Modelling Genetic Benefits and Financial Costs of Integrating Biobanking into the Captive Management of Koalas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Study Species
2.2. Modelling and Analyses
2.3. Cost Modelling
2.4. Genetic Modelling
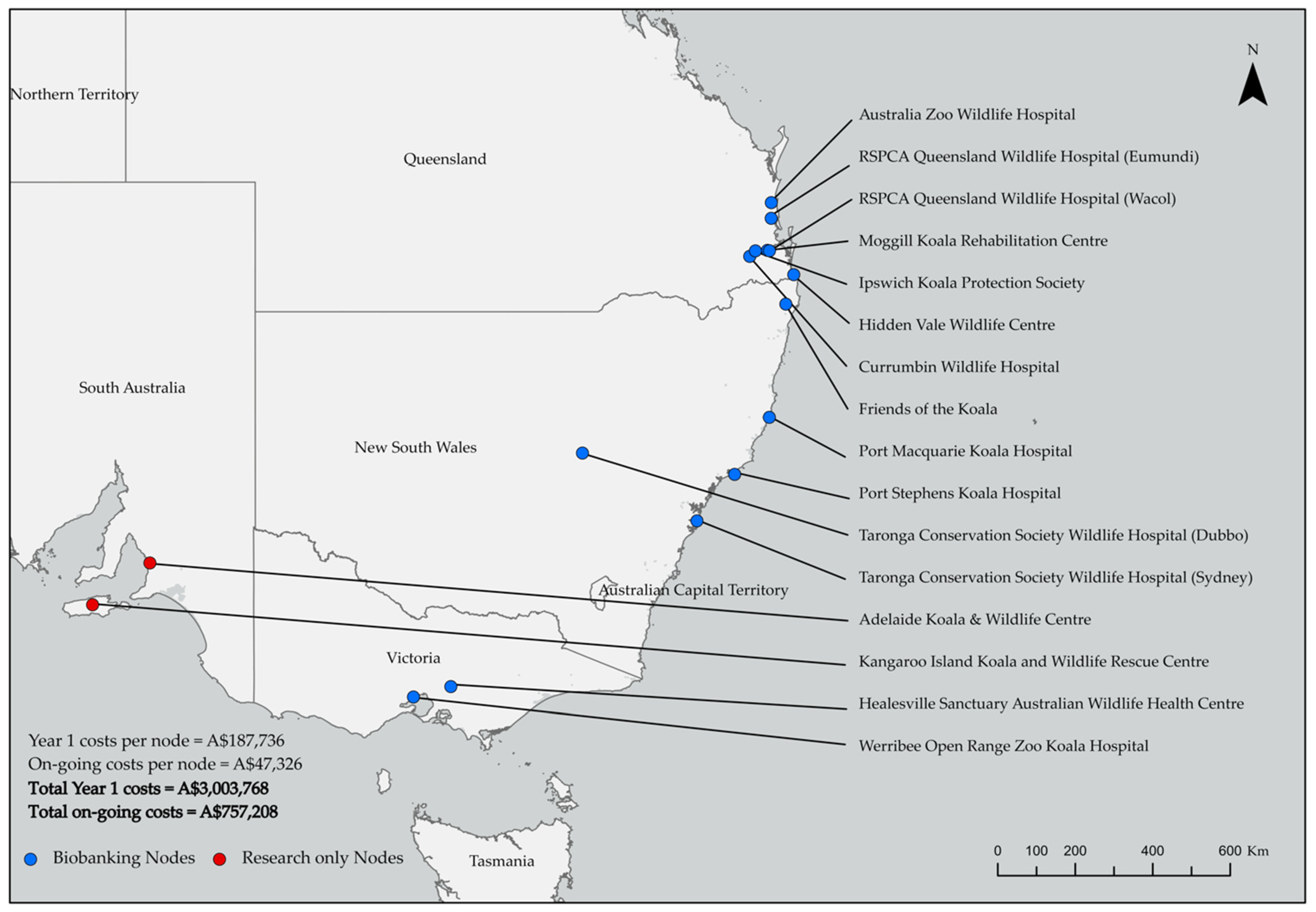
2.5. Mapping Costs of Biobanking and Research Nodes across a Zoo and Wildlife Hospital Network
3. Results
3.1. Modelling Costs and Genetics in Koalas
3.2. Mapping Costs of Biobanking and Research Nodes across A Zoo and Wildlife Hospital Network
4. Discussion
- Recover and make use of genetic material from koalas that can no longer contribute to the population (e.g., koalas that are diseased, recently deceased, or long dead);
- Proactively capture the valuable genetic material from important or imperiled wild koala populations and produce genetically fit koalas for release to the wild;
- Increase the quality of the reproductive output by ensuring surrogate females can give birth to young;
- Overcome geographic separation and behavioral incompatibility of desirable breeding combinations and genetic pairings [62] which could also allow those koalas admitted to hospitals, zoos or captured from the wild during opportunistic research to contribute genetic material for the management of the broader wild population [60,61];
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harley, D.; Mawson, P.R.; Olds, L.; McFadden, M.; Hogg, C.J. The Contribution of Captive Breeding in Zoos to the Conservation of Australia’s Threatened Fauna. In Recovering Australian Threatened Species: A Book of Hope; Garnett, S., Latch, P., Lindenmayer, D., Woinarski, J., Eds.; CSIRO Publishing: Melbourne, VIC, Australia, 2018. [Google Scholar]
- Mawson, P.R.; Lambert, C. Challenges of operating a multi-species breeding-for-release facility at Perth Zoo, Australia. Int. Zoo Yearb. 2017, 51, 165–174. [Google Scholar] [CrossRef]
- Howell, L.G.; Mawson, P.R.; Frankham, R.; Rodger, J.C.; Upton, R.M.O.; Witt, R.R.; Calatayud, N.E.; Clulow, S.; Clulow, J. Integrating biobanking could produce significant cost benefits and minimise inbreeding for Australian amphibian captive breeding programs. Reprod. Fertil. Dev. 2021, 33, 573–587. [Google Scholar] [CrossRef]
- Howell, L.G.; Frankham, R.; Rodger, J.C.; Witt, R.R.; Clulow, S.; Upton, R.M.O.; Clulow, J. Integrating biobanking minimises inbreeding and produces significant cost benefits for a threatened frog captive breeding programme. Conserv. Lett. 2021, 14, e12776. [Google Scholar] [CrossRef]
- Ralls, K.; Ballou, J.D.; Templeton, A. Estimates of lethal equivalents and the cost of inbreeding in mammals. Conserv. Biol. 1988, 2, 185–193. [Google Scholar] [CrossRef]
- Robert, A. Captive breeding genetics and reintroduction success. Biol. Conserv. 2009, 142, 2915–2922. [Google Scholar] [CrossRef]
