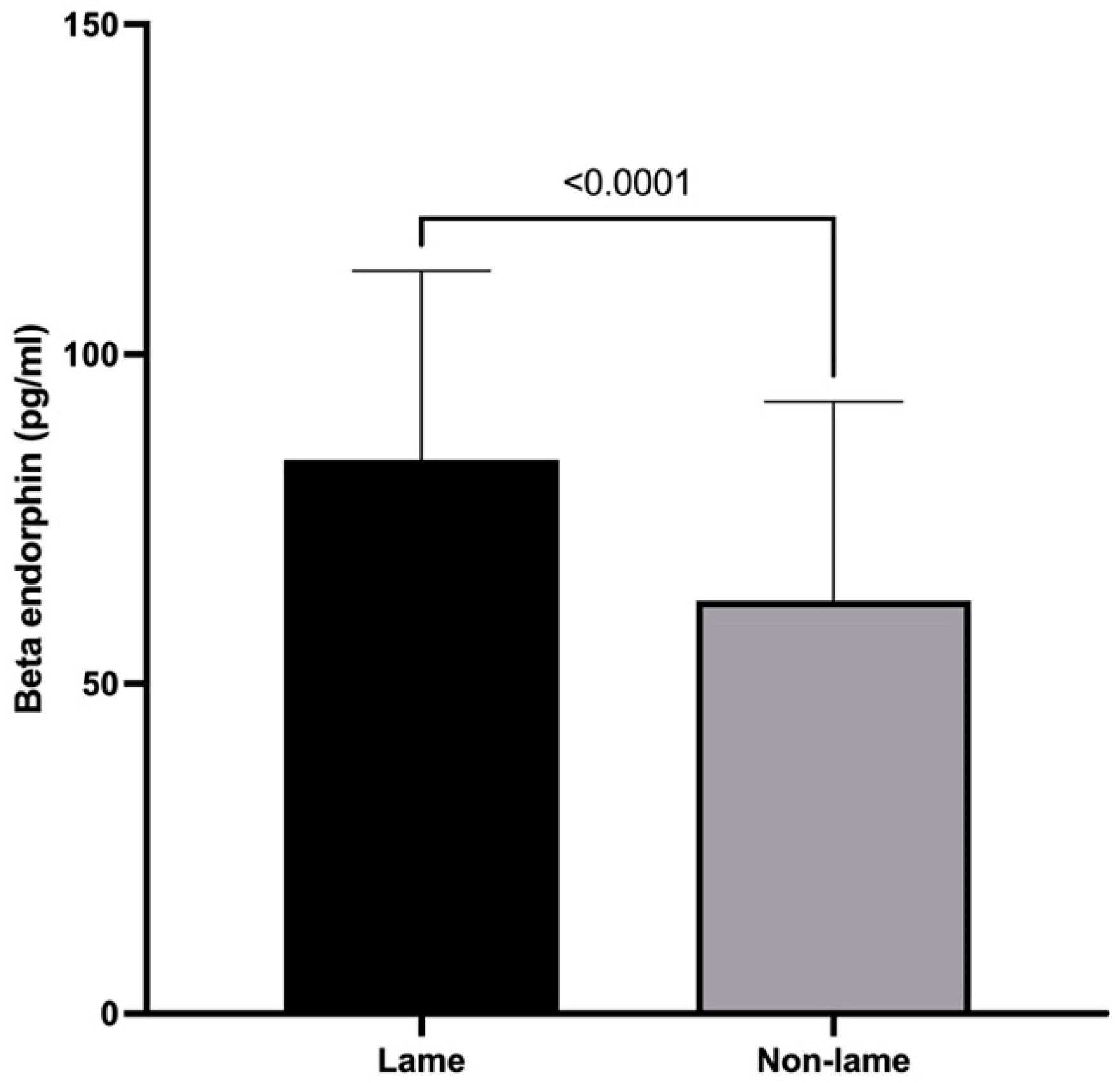
3.2. β-Endorphin Spinal Cord Concentrations
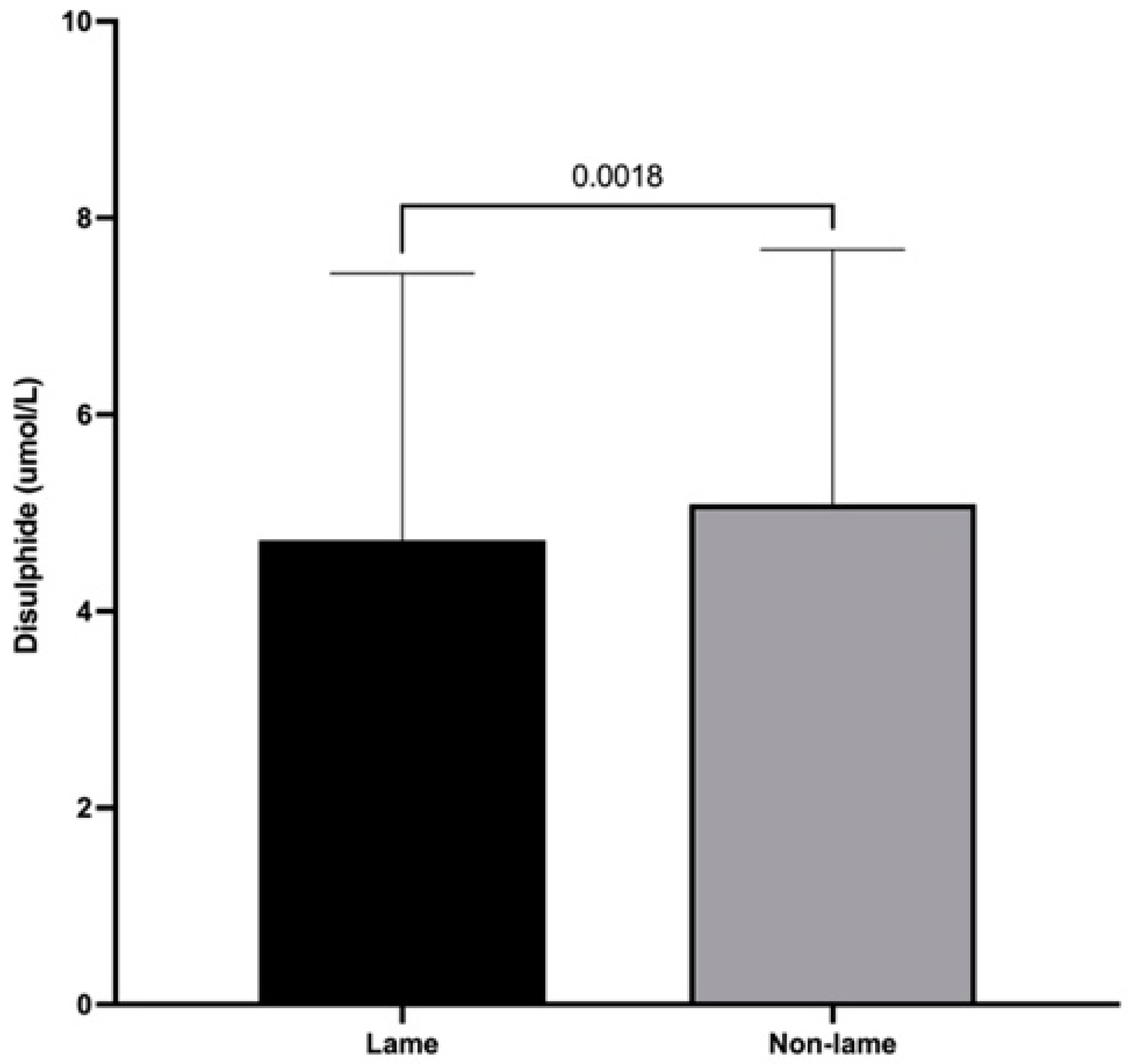
3.3. Dynamic Thiol/Disulfide Homeostasis
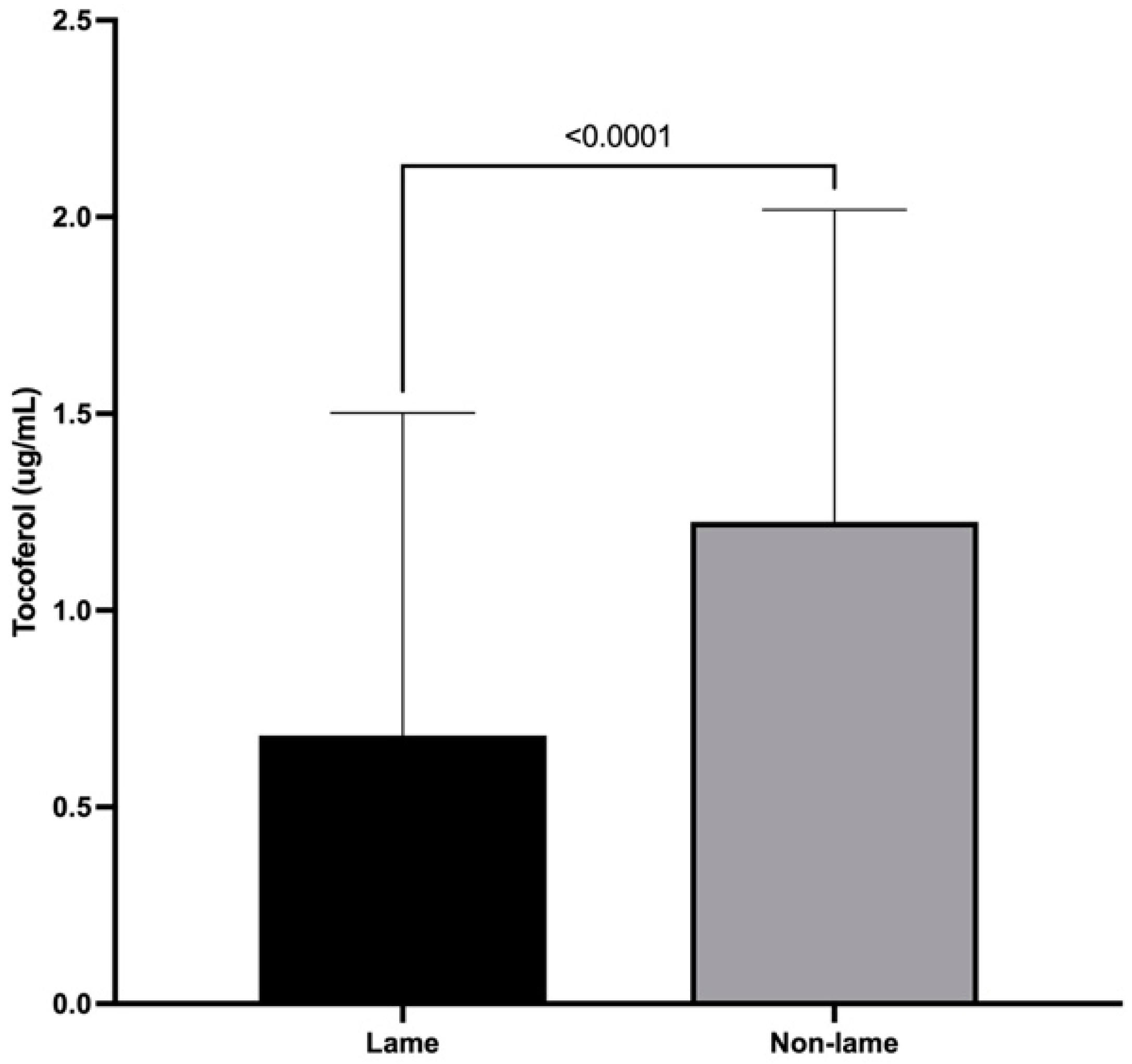
3.4. α-Tocopherol Spinal Cord Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coetzee, J.F.; Shearer, J.K.; Stock, M.L.; Kleinhenz, M.D.; van Amstel, S.R. An Update on the Assessment and Management of Pain Associated with Lameness in Cattle. Vet. Clin. N. Am.—Food Anim. Pract. 2017, 33, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.R.; Herzberg, D.E.; Werner, M.P.; Müller, H.Y.; Bustamante, H.A. Plasma concentration of norepinephrine, β-endorphin, and substance P in lame dairy cows. J. Vet. Res. 2018, 62, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, D.; Strobel, P.; Chihuailaf, R.; Ramirez-Reveco, A.; Müller, H.; Werner, M.; Bustamante, H. Spinal reactive oxygen species and oxidative damage mediate chronic pain in lame dairy cows. Animals 2019, 9, 693. [Google Scholar] [CrossRef]
