Influence of Condensed and Hydrolysable Tannins on the Bacterial Community, Protein Degradation, and Fermentation Quality of Alfalfa Silage
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fresh Alfalfa and Ensiling
2.2. Fermentation Characteristics and Chemical Composition
2.3. Bacterial Community Composition
2.4. Statistical Analysis
3. Results
3.1. The Chemical Composition and Epiphytic Microflora of Wilted Alfalfa
3.2. Silage Bacterial Community
3.3. Fermentation Characteristics and Chemical Composition of Alfalfa Silages Ensiled for 60 d
3.4. Correlation between the Microorganism and Fermentation Parameters
4. Discussion
4.1. Diversity and Composition of Bacterial Community
4.2. Fermentation Characteristics of Alfalfa Silage
4.3. Nitrogen Components and Protease Activity
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohshima, M.; McDonald, P. A review of the changes in nitrogenous compounds of herbage during ensilage. J. Sci. Food Agric. 1978, 29, 497–505. [Google Scholar] [CrossRef]
- Getachew, G.; Depeters, E.J.; Pittroff, W.; Putnam, D.H.; Dandekar, A.M. REVIEW: Does protein in alfalfa need protection from rumen microbes? Prof. Anim. Sci. 2006, 22, 364–373. [Google Scholar] [CrossRef]
- Nkosi, B.D.; Meeske, R.; Langa, T.; Thomas, R.S. Effects of bacterial silage inoculants on wholecrop maize silage fermentation and silage digestibility in rams. S. Afr. J. Anim. Sci. 2011, 41, 350–359. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Oba, M.; Koenig, K.M.; Zhao, G.Y.; Beauchemin, K.A. Use of gallic acid and hydrolyzable tannins to reduce methane emission and nitrogen excretion in beef cattle fed a diet containing alfalfa silage. J. Anim. Sci. 2019, 97, 2230–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar]
- He, L.; Lv, H.; Xing, Y.; Chen, X.; Zhang, Q. Intrinsic tannins affect ensiling characteristics and proteolysis of Neolamarckia cadamba leaf silage by largely altering bacterial community. Bioresour. Technol. 2020, 311, 123496. [Google Scholar] [CrossRef]