- Frankham, R. Genetic adaptation to captivity in species conservation programs. Mol. Ecol. 2008, 17, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Rails, K.; Ballou, J. Effects of inbreeding on infant mortality in captive primates. Int. J. Primatol. 1982, 3, 491–505. [Google Scholar] [CrossRef]
- Farquharson, K.A.; Hogg, C.J.; Grueber, C.E. A meta-analysis of birth-origin effects on reproduction in diverse captive environments. Nat. Commun. 2018, 9, 1055. [Google Scholar] [CrossRef]
- Araki, H.; Cooper, B.; Blouin, M.S. Carry-over effect of captive breeding reduces reproductive fitness of wild-born descendants in the wild. Biol. Lett. 2009, 5, 621–624. [Google Scholar] [CrossRef]
- Clulow, J.; Upton, R.; Trudeau, V.L.; Clulow, S. Amphibian Assisted Reproductive Technologies: Moving from Technology to Application. In Reproductive Sciences in Animal Conservation. Advances in Experimental Medicine and Biology; Comizzoli, P., Brown, J., Holt, W., Eds.; Springer: Cham, Switzerland, 2019; Volume 1200, pp. 413–463. [Google Scholar]
- Rodger, J.C. Marsupials: Progress and Prospects. In Reproductive Sciences in Animal Conservation. Advances in Experimental Medicine and Biology; Comizzoli, P., Brown, J., Holt, W., Eds.; Springer: Cham, Switzerland, 2019; Volume 1200, pp. 309–325. [Google Scholar]
- Comizzoli, P.; Brown, J.L.; Holt, W.V. Reproductive Sciences in Animal Conservation. Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2019; Volume 1200, ISBN 3030236331. [Google Scholar]
- Allen, C.D.; Burridge, M.; Mulhall, S.; Chafer, M.L.; Nicolson, V.N.; Pyne, M.; Zee, Y.P.; Jago, S.C.; Lundie-Jenkins, G.; Holt, W.V. Successful artificial insemination in the koala (Phascolarctos cinereus) using extended and extended-chilled semen collected by electroejaculation. Biol. Reprod. 2008, 78, 661–666. [Google Scholar] [CrossRef]
- Johnston, S.D.; McGowan, M.R.; O’Callaghan, P.; Cox, R.; Houlden, B.; Haig, S.; Taddeo, G. Birth of Koalas Phascolarctos cinereus at Lone Pine Koala Sanctuary following artificial insemination. Int. Zoo Yearb. 2003, 38, 160–172. [Google Scholar] [CrossRef]
- Johnston, S.D.; Holt, W.V. Using the koala (Phascolarctos cinereus) as a case study to illustrate the development of artificial breeding technology in marsupials: An update. Reprod. Sci. Anim. Conserv. 2019, 327–362. [Google Scholar] [CrossRef]
- Clulow, J.; Clulow, S. Cryopreservation and other assisted reproductive technologies for the conservation of threatened amphibians and reptiles: Bringing the ARTs up to speed. Reprod. Fertil. Dev. 2016, 28, 1116–1132. [Google Scholar] [CrossRef] [PubMed]
- Howell, L.G.; Mawson, P.R.; Comizzoli, P.; Witt, R.R.; Frankham, R.; Clulow, S.; O’Brien, J.K.; Clulow, J.; Rodger, J.C. Modelling genetic benefits and financial costs of integrating biobanking into the conservation breeding of managed marsupials. Conserv. Biol. 2021; in review. [Google Scholar]
