Associations of Protein Molecular Structures with Their Nutrient Supply and Biodegradation Characteristics in Different Byproducts of Seed-Used Pumpkin
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Chemical Analysis
2.3. In Situ Rumen Degradation
2.4. Spectral Data Collection and Analysis
2.5. Statistical Analyses
3. Results
3.1. Chemical Composition and CNCPS Protein Components of Seed-Used Pumpkin Byproducts
3.2. Rumen Degradation Parameters of DM and CP of Seed-Used Pumpkin Byproducts
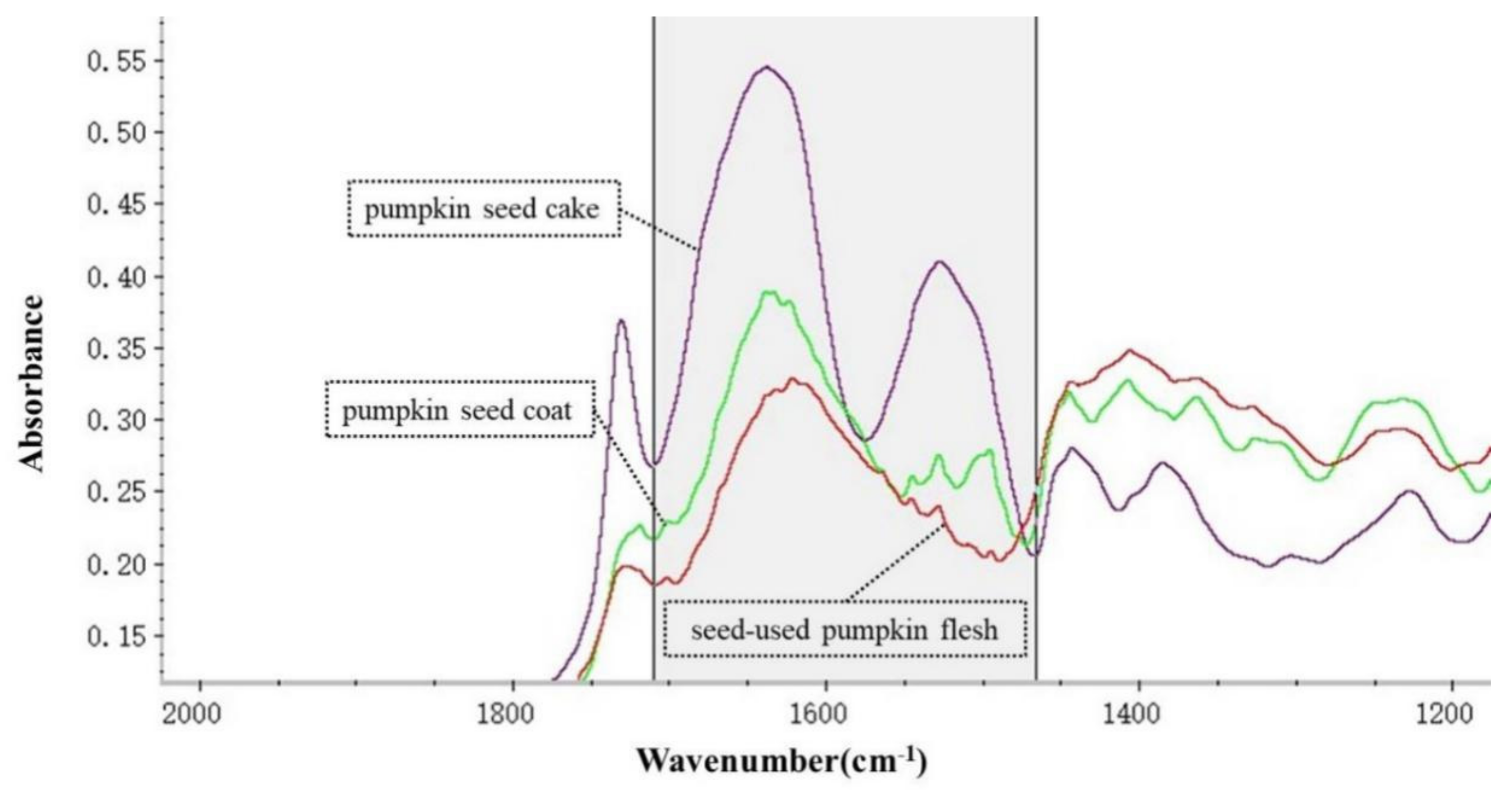
3.3. Spectral Parameters of the Protein Molecular Structure of Seed-Used Pumpkin Byproducts
3.4. Correlations between the Protein Molecular Structure of Seed-Used Pumpkin Byproducts and Their Conventional Chemical Components, CNCPS Protein Components, and Rumen Degradation Characteristics
3.5. Regression Relationship between the Protein Molecular Structure of Seed-Used Pumpkin Byproducts and Their Nutritional Value and Rumen Degradation Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Yao, E.Y.; Zhang, G.N.; Zhang, Y.G. Feasibility analysis of by-products of seed pumpkin as a feedstuff source for ruminants. Chin. J. Anim. Nutr. 2019, 31, 994–1000. [Google Scholar]
- Keller, M.; Reidy, B.; Scheurer, A.; Eggerschwiler, L.; Morel, I.; Giller, K. Soybean meal can be replaced by faba beans, pumpkin seed cake, spirulina or be completely omitted in a forage-based diet for fattening bulls to achieve comparable performance, carcass and meat quality. Animals 2021, 11, 1588. [Google Scholar] [CrossRef] [PubMed]
- Fung, L.; Urriola, P.E.; Baker, L.; Shurson, G.C. Estimated energy and nutrient composition of different sources of food waste and their potential for use in sustainable swine feeding programs. Transl. Anim. Sci. 2019, 3, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Klir, Z.; Castro-Montoya, J.M.; Novoselec, J.; Molkentin, J.; Domacinovic, M.; Mioc, B.; Dickhoefer, U.; Antunovic, Z. Influence of pumpkin seed cake and extruded linseed on milk production and milk fatty acid profile in Alpine goats. Animal 2017, 11, 1772–1778. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.Q.; Lyu, Y.; Bi, J.F.; Wu, X.Y.; Jin, X.; Qiao, Y.N.; Hou, H.N.; Lyu, C.M. Quality assessment and variety classification of seed-used pumpkin by-products: Potential values to deep processing. Food Sci. Nutr. 2019, 7, 4095–4104. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Y.; Bi, J.F.; Chen, Q.Q.; Wu, X.Y.; Qiao, Y.N.; Hou, H.N.; Zhang, X. Bioaccessibility of carotenoids and antioxidant capacity of seed-used pumpkin byproducts powders as affected by particle size and corn oil during in vitro digestion process. Food Chem. 2021, 343, 128541. [Google Scholar] [CrossRef]