- Whay, H.R.; Shearer, J.K. The Impact of Lameness on Welfare of the Dairy Cow. Vet. Clin. N. Am.—Food Anim. Pract. 2017, 33, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Salvemini, D.; Little, J.W.; Doyle, T.; Neumann, W.L. Roles of reactive oxygen and nitrogen species in pain. Free Radic. Biol. Med. 2011, 51, 951–966. [Google Scholar] [CrossRef]
- Haddad, J.J.; Land, S.C. Redox/ROS regulation of lipopolysaccharide-induced mitogen-activated protein kinase (MAPK) activation and MAPK-mediated TNF-a biosynthesis. Br. J. Pharmacol. 2002, 135, 520–536. [Google Scholar] [CrossRef]
- Gao, X.; Kim, H.K.; Chung, J.M.; Chung, K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain 2007, 131, 262–271. [Google Scholar] [CrossRef]
- Kaur, U.; Banerjee, P.; Bir, A.; Sinha, M.S.; Biswas, A.; Chakrabarti, S. Reactive Oxygen Species, Redox Signaling and Neuroinflammation in Alzheimer’s Disease: The NF-κB Connection. Curr. Top. Med. Chem. 2015, 15, 446–457. [Google Scholar] [CrossRef]
- Nishio, N.; Taniguchi, W.; Sugimura, Y.; Takiguchi, N.; Yamanaka, M.; Kiyoyuki, Y.; Yamada, H.; Miyazaki, N.; Yoshida, M.; Nakatsuka, T. Reactive oxygen species enhance excitatory synaptic transmission in rat spinal dorsal horn neurons by activating TRPA1 and TRPV1 channels. Neuroscience 2013, 247, 201–212. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Sen, C.K.; Packer, L. Thiol homeostasis and supplements in physical exercise. Am. J. Clin. Nutr. 2000, 72, 653S–669S. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D.S.; Borges, C.R. Cysteine sulfenic acid as an intermediate in disulfide bond formation and nonenzymatic protein folding. Biochemistry 2010, 49, 7748–7755. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Liang, Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic. Biol. Med. 2009, 47, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Erel, O.; Neselioglu, S. A novel and automated assay for thiol/disulphide homeostasis. Clin. Biochem. 2014, 47, 326–332. [Google Scholar] [CrossRef]