- Soulé, M.; Gilpin, M.; Conway, W.; Foose, T. The millenium ark: How long a voyage, how many staterooms, how many passengers? Zoo Biol. 1986, 5, 101–113. [Google Scholar] [CrossRef]
- Holt, W.V. The black-footed ferret recovery program: A strong advocate for establishing semen banking programs as support tools for small population welfare. Anim. Conserv. 2016, 19, 116–117. [Google Scholar] [CrossRef]
- Howard, J.G.; Lynch, C.; Santymire, R.M.; Marinari, P.E.; Wildt, D.E. Recovery of gene diversity using long-term cryopreserved spermatozoa and artificial insemination in the endangered black-footed ferret. Anim. Conserv. 2016, 19, 102–111. [Google Scholar] [CrossRef]
- Santymire, R.M.; Livieri, T.M.; Branvold-Faber, H.; Marinari, P.E. The black-footed ferret: On the brink of recovery? In Reproductive Sciences in Animal Conservation. Advances in Experimental Medicine and Biology; Holt, W., Brown, J., Comizzoli, P., Eds.; Springer: New York, NY, USA, 2014; Volume 753, pp. 119–134. [Google Scholar]
- Johnston, S.D.; López-Fernández, C.; Gosálbez, A.; Zee, Y.; Holt, W.V.; Allen, C.; Gosálvez, J. The relationship between sperm morphology and chromatin integrity in the koala (Phascolarctos cinereus) as assessed by the sperm chromatin dispersion test (SCDt). J. Androl. 2007, 28, 891–899. [Google Scholar] [CrossRef]
- Howard, J.G.; Marinari, P.E.; Wildt, D.E. Black-footed ferret: Model for assisted reproductive technologies contributing to in situ conservation. In Reproductive Science and Integrated Conservation; Holt, W., Pickard, A., Rodger, J., Wildt, D., Eds.; Cambridge University Press: Cambridge, UK, 2003; pp. 249–266. [Google Scholar]
- Rodger, J.C.; Clulow, J. Resetting the paradigm of reproductive science and conservation. Anim. Reprod. Sci. 2021, 106911. [Google Scholar] [CrossRef]
- Markwell, K. Getting close to a national icon: An examination of the involvement of the koala (Phascolarctos cinereus) in Australian tourism. Tour. Recreat. Res. 2021, 46, 473–486. [Google Scholar] [CrossRef]
- Knott, T.; Lunney, D.; Coburn, D.; Callaghan, J. An ecological history of koala habitat in Port Stephens Shire and the Lower Hunter on the central coast of New South Wales, 1801–1998. Pacific Conserv. Biol. 1998, 4, 354–368. [Google Scholar] [CrossRef]
- McAlpine, C.; Lunney, D.; Melzer, A.; Menkhorst, P.; Phillips, S.; Phalen, D.; Ellis, W.; Foley, W.; Baxter, G.; De Villiers, D. Conserving koalas: A review of the contrasting regional trends, outlooks and policy challenges. Biol. Conserv. 2015, 192, 226–236. [Google Scholar] [CrossRef]
- Polkinghorne, A.; Hanger, J.; Timms, P. Recent advances in understanding the biology, epidemiology and control of chlamydial infections in koalas. Vet. Microbiol. 2013, 165, 214–223. [Google Scholar] [CrossRef] [PubMed]
- McCallum, H.; Kerlin, D.H.; Ellis, W.; Carrick, F. Assessing the significance of endemic disease in conservation—Koalas, chlamydia, and koala retrovirus as a case study. Conserv. Lett. 2018, 11, e12425. [Google Scholar] [CrossRef]