- Dou, X.J.; Ma, Z.W.; Yan, D.; Gao, N.; Li, Z.Y.; Li, Y.; Feng, X.J.; Meng, L.X.; Shan, A.S. Sodium butyrate alleviates intestinal injury and microbial flora disturbance induced by lipopolysaccharides in rats. Food Funct. 2022, 13, 1360–1369. [Google Scholar] [CrossRef]
- Xin, H.S.; Ding, X.; Zhang, L.Y.; Wang, X.F.; Zhang, Y.G.; Sun, F. Investigation of the spectroscopic information on functional groups related to carbohydrates in different morphological fractions of corn stover and their relationship to nutrient supply and biodegradation characteristics. J. Agric. Food Chem. 2017, 65, 4035–4043. [Google Scholar] [CrossRef]
- Prates, L.L.; Lei, Y.G.; Refat, B.; Zhang, W.X.; Yu, P.Q. Effects of heat processing methods on protein subfractions and protein degradation kinetics in dairy cattle in relation to protein molecular structure of barley grain using advanced molecular spectroscopy. J. Cereal Sci. 2018, 80, 212–220. [Google Scholar] [CrossRef]
- Shi, H.T.; Yu, P.Q. Comparison of grating-based near-infrared (NIR) and Fourier transform mid-infrared (ATR-FT/MIR) spectroscopy based on spectral preprocessing and wavelength selection for the determination of crude protein and moisture content in wheat. Food Control 2017, 82, 57–65. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, W.; Zhang, B.; Du, C.G.; Wei, N.N.; Liang, D.; Sun, K.; Tu, K.; Peng, J.; Pan, L.Q. Determination of total protein and wet gluten in wheat flour by Fourier transform infrared photoacoustic spectroscopy with multivariate analysis. J. Food Compos. Anal. 2021, 106, 104349. [Google Scholar] [CrossRef]
- Guevara Oquendo, V.H.; Rodriguez Espinosa, M.E.; Yu, P.Q. Research progress on faba bean and faba forage in food and feed types, physiochemical, nutritional, and molecular structural characteristics with molecular spectroscopy. Crit. Rev. Food Sci. Nutr. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jonker, A.; Gruber, M.Y.; Wang, Y.; Coulman, B.; Mckinnon, J.J.; Christensen, D.A.; Yu, P. Foam stability of leaves from anthocyanidin-accumulating Lc-alfalfa and relation to molecular structures detected by fourier-transformed infrared-vibration spectroscopy. Grass Forage Sci. 2012, 67, 369–381. [Google Scholar] [CrossRef]
- Abeysekara, S.; Christensen, D.A.; Yu, P.Q. Characterizations of structural, biochemical, and nutritive profiles in silage among cool-season corn cultivars in relation to heat units (aCHU, dCHU) with curvilinear response and multivariate analyses. J. Agric. Food Chem. 2013, 61, 12315–12326. [Google Scholar] [CrossRef]