- Aguerre, M.J.; Capozzolo, M.C.; Lencioni, P.; Cabral, C.; Wattiaux, M.A. Effect of quebracho-chestnut tannin extracts at 2 dietary crude protein levels on performance, rumen fermentation, and nitrogen partitioning in dairy cows. J. Dairy Sci. 2016, 99, 4476–4486. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; He, L.; Xing, Y.; Zhou, W.; Yang, F.; Chen, X.; Zhang, Q. Effects of mixing Neolamarckia cadamba leaves on fermentation quality, microbial community of high moisture alfalfa and stylo silage. Microb. Biotechnol. 2019, 12, 869–878. [Google Scholar] [CrossRef] [Green Version]
- Beauchemin, K.A.; Kreuzer, M.; O’Mara, F.; McAllister, T.A. Nutritional management for enteric methane abatement: A review. Aust. J. Exp. Agric. 2008, 48, 21–27. [Google Scholar] [CrossRef]
- Yang, K.; Wei, C.; Zhao, G.Y.; Xu, Z.W.; Lin, S.X. Effects of dietary supplementing tannic acid in the ration of beef cattle on rumen fermentation, methane emission, microbial flora and nutrient digestibility. J. Anim. Physiol. Anim. Nutr. 2017, 101, 302–310. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Zhang, Q.; Jiang, Y.; Wu, Z.; Yu, Z. Effects of mixing red clover with alfalfa at different ratios on dynamics of proteolysis and protease activities during ensiling. J. Dairy Sci. 2018, 101, 8954–8964. [Google Scholar] [CrossRef] [PubMed]
- Farha, A.K.; Yang, Q.Q.; Kim, G.; Li, H.-B.; Zhu, F.; Liu, H.Y.; Gan, R.Y.; Corke, H. Tannins as an alternative to antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Herremans, S.; Decruyenaere, V.; Beckers, Y.; Froidmont, E. Silage additives to reduce protein degradation during ensiling and evaluation of in vitro ruminal nitrogen degradability. Grass Forage Sci. 2019, 74, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Winters, A.L.; Cockburn, J.E.; Dhanoa, M.S.; Merry, R.J. Effects of lactic acid bacteria in inoculants on changes in amino acid composition during ensilage of sterile and non-sterile ryegrass. J. Appl. Microbiol. 2020, 98, 442–451. [Google Scholar] [CrossRef]