- Dique, D.S.; Thompson, J.; Preece, H.J.; Penfold, G.C.; de Villiers, D.L.; Leslie, R.S. Koala mortality on roads in south-east Queensland: The koala speed-zone trial. Wildl. Res. 2003, 30, 419–426. [Google Scholar] [CrossRef]
- Adams-Hosking, C.; Grantham, H.S.; Rhodes, J.R.; McAlpine, C.; Moss, P.T. Modelling climate-change-induced shifts in the distribution of the koala. Wildl. Res. 2011, 38, 122–130. [Google Scholar] [CrossRef]
- Lunney, D.; Gresser, S.M.; Mahon, P.S.; Matthews, A. Post-fire survival and reproduction of rehabilitated and unburnt koalas. Biol. Conserv. 2004, 120, 567–575. [Google Scholar] [CrossRef]
- Matthews, A.; Lunney, D.; Gresser, S.; Maitz, W. Movement patterns of koalas in remnant forest after fire. Aust. Mammal. 2016, 38, 91–104. [Google Scholar] [CrossRef]
- NSW Legislative Council. Koala Populations and Habitat in New South Wales/Portfolio Committe No. 7-Planning and Environment; NSW Legislative Council: Sydney, NSW, Australia, 2020. [Google Scholar]
- Dickman, C.; Driscoll, D.; Garnett, S.; Keith, D.; Legge, S.; Lindenmayer, D.; Maron, M.; Reside, A.; Ritchie, E.; Watson, J.; et al. After the catastrophe: A blueprint for a conservation response to large-scale ecological disaster. Threat. Species Recovery Hub 2020. Available online: https://www.nespthreatenedspecies.edu.au/media/0akfale0/after-the-catastrophe-report_v5.pdf (accessed on 17 March 2022).
- Abts, K.C.; Ivy, J.A.; DeWoody, J.A. Demographic, environmental and genetic determinants of mating success in captive koalas (Phascolarctos cinereus). Zoo Biol. 2018, 37, 416–433. [Google Scholar] [CrossRef]
- Littleford-Colquhoun, B.L.; Weyrich, L.S.; Hohwieler, K.; Cristescu, R.; Frère, C.H. How microbiomes can help inform conservation: Landscape characterisation of gut microbiota helps shed light on additional population structure in a specialist folivore. Anim. Microbiome 2022, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Adams-Hosking, C.; McAlpine, C.A.; Rhodes, J.R.; Moss, P.T.; Grantham, H.S. Prioritizing regions to conserve a specialist folivore: Considering probability of occurrence, food resources, and climate change. Conserv. Lett. 2015, 8, 162–170. [Google Scholar] [CrossRef]
- Shipley, L.A.; Forbey, J.S.; Moore, B.D. Revisiting the dietary niche: When is a mammalian herbivore a specialist? Integr. Comp. Biol. 2009, 49, 274–290. [Google Scholar] [CrossRef] [PubMed]
- Office of Environment and Heritage NSW Koala Strategy. 2018. Available online: https://www.environment.nsw.gov.au/research-and-publications/publications-search/nsw-koala-strategy (accessed on 17 March 2022).
- Woinarski, J.; Burbidge, A.A. Phascolarctos cinereus. The IUCN Red List of Threatened Fauna Species 2016: E T16892A21960344. 2016. Available online: https://www.academia.edu/29408670/Phascolarctos_cinereus_Koala_THE_IUCN_RED_LIST_OF_THREATENED_SPECIES (accessed on 17 March 2020).