- Xin, H.S.; Qu, Y.L.; Wu, H.N.; Yu, P.Q.; Zhang, Y.G. Univariate and multi-variate comparisons of protein and carbohydrate molecular structural conformations and their associations with nutritive factors in typical by-products. J. Sci. Food Agric. 2016, 96, 4736–4748. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysts, 17th ed.; AOAC International: Arlington, VA, USA, 2000; Volume 1. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Soest, P. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Sniffen, C.J.; O’Connor, J.D.; Soest, P.J.V.; Fox, D.G.; Russell, J.B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef]
- Zhang, G.N.; Li, Y.; Zhao, C.; Fang, X.P.; Zhang, Y.G. Effect of substituting wet corn gluten feed and corn stover for alfalfa hay in total mixed ration silage on lactation performance in dairy cows. Animal 2021, 15, 100013. [Google Scholar] [CrossRef]
- Ørskov, E.R.; Debhovell, F.D.; Mould, F. The use of the nylon bag technique for the evaluation of feedstuffs. Trop. Anim. Prod. 1980, 5, 195–213. [Google Scholar]
- Cameron, D.G.; Moffatt, D.J.; Mantsch, H.H.; Kauppinen, J.K. Fourier self-deconvolution: A method for resolving intrinsically overlapped bands. Appl. Spectrosc. 1981, 35, 271–276. [Google Scholar]
- Li, X.X.; Zhang, Y.G.; Yu, P.Q. Association of bioenergy processing-induced protein molecular structure changes with CNCPS-based protein degradation and digestion of coproducts in dairy cows. J. Agric. Food Chem. 2016, 64, 4086–4094. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.A. Understanding the milk-to-feed price ratio as a proxy for dairy farm profitability. J. Dairy Sci. 2010, 93, 4942–4948. [Google Scholar] [CrossRef]
- Hao, X.Y.; Yu, S.C.; Mu, C.T.; Wu, X.D.; Zhang, J.X. Replacing soybean meal with flax seed meal: Effects on nutrient digestibility, rumen microbial protein synthesis and growth performance in sheep. Animal 2020, 14, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, G.N.; Fang, X.P.; Zhao, C.; Wu, H.Y.; Lan, Y.X.; Che, L.; Sun, Y.K.; Lv, J.Y.; Zhang, Y.G. Effects of replacing soybean meal with pumpkin seed cake and dried distillers grains with solubles on milk performance and antioxidant functions in dairy cows. Animal 2021, 15, 100004. [Google Scholar] [CrossRef] [PubMed]
- Greiling, A.M.; Schwarz, C.; Gierus, M.; Rodehutscord, M. Pumpkin seed cake as a fishmeal substitute in fish nutrition: Effects on growth performance, morphological traits and fillet colour of two freshwater salmonids and two catfish species. Arch. Anim. Nutr. 2018, 72, 239–259. [Google Scholar] [CrossRef]
- Antunović, Z.; Klir, Ž.; Šperanda, M.; Sičaja, V.; Čolović, D.; Mioč, B.; Novoselec, J. Partial replacement of soybean meal with pumpkin seed cake in lamb diets: Effects on carcass traits, haemato-chemical parameters and fatty acids in meat. S. Afr. J. Anim. Sci. 2018, 48, 695–704. [Google Scholar] [CrossRef]