- Eroglu, S.; Haskul, I.; Aziz, V.; Yurtcu, E.; Karatas, F.; Neşelioğlu, S.; Erel, O. Dynamic thiol/disulphide homeostasis in patients with Uterine Myoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 216, 24–26. [Google Scholar] [CrossRef]
- Karatas, G.; Gunduz, R.; Haskul, I.; Ustun, B.; Neselioglu, S.; Karatas, F.; Akyuz, M.; Erel, O. Dynamic thiol and disulphide homoeostasis in fibromyalgia. Arch. Med. Sci. 2020, 16, 597–602. [Google Scholar] [CrossRef]
- Tuzcu, A.; Baykara, R.A.; Alışık, M.; Omma, A.; Acet, G.K.; Dogan, E.; Cure, M.C.; Duygun, F.; Cure, E.; Erel, O. Alteration of Thiol-Disulfide Homeostasis in Fibromyalgia Syndrome. Acta Med. 2019, 62, 12–18. [Google Scholar] [CrossRef]
- Chudinova, V.V.; Alekseev, S.M.; I Zakharova, E.; Evstigneeva, R.P. Lipid peroxidation and mechanism of antioxidant effect of vitamin E. Bioorg. Khim. 1994, 20, 1029–1046. Available online: https://pubmed.ncbi.nlm.nih.gov/7826404/ (accessed on 16 December 2022).