- McKersie, B.D. Proteinases and pepsidases of alfalfa herbage. Can. J. Plant Sci. 1981, 61, 53–59. [Google Scholar]
- Liu, Q. Effects of Hydrolysable Tannin with or without Condensed Tannin on Alfalfa silage fermentation characteristics and in vitro ruminal methane production, fermentation patterns, and microbiota. Animals 2021, 11, 1967. [Google Scholar] [CrossRef]
- Guo, X.S.; Ke, W.C.; Ding, W.R.; Ding, L.M.; Xu, D.M.; Wang, W.W.; Zhang, P.; Yang, F.Y. Profiling of metabolome and bacterial community dynamics in ensiled medicago sativa inoculated without or with Lactobacillus plantarum or Lactobacillus buchneri. Sci. Rep. 2018, 8, 357. [Google Scholar] [CrossRef] [Green Version]
- Colak, S.M.; Yapici, B.M.; Yapici, A.N. Determination of antimicrobial activity of tannic acid in pickling process. Rom. Biotechnol. Lett. 2010, 15, 5325–5330. [Google Scholar]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, H.; Yu, Z.; Zhang, Y. Changes in the distribution of nitrogen and plant enzymatic activity during ensilage of lucerne treated with different additives. Grass Forage Sci. 2007, 62, 35–43. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Association of Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Thomas, T.A. An automated procedure for the determination of soluble carbohydrates in herbage. J. Sci. Food Agric. 1977, 28, 639–642. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses. Agriculture Handbook No 379; USDA: Washington, DC, USA, 1970.
- Yang, L.; Yuan, X.; Li, J.; Dong, Z.; Shao, T. Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour. Technol. 2019, 275, 280–287. [Google Scholar] [CrossRef]
- Du, Z.; Sun, L.; Chen, C.; Lin, J.; Yang, F.; Cai, Y. Exploring microbial community structure and metabolic gene clusters during silage fermentation of paper mulberry, a high-protein woody plant. Anim. Feed Sci. Technol. 2021, 275, 114766. [Google Scholar] [CrossRef]
- Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production—progress and challenges. Anim. Feed Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Wang, Y.; McAllister, T.A.; Acharya, S. Condensed tannins in sainfoin: Composition, concentration, and effects on nutritive and feeding value of sainfoin forage. Crop Sci. 2015, 55, 13–22. [Google Scholar] [CrossRef]
- Hu, Z.; Niu, H.; Tong, Q.; Chang, J.; Yu, J.; Li, S.; Zhang, S.; Ma, D. The microbiota dynamics of alfalfa silage during ensiling and after air exposure, and the metabolomics after air exposure are affected by Lactobacillus casei and cellulase addition. Front. Microbiol. 2020, 11, 519121. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, T.V.C.; Bezerra, L.R.; Menezes, D.R.; Lucena, A.R.F.; Queiroz, M.A.Á.; Trajano, J.S.; Oliveira, R.L. Condensed tannin-amended cassava silage: Fermentation characteristics, degradation kinetics and in-vitro gas production with rumen liquor. J. Agric. Sci. 2018, 156, 83–91. [Google Scholar] [CrossRef]
- Ni, K.; Minh, T.T.; Tu, T.T.; Tsuruta, T.; Pang, H.; Nishino, N. Comparative microbiota assessment of wilted Italian ryegrass, whole crop corn, and wilted alfalfa silage using denaturing gradient gel electrophoresis and next-generation sequencing. Appl. Microbiol. Biotechnol. 2017, 101, 1385–1394. [Google Scholar] [CrossRef]
- Tabasco, R.; Sanchez-Patan, F.; Monagas, M.; Bartolome, B.; Moreno-Arribas, M.V.; Pelaez, C.; Requena, T. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microbiol. 2011, 28, 1345–1352. [Google Scholar] [CrossRef]
- Jayanegara, A.; Sujarnoko, T.U.P.; Ridla, M.; Kondo, M.; Kreuzer, M. Silage quality as influenced by concentration and type of tannins present in the material ensiled: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2019, 103, 456–465. [Google Scholar] [CrossRef]
- Chen, L.; Bao, X.; Guo, G.; Huo, W.; Xu, Q.; Wang, C.; Liu, Q. Treatment of alfalfa silage with tannin acid at different levels modulates ensiling characteristics, methane mitigation, ruminal fermentation patterns and microbiota. Anim. Feed Sci. Technol. 2021, 278, 114997. [Google Scholar] [CrossRef]