- Chrenková, M.; Cerešnáková, Z.; Weisbjerg, M.R.; Formelová, Z.; Vondráková, M. Characterization of proteins in feeds according to the CNCPS and comparison to in situ parameters. Czech J. Anim. Sci. 2014, 59, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Kleinschmit, D.H.; Anderson, J.L.; Schingoethe, D.J.; Kalscheur, K.F.; Hippen, A.R. Ruminal and intestinal degradability of distillers grains plus solubles varies by source. J. Dairy Sci. 2007, 90, 2909–2918. [Google Scholar] [CrossRef]
- Doiron, K.; Yu, P.Q.; Mckinnon, J.J.; Christensen, D.A. Heat-induced protein structure and subfractions in relation to protein degradation kinetics and intestinal availability in dairy cattle. J. Dairy Sci. 2009, 92, 3319–3330. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.Q.; Nuez-Ortín, W.G. Relationship of protein molecular structure to metabolisable proteins in different types of dried distillers grains with solubles: A novel approach. Br. J. Nutr. 2010, 104, 1429–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbonaro, M.; Nucara, A. Secondary structure of food proteins by Fourier transform spectroscopy in the mid-infrared region. Amino Acids 2010, 38, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Theodoridou, K.; Yu, P.Q. Application potential of ATR-FT/IR molecular spectroscopy in animal nutrition: Revelation of protein molecular structures of canola meal and presscake, as affected by heat-processing methods, in relationship with their protein digestive behavior and utiliz. J. Agric. Food Chem. 2013, 61, 5449–5458. [Google Scholar] [CrossRef] [PubMed]
- Damiran, D.; Yu, P.Q. Molecular basis of structural makeup of hulless barley in relation to rumen degradation kinetics and intestinal availability in dairy cattle: A novel approach. J. Dairy Sci. 2011, 94, 5151–5159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samadi; Yu, P.Q. Dry and moist heating-induced changes in protein molecular structure, protein subfraction, and nutrient profiles in soybeans. J. Dairy Sci. 2011, 94, 6092–6102. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.S.; Yu, P.Q. Detect changes in lipid-related structure of brown- and yellow-seeded Brassica Carinata seed during rumen fermentation in relation to basic chemical profile using ATR-FT/IR molecular spectroscopy with chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.S.; Khan, N.A.; Sun, K.J.; Sun, F.; Hu, G.; Rahman, S.U.; Fu, Q.; Li, Y.; Zhang, Y.G.; Hu, G.H. Batch-to-batch variation in protein molecular structures, nutritive value and ruminal metabolism in corn coproducts. Anim. Feed Sci. Technol. 2020, 263, 114428. [Google Scholar] [CrossRef]
- Yan, X.G.; Khan, N.A.; Zhang, F.Y.; Ling, Y.; Yu, P.Q. Microwave irradiation induced changes in protein molecular structures of barley grains: Relationship to changes in protein chemical profile, protein subfractions, and digestion in dairy cows. J. Agric. Food Chem. 2014, 62, 6546–6555. [Google Scholar] [CrossRef]