- Herrera, E.; Barbas, C. Vitamin E: Action, metabolism and perspectives. J. Physiol. Biochem. 2001, 57, 43–56. [Google Scholar] [CrossRef]
- Wefers, H.; Sies, H. The protection by ascorbate and glutathione against microsomal lipid peroxidation is dependent on vitamin E. Eur. J. Biochem. 1988, 174, 353–357. [Google Scholar] [CrossRef]
- Schneider, C. Chemistry and biology of vitamin E. Mol. Nutr. Food Res. 2005, 49, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Tasinato, A.; Boscoboinik, D.; Bartoli, G.M.; Maroni, P.; Azzi, A. d-α-tocopherol inhibition of vascular smooth muscle cell proliferation occurs at physiological concentrations, correlates with protein kinase C inhibition, and is independent of its antioxidant properties. Proc. Natl. Acad. Sci. USA 1995, 92, 12190–12194. [Google Scholar] [CrossRef] [PubMed]
- Morani, A.S.; Bodhankar, S.L. Early co-administration of vitamin E acetate and methylcobalamin improves thermal hyperalgesia and motor nerve conduction velocity following sciatic nerve crush injury in rats. Pharmacol. Rep. 2010, 62, 405–409. [Google Scholar] [CrossRef]
- Milligan, E.D.; Watkins, L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009, 10, 23–36. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F. Immune regulation of central nervous system functions: From sickness responses to pathological pain. J. Intern. Med. 2005, 257, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Guo, T.Z.; Shi, X.; Sun, Y.; Wei, T.; Clark, D.; Kingery, W. Substance P spinal signaling induces glial activation and nociceptive sensitization after fracture. Neuroscience 2015, 310, 73–90. [Google Scholar] [CrossRef]
- Drew, G.M.; Lau, B.K.; Vaughan, C.W. Substance P drives endocannabinoid-mediated disinhibition in a midbrain descending analgesic pathway. J. Neurosci. 2009, 29, 7220–7229. [Google Scholar] [CrossRef]
- Bán, E.G.; Brassai, A.; Vizi, E.S. The role of the endogenous neurotransmitters associated with neuropathic pain and in the opioid crisis: The innate pain-relieving system. Brain Res. Bull. 2020, 155, 129–136. [Google Scholar] [CrossRef]
- De Felipe, C.; Herrero, J.F.; O’Brien, J.A.; Palmer, J.A.; Doyle, C.A.; Smith, A.J.H.; Laird, J.M.A.; Belmonte, C.; Cervero, F.; Hunt, S.P. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature 1998, 392, 394–397. [Google Scholar] [CrossRef]
- Holden, J.E.; Jeong, Y.; Forrest, J.M. The endogenous opioid system and clinical pain management. AACN Clin. Issues 2005, 16, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Cesselin, F.; Benoliel, J.; Bourgoin, S.; Collin, E.; Pohl, M.; Hamon, M. Spinal mechanisms of opioid analgesia. In Opioids in Pain Control: Basic and Clinical Aspects; Stein, C., Ed.; Cambridge University Press: Cambridge, UK, 1990; pp. 70–95. [Google Scholar]
- Niikura, K.; Narita, M.; Butelman, E.R.; Kreek, M.J.; Suzuki, T. Neuropathic and chronic pain stimuli downregulate central μ-opioid and dopaminergic transmission. Trends Pharmacol. Sci. 2010, 31, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Veening, J.G.; Gerrits, P.O.; Barendregt, H.P. Volume transmission of beta-endorphin via the cerebrospinal fluid; a review. Fluids Barriers CNS 2012, 9, 16. [Google Scholar] [CrossRef]
- Reader, J.D.; Green, M.J.; Kaler, J.; Mason, S.A.; Green, L.E. Effect of mobility score on milk yield and activity in dairy cattle. J. Dairy Sci. 2011, 94, 5045–5052. [Google Scholar] [CrossRef] [PubMed]
- Chihuailaf, R.H.; González, C.S.; Wittwer, F.; Contreras, P.A. Plasma retinol concentration in grazing heifers: First data obtained from a dairy herd in the south of Chile. Concentraciones plasmáticas de retinol en vaquillas a pastoreo: Primeros valores obtenidos en un rebaño del sur de Chile. Arch. Med. Vet. 2008, 68, 65–68. [Google Scholar]
- Bekhbat, M.; Rowson, S.A.; Neigh, G.N. Checks and balances: The glucocorticoid receptor and NFĸB in good times and bad. Front. Neuroendocrinol. 2017, 46, 15–31. [Google Scholar] [CrossRef]
- Yang, K.; Takeuchi, K.; Wei, F.; Dubner, R.; Ren, K. Activation of group i mGlu receptors contributes to facilitation of NMDA receptor membrane current in spinal dorsal horn neurons after hind paw inflammation in rats. Eur. J. Pharmacol. 2011, 670, 509–518. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef]