- Cavallarin, L.; Tabacco, E.; Borreani, G. Forage and grain legume silages as a valuable source of proteins for dairy cows. Ital. J. Anim. Sci. 2007, 6, 282–284. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Huang, X.D.; Liang, J.B.; Tan, H.Y.; Yahya, R.; Khamseekhiew, B.; Ho, Y.W. Molecular weight and protein binding affinity of Leucaena condensed tannins and their effects on in vitro fermentation parameters. Anim. Feed Sci. Technol. 2010, 159, 81–87. [Google Scholar] [CrossRef]
- Liu, B.; Huan, H.; Gu, H.; Xu, N.; Shen, Q.; Ding, C. Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 2019, 273, 212–219. [Google Scholar] [CrossRef]
- McKersie, B.D.; Smith, J.B. Changes in the levels of proteolytic enzymes in ensiled alfalfa forage. Can. J. Plant Sci. 1982, 62, 111–116. [Google Scholar] [CrossRef]
- Ogunade, I.M.; Jiang, Y.; Cervantes, A.A.P.; Kim, D.H.; Oliveira, A.S.; Vyas, D.; Weinberg, Z.G.; Jeong, K.C.; Adesogan, A.T. Bacterial diversity and composition of alfalfa silage as analyzed by illumina MiSeq sequencing: Effects of Escherichia coli O157:H7 and silage additives. J. Dairy Sci. 2018, 101, 2048–2059. [Google Scholar] [CrossRef]
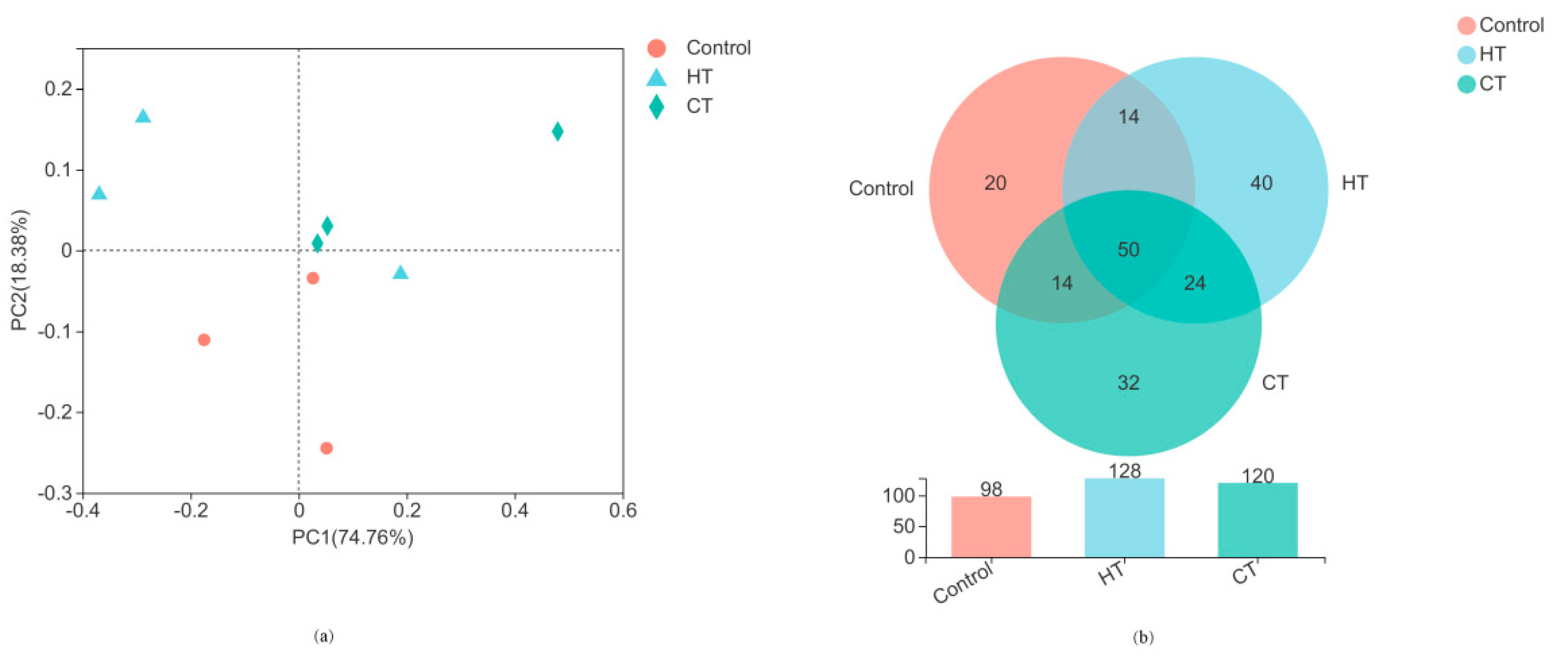
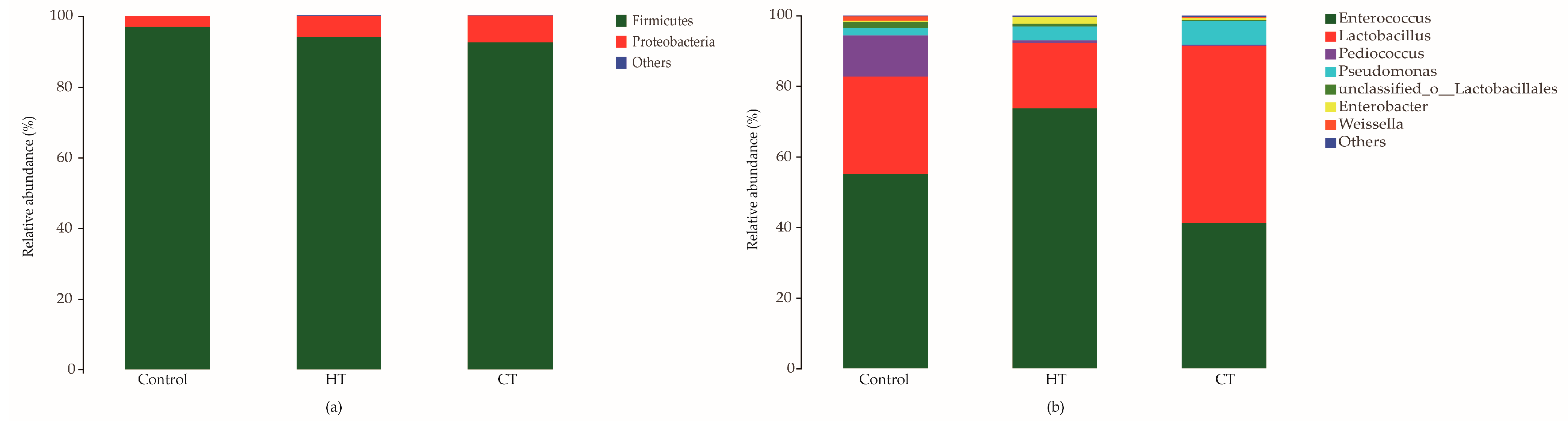
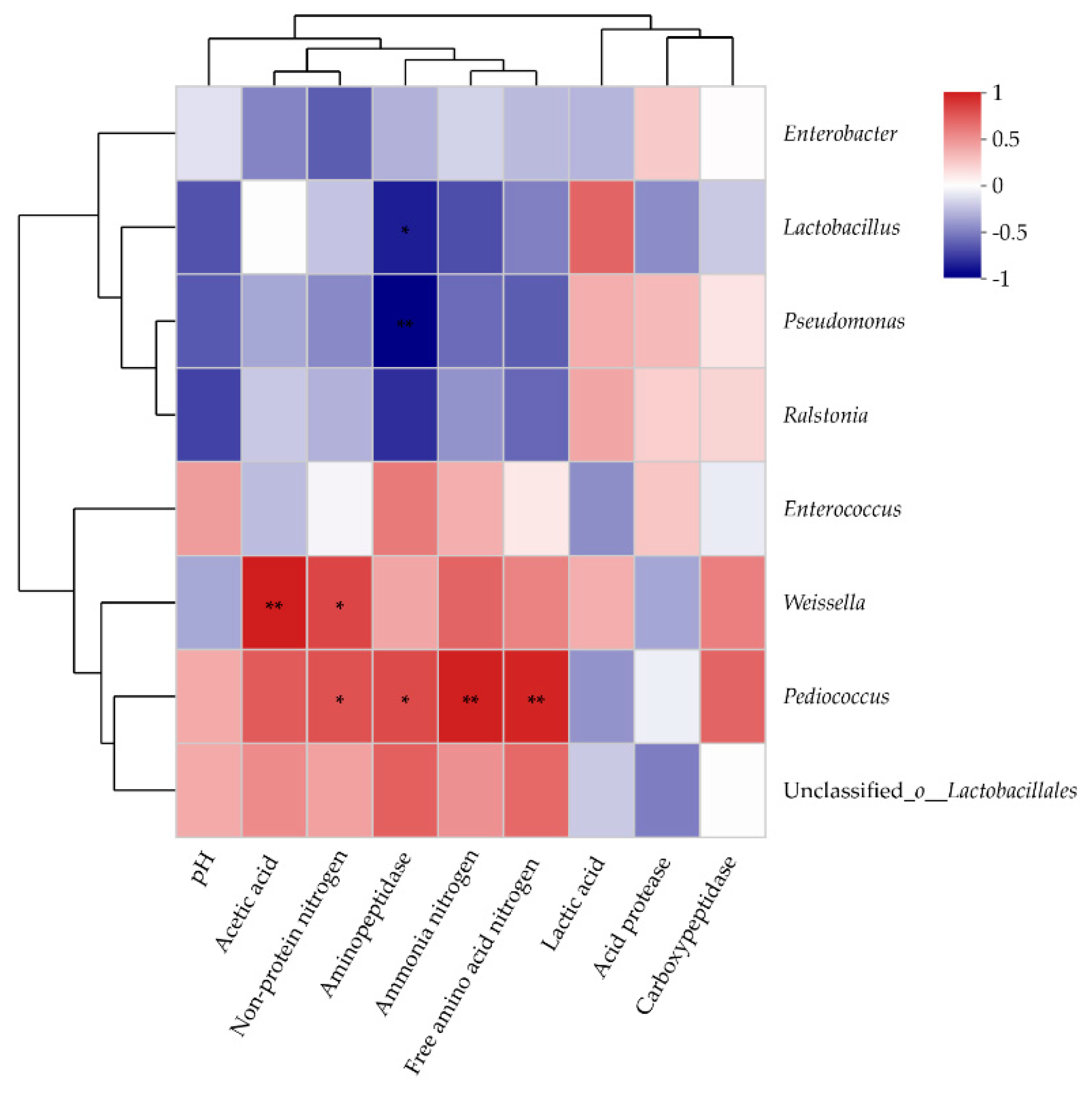
| Item 1 | Alfalfa |
|---|---|
| LAB (log10 cfu/g of FM) | 3.86 ± 0.11 |
| Yeasts (log10 cfu/g of FM) | 4.01 ± 0.05 |
| Coliform bacteria (log10 cfu/g of FM) | - |
| Molds (log10 cfu/g of FM) | - |
| DM (%) | 33.3 ± 1.06 |
| CP (% DM) | 22.8 ± 0.21 |
| WSC (% DM) | 1.79 ± 0.12 |
| NDF (% DM) | 41.9 ± 2.48 |
| ADF (% DM) | 27.0 ± 0.68 |
| Item 1 | Alfalfa Silage 2 | SEM 3 | p-Value | ||
|---|---|---|---|---|---|
| Control | HT | CT | |||
| CCS | 52,740 | 52,467 | 48,431 | 3382 | 0.62 |
| Shannon index | 1.24 | 0.69 | 0.99 | 0.15 | 0.11 |
| Chao1 index | 83.3 | 120 | 110 | 24.7 | 0.58 |
| Coverage index | 0.99 | 0.99 | 0.99 | 0.00 | 0.62 |
| Item 1 | Alfalfa Silages 2,3 | SEM 4 | p-Value | ||
|---|---|---|---|---|---|