- Yu, P.Q.; Mckinnon, J.J.; Christensen, C.R.; Christensen, D.A.; Marinkovic, N.S.; Miller, L.M. Chemical imaging of microstructures of plant tissues within cellular dimension using synchrotron infrared microspectroscopy. J. Agric. Food Chem. 2003, 51, 6062–6067. [Google Scholar] [CrossRef]
| Pumpkin Seed Cake | Pumpkin Seed Coat | Seed-Used Pumpkin Flesh | SEM 1 | p | |
|---|---|---|---|---|---|
| Chemical composition (g/100 g DM) 2 | |||||
| DM | 95.36 a | 87.84 c | 89.52 b | 0.016 | <0.0001 |
| CP | 55.31 a | 16.26 b | 11.40 c | 0.386 | <0.0001 |
| SCP | 11.98 a | 3.55 c | 6.51 b | 0.337 | <0.0001 |
| NPN | 4.17 b | 2.25 c | 5.48 a | 0.377 | 0.0027 |
| ADICP | 0.18 c | 8.10 a | 0.87 b | 0.083 | <0.0001 |
| NDICP | 6.82 b | 9.92 a | 2.64 c | 0.363 | <0.0001 |
| subfractions of protein (g/100 g CP, using the CNCPS) 3 | |||||
| PA | 7.53 c | 13.82 b | 47.92 a | 1.453 | <0.0001 |
| PB1 | 14.13 a | 8.01 b | 9.09 b | 1.312 | 0.0347 |
| PB2 | 66.02 a | 17.15 b | 19.77 b | 0.958 | <0.0001 |
| PB3 | 12.01 | 11.18 | 15.59 | 1.276 | 0.1042 |
| PC | 0.32 c | 49.84 a | 7.64 b | 0.614 | <0.0001 |
| Pumpkin Seed Cake | Pumpkin Seed Coat | Seed-Used Pumpkin Flesh | SEM 1 | p | |
|---|---|---|---|---|---|
| in situ dry matter (DM) rumen degradation kinetics 2 | |||||
| a (g/kg) | 565 b | 235 c | 596 a | 5.8 | <0.0001 |
| b (g/kg) | 391 a | 140 b | 385 a | 9.6 | <0.0001 |
| a + b (g/kg) | 957 b | 375 c | 982 a | 6.0 | <0.0001 |
| c (g/kg h−1) | 142 a | 88.5 c | 109 b | 3.77 | 0.0002 |
| ED (g/kg) | 840 a | 377 b | 844 a | 3.6 | <0.0001 |
| in situ dry matter (CP) rumen degradation kinetics | |||||
| a (g/kg) | 510 b | 271 c | 566 a | 6.4 | <0.0001 |
| b (g/kg) | 441 a | 122 c | 398 b | 10.5 | <0.0001 |
| a + b (g/kg) | 951 a | 394 b | 964 a | 6.3 | <0.0001 |
| c (g/kg h−1) | 174 a | 51.5 c | 108 b | 6.23 | <0.0001 |
| ED (g/kg) | 837 a | 336 c | 822 b | 3.3 | <0.0001 |
| Pumpkin Seed Cake | Pumpkin Seed Coat | Seed-Used Pumpkin Flesh | SEM 1 | p | |
|---|---|---|---|---|---|
| molecular spectral features 2 | |||||
| A_Amide I | 26.6 a | 11.0 b | 12.5 b | 1.54 | 0.0007 |
| A_Amide II | 14.0 a | 2.56 b | 0.70 b | 0.77 | <0.0001 |
| A_Amide I + II | 40.6 a | 13.6 b | 13.2 b | 2.28 | 0.0002 |
| H_Amide I | 0.33 a | 0.13 b | 0.13 b | 0.019 | 0.0004 |
| H_Amide II | 0.21 a | 0.05 b | 0.03 b | 0.012 | <0.0001 |
| H_α-helix | 0.32 a | 0.09 b | 0.12 b | 0.018 | 0.0002 |
| H_β-sheet | 0.28 a | 0.13 b | 0.13 b | 0.017 | 0.001 |
| spectral ratio profiles | |||||
| A_Amide I/Amide II ratio | 1.90 c | 4.29 b | 18.03 a | 0.394 | <0.0001 |
| H_Amide I/Amide II ratio | 1.57 c | 2.59 b | 4.28 a | 0.175 | 0.0001 |
| H_α-helix/β-sheet ratio | 1.14 a | 0.71 c | 0.97 b | 0.007 | <0.0001 |