- Johnson, R.N.; O’Meally, D.; Chen, Z.; Etherington, G.J.; Ho, S.Y.W.; Nash, W.J.; Grueber, C.E.; Cheng, Y.; Whittington, C.M.; Dennison, S.; et al. Adaptation and conservation insights from the koala genome. Nat. Genet. 2018, 50, 1102–1111. [Google Scholar] [CrossRef]
- Johnston, S. Challenges associated with the development and transfer of assisted breeding technology in marsupials and monotremes: Lessons from the koala, wombat and short-beaked echidna. Reprod. Fertil. Dev. 2019, 31, 1305–1314. [Google Scholar] [CrossRef]
- Allen, C.D.; Burridge, M.; Chafer, M.L.; Nicolson, V.N.; Jago, S.C.; Booth, R.J.; Fraser, G.; Ensabella, T.-J.; Zee, Y.P.; Lundie-Jenkins, G. Control of the koala (Phascolarctos cinereus) anterior pituitary-gonadal axis with analogues of GnRH. Reprod. Fertil. Dev. 2008, 20, 598–605. [Google Scholar] [CrossRef]
- Frankham, R.; Ballou, J.D.; Briscoe, D.A. Introduction to Conservation Genetics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Mace, G. Genetic management of small populations. Int. Zoo Yearb. 1986, 24, 167–174. [Google Scholar] [CrossRef]
- Crow, J.F.; Kimura, M. An Introduction to Population Genetics Theory; Harper and Rowe: New York, NY, USA, 1970. [Google Scholar]
- Cristescu, R.; Cahill, V.; Sherwin, W.B.; Handasyde, K.; Carlyon, K.; Whisson, D.; Herbert, C.A.; Carlsson, B.L.J.; Wilton, A.N.; Cooper, D.W. Inbreeding and testicular abnormalities in a bottlenecked population of koalas (Phascolarctos cinereus). Wildl. Res. 2009, 36, 299–308. [Google Scholar] [CrossRef]
- Reed, D.H.; Frankham, R. Correlation between fitness and genetic diversity. Conserv. Biol. 2003, 17, 230–237. [Google Scholar] [CrossRef]
- Couvet, D. Deleterious effects of restricted gene flow in fragmented populations. Conserv. Biol. 2002, 16, 369–376. [Google Scholar] [CrossRef]
- Seymour, A.M.; Montgomery, M.E.; Costello, B.H.; Ihle, S.; Johnsson, G.; John, B.S.; Taggart, D.; Houlden, B.A. High effective inbreeding coefficients correlate with morphological abnormalities in populations of South Australian koalas (Phascolarctos cinereus). In Proceedings of the Animal Conservation Forum; Cambridge University Press: Cambridge, UK, 2001; Volume 4, pp. 211–219. [Google Scholar]
- Farquharson, K.A.; Hogg, C.J.; Grueber, C.E. Offspring survival changes over generations of captive breeding. Nat. Commun. 2021, 12, 3045. [Google Scholar] [CrossRef] [PubMed]
- Spielman, D.; Brook, B.W.; Briscoe, D.A.; Frankham, R. Does inbreeding and loss of genetic diversity decrease disease resistance? Conserv. Genet. 2004, 5, 439–448. [Google Scholar] [CrossRef]
- Glassock, G.L.; Grueber, C.E.; Belov, K.; Hogg, C.J. Reducing the extinction risk of populations threatened by infectious diseases. Diversity 2021, 13, 63. [Google Scholar] [CrossRef]
- Franklin, I.R.; Frankham, R. How large must populations be to retain evolutionary potential? Anim. Conserv. 1998, 1, 69–70. [Google Scholar] [CrossRef]
- Razgour, O.; Taggart, J.B.; Manel, S.; Juste, J.; Ibanez, C.; Rebelo, H.; Alberdi, A.; Jones, G.; Park, K. An integrated framework to identify wildlife populations under threat from climate change. Mol. Ecol. Resour. 2018, 18, 18–31. [Google Scholar] [CrossRef]
- Weeks, A.R.; Heinze, D.; Perrin, L.; Stoklosa, J.; Hoffmann, A.A.; van Rooyen, A.; Kelly, T.; Mansergh, I. Genetic rescue increases fitness and aids rapid recovery of an endangered marsupial population. Nat. Commun. 2017, 8, 1071. [Google Scholar] [CrossRef]
- Beauclerc, K.B.; Johnson, B.; White, B.N. Genetic rescue of an inbred captive population of the critically endangered Puerto Rican crested toad (Peltophryne lemur) by mixing lineages. Conserv. Genet. 2010, 11, 21–32. [Google Scholar] [CrossRef]