- Whay, H.; Waterman, A.; Webster, A.; O’Brien, J. The influence of lesion type on the duration of hyperalgesia associated with hindlimb lameness in dairy cattle. Vet. J. 1998, 156, 23–29. [Google Scholar] [CrossRef]
- Tadich, N.; Flor, E.; Green, L. Associations between hoof lesions and locomotion score in 1098 unsound dairy cows. Vet. J. 2010, 184, 60–65. [Google Scholar] [CrossRef]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic Pain: A Maladaptive Response of the Nervous System to Damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef]
- Niikura, K.; Narita, M.; Narita, M.; Nakamura, A.; Okutsu, D.; Ozeki, A.; Kurahashi, K.; Kobayashi, Y.; Suzuki, M.; Suzuki, T. Direct evidence for the involvement of endogenous β-endorphin in the suppression of the morphine-induced rewarding effect under a neuropathic pain-like state. Neurosci. Lett. 2008, 435, 257–262. [Google Scholar] [CrossRef]
- Le Roy, C.; Laboureyras, E.; Gavello-Baudy, S.; Chateauraynaud, J.; Laulin, J.P.; Simonnet, G. Endogenous opioids released during non-nociceptive environmental stress induce latent pain sensitization via a NMDA-dependent process. J. Pain 2011, 12, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Fidan, F.; Alkan, B.M.; Uğurlu, F.G.; Bozkurt, S.; Sezer, N.; Biçer, C.; Erel, Ö.; Ardiçoğlu, Ö.; Akkuş, S. Dynamic thiol/disulphide homeostasis in patients with fibromyalgia. Arch. Rheumatol. 2017, 32, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, E.; Giampietro, O. Thiol signalling network with an eye to diabetes. Molecules 2010, 15, 8890–8903. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.; Sarcar, B.; Kahali, S.; Yuan, Z.; Johnson, J.J.; Adam, K.-P.; Kensicki, E.; Chinnaiyan, P. Cysteine catabolism: A novel metabolic pathway contributing to glioblastoma growth. Cancer Res. 2014, 74, 787–796. [Google Scholar] [CrossRef]
- Smeyne, M.; Smeyne, R.J. Glutathione metabolism and Parkinson’s disease. Free Radic. Biol. Med. 2013, 62, 13–25. [Google Scholar] [CrossRef]
- Lemke, M.; Frei, B.; Ames, B.N.; Faden, A.I. Decreases in tissue levels of ubiquinol-9 and -10, ascorbate and α-tocopherol following spinal cord impact trauma in rats. Neurosci. Lett. 1990, 108, 201–206. [Google Scholar] [CrossRef]
- Riffel, A.P.K.; Santos, M.; De Souza, J.; Scheid, T.; Horst, A.; Kolberg, C.; Bello-Klein, A.; Partata, W. Treatment with ascorbic acid and α-tocopherol modulates oxidative-stress markers in the spinal cord of rats with neuropathic pain. Braz. J. Med. Biol. Res. 2018, 51, e7079. [Google Scholar] [CrossRef]
- Park, E.S.; Gao, X.; Chung, J.M.; Chung, K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci. Lett. 2006, 391, 108–111. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, H.; Herzberg, D.; Chihuailaf, R.; Strobel, P.; Werner, M.; Bustamante, H. Changes in Dynamic Thiol/Disulfide Homeostasis, and Substance P, B-Endorphin and α-Tocopherol Concentrations in the Spinal Cord of Chronically Lame Dairy Cows. Animals 2023, 13, 1620. https://doi.org/10.3390/ani13101620
Müller H, Herzberg D, Chihuailaf R, Strobel P, Werner M, Bustamante H. Changes in Dynamic Thiol/Disulfide Homeostasis, and Substance P, B-Endorphin and α-Tocopherol Concentrations in the Spinal Cord of Chronically Lame Dairy Cows. Animals. 2023; 13(10):1620. https://doi.org/10.3390/ani13101620
Chicago/Turabian StyleMüller, Heine, Daniel Herzberg, Ricardo Chihuailaf, Pablo Strobel, Marianne Werner, and Hedie Bustamante. 2023. "Changes in Dynamic Thiol/Disulfide Homeostasis, and Substance P, B-Endorphin and α-Tocopherol Concentrations in the Spinal Cord of Chronically Lame Dairy Cows" Animals 13, no. 10: 1620. https://doi.org/10.3390/ani13101620
APA StyleMüller, H., Herzberg, D., Chihuailaf, R., Strobel, P., Werner, M., & Bustamante, H. (2023). Changes in Dynamic Thiol/Disulfide Homeostasis, and Substance P, B-Endorphin and α-Tocopherol Concentrations in the Spinal Cord of Chronically Lame Dairy Cows. Animals, 13(10), 1620. https://doi.org/10.3390/ani13101620