| A_Amide I | A_Amide II | H_Amide I | H_Amide II | H_α-Helix | H_β-Sheet | A_Amide I + II | A_Amide I/Amide II Ratio | H_Amide I/Amide II Ratio | H_α-Helix/β-Sheet Ratio | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| protein chemical profiles 2 (g/100g DM−1) | ||||||||||||||||||||
| DM | −0.70 | 0.04 | −0.56 | 0.12 | −0.64 | 0.06 | −0.17 | 0.66 | −0.64 | 0.06 | −0.58 | 0.10 | 0.06 | 0.87 | −0.74 | 0.02 | −0.63 | 0.07 | −0.98 | <0.0001 |
| CP | 0.95 | 0.0001 | 0.99 | <0.0001 | 0.96 | <0.0001 | 0.98247 | <0.0001 | 0.95 | <0.0001 | 0.95 | <0.0001 | 0.97 | <0.0001 | −0.69 | 0.04 | −0.83 | 0.006 | 0.73 | 0.03 |
| SCP | 0.95 | 0.0001 | 0.88 | 0.002 | 0.92 | 0.0004 | 0.89171 | 0.001 | 0.97 | <0.0001 | 0.92 | 0.001 | 0.92 | 0.0004 | −0.30 | 0.43 | −0.51 | 0.16 | 0.94 | 0.0002 |
| NPN | 0.26 | 0.49 | 0.03 | 0.95 | 0.17 | 0.66 | 0.05868 | 0.88 | 0.29 | 0.46 | 0.19 | 0.62 | 0.16 | 0.69 | 0.66 | 0.05 | 0.47 | 0.20 | 0.62 | 0.08 |
| ADICP | −0.61 | 0.08 | −0.45 | 0.23 | −0.54 | 0.14 | −0.4679 | 0.20 | −0.65 | 0.06 | −0.53 | 0.14 | -0.54 | 0.13 | −0.30 | 0.43 | −0.06 | 0.88 | −0.95 | 0.0001 |
| NDICP | 0.01 | 0.98 | 0.22 | 0.58 | 0.10 | 0.80 | 0.1898 | 0.62 | −0.03 | 0.94 | 0.10 | 0.80 | 0.10 | 0.79 | −0.82 | 0.006 | −0.65 | 0.06 | −0.52 | 0.15 |
| protein fractions partitioned by the CNCPS 3 (g/100g CP−1) | ||||||||||||||||||||
| PA | −0.50 | 0.17 | −0.69 | 0.04 | −0.58 | 0.10 | −0.67 | 0.05 | −0.48 | 0.19 | −0.57 | 0.11 | −0.59 | 0.09 | 0.99 | <0.0001 | 0.94 | 0.0002 | −0.03 | 0.94 |
| PB1 | 0.75 | 0.02 | 0.77 | 0.02 | 0.75 | 0.02 | 0.77 | 0.02 | 0.77 | 0.02 | 0.73 | 0.03 | 0.76 | 0.02 | −0.39 | 0.30 | −0.53 | 0.14 | 0.74 | 0.02 |
| PB2 | 0.94 | 0.0001 | 0.96 | <0.0001 | 0.95 | <0.0001 | 0.96 | <0.0001 | 0.96 | <0.0001 | 0.94 | 0.0002 | 0.96 | <0.0001 | −0.57 | 0.11 | −0.74 | 0.02 | 0.83 | 0.006 |
| PB3 | −0.20 | 0.61 | −0.32 | 0.40 | −0.26 | 0.51 | −0.31 | 0.41 | −0.15 | 0.70 | −0.25 | 0.52 | −0.25 | 0.51 | 0.70 | 0.04 | 0.64 | 0.06 | 0.22 | 0.56 |
| PC | −0.65 | 0.06 | −0.50 | 0.17 | −0.58 | 0.10 | −0.52 | 0.15 | −0.69 | 0.04 | −0.58 | 0.10 | −0.59 | 0.10 | −0.24 | 0.53 | 0.00 | 0.99 | −0.96 | <0.0001 |
| in situ DM rumen degradation kinetics 4 | ||||||||||||||||||||
| a (g/kg) | 0.49 | 0.18 | 0.31 | 0.42 | 0.41 | 0.27 | 0.33 | 0.38 | 0.53 | 0.1386 | 0.41 | 0.27 | 0.41 | 0.27 | 0.45 | 0.23 | 0.21 | 0.58 | 0.88 | 0.002 |
| b (g/kg) | 0.55 | 0.13 | 0.39 | 0.30 | 0.48 | 0.19 | 0.41 | 0.27 | 0.59 | 0.0925 | 0.47 | 0.20 | 0.48 | 0.19 | 0.35 | 0.35 | 0.11 | 0.78 | 0.92 | 0.0004 |
| a + b (g/kg) | 0.52 | 0.15 | 0.34 | 0.37 | 0.44 | 0.23 | 0.37 | 0.33 | 0.56 | 0.1161 | 0.44 | 0.24 | 0.44 | 0.23 | 0.41 | 0.28 | 0.17 | 0.66 | 0.90 | 0.0008 |
| c (g/kg h−1) | 0.91 | 0.0006 | 0.85 | 0.004 | 0.89 | 0.001 | 0.86 | 0.003 | 0.93 | 0.0003 | 0.88 | 0.002 | 0.89 | 0.001 | −0.27 | 0.48 | −0.48 | 0.19 | 0.93 | 0.0003 |