- Johnston, S.; Beagley, K.; Seddon, J.; Schultz, B.; Keeley, T.; Hulse, L.; Mucci, A. A new paradigm for koala conservation in SE Queensland: Establishment of a living koala genome bank. In Proceedings of the Queensland Environmental Law Association 2018 Conference, Gold Coast, QLD, Australia, 23–25 May 2018; pp. 23–25. [Google Scholar]
- Johnston, S.; Mucci, A.; Ellis, W. The role of captive koalas in koala conservation. In Conserving Central Queensland’s Koalas; Flint, N., Melzer, A., Eds.; Central Queensland University: Rockhampton, QLD, Autrralia, 2013; pp. 54–59. [Google Scholar]
- Brandies, P.A.; Grueber, C.E.; Ivy, J.A.; Hogg, C.J.; Belov, K. Disentangling the mechanisms of mate choice in a captive koala population. PeerJ 2018, 6, e5438. [Google Scholar] [CrossRef]
- Comizzoli, P.; Crosier, A.E.; Songsasen, N.; Gunther, M.S.; Howard, J.G.; Wildt, D.E. Advances in reproductive science for wild carnivore conservation. Reprod. Domest. Anim. 2009, 44, 47–52. [Google Scholar] [CrossRef]
- Waugh, C.; Hanger, J.; Timms, P.; Polkinghorne, A. Koala translocations and Chlamydia: Managing risk in the effort to conserve native species. Biol. Conserv. 2016, 197, 247–253. [Google Scholar] [CrossRef]
- Howell, L.G.; Witt, R.R. Emerging arguments for assisted reproductive technologies in wildlife and their implications for the assisted breeding of koalas and other managed marsupials. Theriogenology 2022. in preparation. [Google Scholar]
- Patil, S.; Majumdar, B.; Awan, K.H.; Sarode, G.S.; Sarode, S.C.; Gadbail, A.R.; Gondivkar, S. Cancer oriented biobanks: A comprehensive review. Oncol. Rev. 2018, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, L.F.; Gregusson, S.; Guldbrandtsen, B.; Hiemstra, S.J.; Hveem, K.; Kantanen, J.; Lohi, H.; Stroemstedt, L.; Berg, P. Domesticated animal biobanking: Land of opportunity. PLoS Biol. 2016, 14, e1002523. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, H.D. Biobanking genetic material for agricultural animal species. Annu. Rev. Anim. Biosci. 2018, 6, 69–82. [Google Scholar] [CrossRef]
- Larson, L.L.; Ball, P.J.H. Regulation of estrous cycles in dairy cattle: A review. Theriogenology 1992, 38, 255–267. [Google Scholar] [CrossRef]
- Mapletoft, R.J.; Hasler, J.F. Assisted reproductive technologies in cattle: A review. Rev. Sci. Tech. 2005, 24, 393–403. [Google Scholar] [CrossRef]
- Silversides, F.G.; Purdy, P.H.; Blackburn, H.D. Comparative costs of programmes to conserve chicken genetic variation based on maintaining living populations or storing cryopreserved material. Br. Poult. Sci. 2012, 53, 599–607. [Google Scholar] [CrossRef]
- Asdal, Å.; Guarino, L. The Svalbard global seed vault: 10 years—1 million samples. Biopreserv. Biobank. 2018, 16, 391–392. [Google Scholar] [CrossRef]
- Mintzer, J.L.; Kronenthal, C.J.; Kelly, V.; Seneca, M.; Butler, G.; Fecenko-Tacka, K.; Altamuro, D.; Madore, S.J. Preparedness for a natural disaster: How Coriell planned for hurricane Sandy. Biopreserv. Biobank. 2013, 11, 216–220. [Google Scholar] [CrossRef]
- Morrin, H.R.; Robinson, B.A. Sustaining a biobank through a series of earthquake swarms: Lessons learned from our New Zealand experience. Biopreserv. Biobank. 2013, 11, 211–215. [Google Scholar] [CrossRef]
- Della-Togna, G.; Howell, L.G.; Clulow, J.; Langhorne, C.J.; Marcec-Greaves, R.; Calatayud, N.E. Evaluating amphibian biobanking and reproduction for captive breeding programs according to the Amphibian Conservation Action Plan objectives. Theriogenology 2020, 150, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Hodskins, L.G. Annual report on conservation and science Volume 1: Conservation program reports. Bethesda MD Am. Zoo Aquar. Assoc. 1997. Available online: https://link.springer.com/chapter/10.1007/978-3-030-33334-8_15 (accessed on 17 March 2022).