| ED (g/kg) | 0.54 | 0.13 | 0.37 | 0.32 | 0.47 | 0.20 | 0.39 | 0.29 | 0.59 | 0.0971 | 0.47 | 0.21 | 0.47 | 0.20 | 0.38 | 0.31 | 0.14 | 0.71 | 0.92 | 0.0005 |
| in situ CP rumen degradation kinetics | ||||||||||||||||||||
| a (g/kg) | 0.40 | 0.28 | 0.21 | 0.59 | 0.32 | 0.40 | 0.23 | 0.55 | 0.45 | 0.23 | 0.32 | 0.40 | 0.32 | 0.40 | 0.54 | 0.14 | 0.31 | 0.42 | 0.83 | 0.006 |
| b (g/kg) | 0.62 | 0.07 | 0.48 | 0.19 | 0.56 | 0.12 | 0.50 | 0.17 | 0.67 | 0.05 | 0.55 | 0.12 | 0.56 | 0.11 | 0.26 | 0.51 | 0.01 | 0.97 | 0.96 | <0.0001 |
| a + b (g/kg) | 0.53 | 0.14 | 0.36 | 0.35 | 0.45 | 0.22 | 0.38 | 0.32 | 0.57 | 0.11 | 0.45 | 0.22 | 0.45 | 0.22 | 0.39 | 0.29 | 0.16 | 0.69 | 0.91 | 0.0006 |
| c (g/kg h-1) | 0.89 | 0.001 | 0.81 | 0.008 | 0.86 | 0.003 | 0.82 | 0.006 | 0.92 | 0.0005 | 0.85 | 0.004 | 0.86 | 0.003 | −0.18 | 0.64 | −0.40 | 0.28 | 0.97 | <0.0001 |
| ED (g/kg) | 0.57 | 0.11 | 0.40 | 0.28 | 0.50 | 0.17 | 0.42 | 0.26 | 0.61 | 0.08 | 0.49 | 0.18 | 0.50 | 0.17 | 0.35 | 0.36 | 0.11 | 0.78 | 0.93 | 0.0003 |
| Predicted Variable (Y) 1 | Variable in the Model with p < 0.05 2 | Prediction Equations: Y = a + b1x1 + b2x2···..... | Model R2 Value | RSD 3 | p-Value |
|---|---|---|---|---|---|
| Protein chemical profiles | |||||
| DM | H_amide I/amide II and H_α-helix/β-sheet left in the model | Y = 110.289 − 0.550 H_amide I/amide II − 18.934 H_α-helix/β-sheet | 0.995 | 0.0701 | <0.0001 |
| CP (g/100g DM−1) | A_amide II left in the model | Y = 9.007 + 3.239 A_amide II | 0.973 | 13.523 | <0.0001 |
| SCP (g/100g DM−1) | H_α-helix/β-sheet and H_α-helix left in the model | Y = −4.673 + 8.954 H_α-helix/β-sheet + 20.067 H_α-helix | 0.993 | 0.135 | <0.0001 |
| ADICP (g/100g DM−1) | H_α-helix/β-sheet left in the model | Y = 21.250 − 19.319 H_α-helix/β-sheet | 0.896 | 1.714 | 0.0001 |
| NDICP (g/100g DM−1) | A_amide I/amide II left in the model | Y = 9.285 − 0.350 A_amide I/amide II | 0.678 | 3.799 | 0.0064 |
| protein subfractions using the CNCPS system | |||||
| PA (g/100g CP) | A_amide I/amide II left in the model | Y = 3.056 + 2.481 A_amide I/amide II | 0.980 | 8.066 | <0.0001 |
| PB1 (g/100g CP) | H_α-helix left in the model | Y = 6.149 + 23.857 H_α-helix | 0.588 | 5.594 | 0.0159 |
| PB2 (g/100g CP) | H_α-helix/β-sheet and A_amide II left in the model | Y = −18.791 + 2.812 A_amide II-39.182 H_α-helix/β-sheet | 0.972 | 21.238 | <0.0001 |
| PB3 (g/100g CP) | A_amide I/amide II left in the model | Y = 10.852 + 0.257 A_amide I/amide II | 0.484 | 4.589 | 0.0374 |
| PC (g/100g CP) | A_amide I/amide II and H_α-helix/β-sheet left in the model | Y = 138.971 − 120.225 H_α-helix/β-sheet-0.797 A_amide I/amide II | 0.996 | 3.209 | <0.0001 |
| in situ DM rumen degradation kinetics | |||||
| a (g/kg) | A_amide I/amide II and H_α-helix/β-sheet left in the model | Y = −40.263 + 1.058 A_amide I/amide II + 83.074 H_α-helix/β-sheet | 0.994 | 2.534 | <0.0001 |
| b (g/kg) | H_α-helix/β-sheet left in the model | Y = −27.820 + 61.952 H_α-helix/β-sheet | 0.852 | 26.485 | 0.0004 |
| a + b (g/kg) | A_amide I/amide II and H_α-helix/β-sheet left in the model | Y = −73.346 + 1.664 A_amide I/amide II + 145.417 H_α-helix/β-sheet | 0.996 | 4.354 | <0.0001 |
| c (g/kg h−1) | H_amide I and H_α-helix/β-sheet left in the model | Y = 1.951 + 10.091 H_amide I + 7.775 H_α-helix/β-sheet | 0.953 | 0.363 | 0.0001 |
| ED (g/kg) | A_amide I/amide II and H_α-helix/β-sheet left in the model | Y = −63.890 + 1.350 A_amide I/amide II + 127.419 H_α-helix/β-sheet | 0.996 | 3.289 | <0.0001 |
| in situ CP rumen degradation kinetics | |||||
| a (g/kg) | A_amide I/amide II and H_α-helix/β-sheet left in the model | Y = −20.660 + 0.987 A_amide I/amide II + 61.147 H_α-helix/β-sheet | 0.989 | 2.704 | <0.0001 |
| b (g/kg) | H_α-helix/β-sheet left in the model | Y = −40.841 + 77.347 H_α-helix/β-sheet | 0.918 | 21.107 | <0.0001 |
| a + b (g/kg) | A_amide I/amide II and H_α-helix/β-sheet left in the model | Y = −66.178 + 1.525 A_amide I/amide II + 138.842 H_α-helix/β-sheet | 0.997 | 3.133 | <0.0001 |
| c (g/kg h−1) | H_amide I and H_α-helix/β-sheet left in the model | Y = −12.010 + 16.295 H_amide I + 21.082 H_α-helix/β-sheet | 0.976 | 0.924 | <0.0001 |
| ED (g/kg) | A_amide I/amide II and H_α-helix/β-sheet left in the model | Y = −59.821 + 1.193 A_amide I/amide II + 123.848 H_α-helix/β-sheet | 0.996 | 2.981 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, Q.; Lv, J.; Jia, X.; Gao, J.; Zhang, Y.; Wang, L. Associations of Protein Molecular Structures with Their Nutrient Supply and Biodegradation Characteristics in Different Byproducts of Seed-Used Pumpkin. Animals 2022, 12, 956. https://doi.org/10.3390/ani12080956
Li Y, Wu Q, Lv J, Jia X, Gao J, Zhang Y, Wang L. Associations of Protein Molecular Structures with Their Nutrient Supply and Biodegradation Characteristics in Different Byproducts of Seed-Used Pumpkin. Animals. 2022; 12(8):956. https://doi.org/10.3390/ani12080956
Chicago/Turabian StyleLi, Yang, Qinghua Wu, Jingyi Lv, Xiaoman Jia, Jianxu Gao, Yonggen Zhang, and Liang Wang. 2022. "Associations of Protein Molecular Structures with Their Nutrient Supply and Biodegradation Characteristics in Different Byproducts of Seed-Used Pumpkin" Animals 12, no. 8: 956. https://doi.org/10.3390/ani12080956
APA StyleLi, Y., Wu, Q., Lv, J., Jia, X., Gao, J., Zhang, Y., & Wang, L. (2022). Associations of Protein Molecular Structures with Their Nutrient Supply and Biodegradation Characteristics in Different Byproducts of Seed-Used Pumpkin. Animals, 12(8), 956. https://doi.org/10.3390/ani12080956