- Seddon, J.M.; Schultz, B. Koala conservation in Queensland, Australia: A role for assisted gene flow for genetic rescue? In Conservation Genetics in Mammals; Ortega, J., Maldonado, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 331–349. [Google Scholar]
- Whiteley, A.R.; Fitzpatrick, S.W.; Funk, W.C.; Tallmon, D.A. Genetic rescue to the rescue. Trends Ecol. Evol. 2015, 30, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.J.; Bilney, R.; Martin, R.W. Population-structure and reproductive status of koalas on Raymond Island, Victoria. Wildl. Res. 1988, 15, 511–514. [Google Scholar] [CrossRef][Green Version]
- Lee, K.E.; Seddon, J.M.; Johnston, S.; FitzGibbon, S.I.; Carrick, F.; Melzer, A.; Bercovitch, F.; Ellis, W. Genetic diversity in natural and introduced island populations of koalas in Queensland. Aust. J. Zool. 2013, 60, 303–310. [Google Scholar] [CrossRef]
- Lunney, D.; O’Neill, L.; Matthews, A.; Sherwin, W.B. Modelling mammalian extinction and forecasting recovery: Koalas at Iluka (NSW, Australia). Biol. Conserv. 2002, 106, 101–113. [Google Scholar] [CrossRef]
- Hayward, M.W.; Kerley, G.I.H. Fencing for conservation: Restriction of evolutionary potential or a riposte to threatening processes? Biol. Conserv. 2009, 142, 1–13. [Google Scholar] [CrossRef]
- Santymire, R.M. Saving the Black-Footed Ferret from Extinction: In Theory and Practice. In Scientific Foundations of Zoos and Aquariums: Their Role in Conservation and Research; Kaufman, A., Bashaw, M., Maple, T., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 440–474. [Google Scholar]
- McLean, N.; Handasyde, K.A. Sexual maturity, factors affecting the breeding season and breeding in consecutive seasons in populations of overabundant Victorian koalas (Phascolarctos cinereus). Aust. J. Zool. 2007, 54, 385–392. [Google Scholar] [CrossRef]
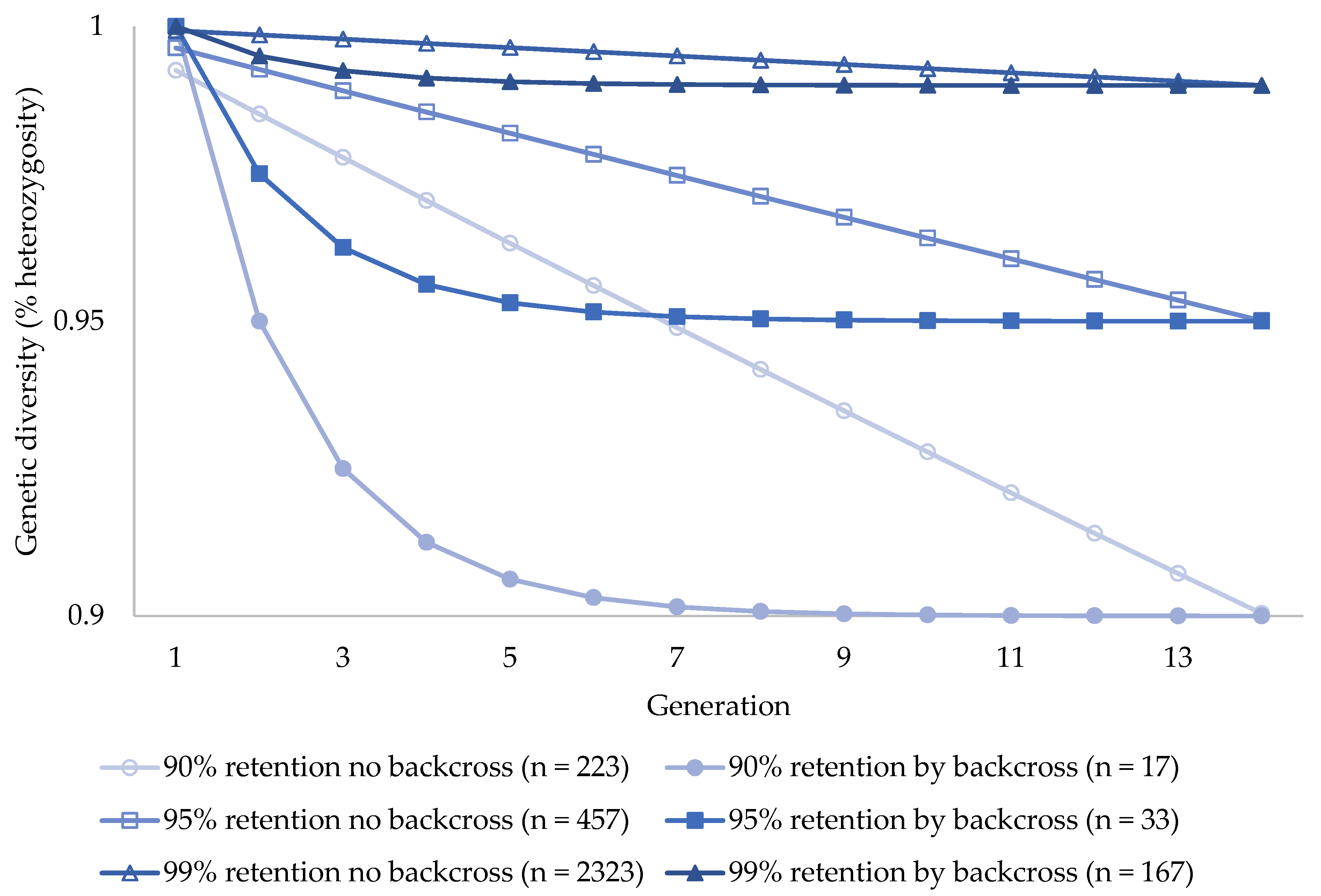
| Backcross Scenario | Ne | N | Ft No Backcross | Ft Backcross | Ht/Ho after 100 Years |
|---|---|---|---|---|---|
| 90% heterozygosity retention with no backcross | 67 | 223 | 0.0996 | n.d | 0.9004 |
| 90% heterozygosity retention by backcrossing every generation (7-year intervals) | n.d | 17 | 0.4999 | 0.1000 | 0.9000 |
| 95% heterozygosity retention with no backcross | 137 | 457 | 0.0499 | n.d | 0.9501 |
| 95% heterozygosity retention by backcrossing every generation (7-year intervals) | n.d | 33 | 0.4999 | 0.0500 | 0.9500 |
| 99% heterozygosity retention with no backcross | 697 | 2323 | 0.0100 | n.d | 0.9900 |
| 99% heterozygosity retention by backcrossing every generation (7-year intervals) | n.d | 167 | 0.4999 | 0.0100 | 0.9900 |
| Backcross Scenario | N | Cost ($) Year 1 | Cost ($) Year 2 | 100-Year Captive Colony Costs ($) | 100-Year Back-Cross Costs ($) | 100-Year Program Costs ($) |
|---|---|---|---|---|---|---|
| 90% heterozygosity retention with no backcross | 223 | A$5,285,556 | A$2,576,923 | A$73,082,167 | n.d | A$73,082,167 |
| 90% heterozygosity retention by backcrossing every generation (7-year intervals) | 17 | A$904,505 | A$350,626 | A$5,453,893 | A$5,158,118 | A$10,612,011 |
| 95% heterozygosity retention with no backcross | 457 | A$10,807,778 | A$5,269,231 | A$149,436,671 | n.d | A$149,436,671 |
| 95% heterozygosity retention by backcrossing every generation (7-year intervals) | 33 | A$1,298,950 | A$542,934 | A$10,907,786 | A$5,158,118 | A$16,065,904 |
| 99% heterozygosity retention with no backcross | 2323 | A$54,985,556 | A$26,807,692 | A$760,272,697 | n.d | A$760,272,697 |
| 99% heterozygosity retention by backcrossing every generation (7-year intervals) | 167 | A$4,454,505 | A$2,081,395 | A$54,538,931 | A$5,158,118 | A$59,697,049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howell, L.G.; Johnston, S.D.; O’Brien, J.K.; Frankham, R.; Rodger, J.C.; Ryan, S.A.; Beranek, C.T.; Clulow, J.; Hudson, D.S.; Witt, R.R. Modelling Genetic Benefits and Financial Costs of Integrating Biobanking into the Captive Management of Koalas. Animals 2022, 12, 990. https://doi.org/10.3390/ani12080990
Howell LG, Johnston SD, O’Brien JK, Frankham R, Rodger JC, Ryan SA, Beranek CT, Clulow J, Hudson DS, Witt RR. Modelling Genetic Benefits and Financial Costs of Integrating Biobanking into the Captive Management of Koalas. Animals. 2022; 12(8):990. https://doi.org/10.3390/ani12080990
Chicago/Turabian StyleHowell, Lachlan G., Stephen D. Johnston, Justine K. O’Brien, Richard Frankham, John C. Rodger, Shelby A. Ryan, Chad T. Beranek, John Clulow, Donald S. Hudson, and Ryan R. Witt. 2022. "Modelling Genetic Benefits and Financial Costs of Integrating Biobanking into the Captive Management of Koalas" Animals 12, no. 8: 990. https://doi.org/10.3390/ani12080990
APA StyleHowell, L. G., Johnston, S. D., O’Brien, J. K., Frankham, R., Rodger, J. C., Ryan, S. A., Beranek, C. T., Clulow, J., Hudson, D. S., & Witt, R. R. (2022). Modelling Genetic Benefits and Financial Costs of Integrating Biobanking into the Captive Management of Koalas. Animals, 12(8), 990. https://doi.org/10.3390/ani12080990