| Control | HT | CT | |||
| Microbial population | |||||
| LAB (log10 cfu g−1 of FM) | 6.74 a | 5.57 c | 6.41 b | 0.05 | <0.01 |
| Yeast (log10 cfu g−1 of FM) | 4.08 a | 3.80 b | 3.53 c | 0.05 | <0.01 |
| Coliform bacteria (log10 cfu g−1 of FM) | - | - | - | - | - |
| Molds (log10 cfu g−1 of FM) | - | - | - | - | - |
| Fermentation parameters | |||||
| pH | 4.86 b | 5.06 a | 4.61 c | 0.02 | <0.01 |
| Lactic acid (% DM) | 2.83 b | 2.14 c | 3.21 a | 0.04 | <0.01 |
| Acetic acid (% DM) | 2.28 a | 1.99 b | 2.06 b | 0.02 | <0.01 |
| Propionic acid (% DM) | 0.67 | - | - | - | - |
| Butyric acid (% DM) | - | - | - | - | - |
| Chemical composition | |||||
| DM (%) | 32.7 | 32.3 | 33.3 | 0.56 | 0.48 |
| NDF (% DM) | 40.3 | 39.9 | 41.3 | 0.58 | 0.30 |
| ADF (%DM) | 27.3 | 29.9 | 28.3 | 2.87 | 0.97 |
| Nitrogen components | |||||
| CP (% DM) | 21.7 | 21.5 | 21.5 | 0.26 | 0.78 |
| NPN (% TN) | 73.1 a | 51.4 b | 50.4 b | 24.14 | <0.01 |
| NH3-N (% TN) | 11.5 a | 8.32 b | 5.71 c | 0.17 | <0.01 |
| AA-N (% TN) | 31.1 a | 20.3 b | 16.8 c | 4.25 | <0.01 |
| Protease activity | |||||
| Acid proteases (unit h−1 g−1 DM) | 7.99 a | 6.39 b | 8.07 a | 0.18 | <0.01 |
| Carboxypeptidases (unit h−1 g−1 DM) | 17.6 a | 8.53 b | 8.35 b | 1.76 | 0.02 |
| Aminopeptidases (unit h−1 g−1 DM) | 24.0 a | 24.4 a | 15.8 b | 0.67 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, W.; Zhang, H.; Li, S.; Xue, Y.; Wang, Y.; Dong, W.; Cai, Y.; Zhang, G. Influence of Condensed and Hydrolysable Tannins on the Bacterial Community, Protein Degradation, and Fermentation Quality of Alfalfa Silage. Animals 2022, 12, 831. https://doi.org/10.3390/ani12070831
Ke W, Zhang H, Li S, Xue Y, Wang Y, Dong W, Cai Y, Zhang G. Influence of Condensed and Hydrolysable Tannins on the Bacterial Community, Protein Degradation, and Fermentation Quality of Alfalfa Silage. Animals. 2022; 12(7):831. https://doi.org/10.3390/ani12070831
Chicago/Turabian StyleKe, Wencan, Huan Zhang, Shengnan Li, Yanlin Xue, Yan Wang, Wencheng Dong, Yimin Cai, and Guijie Zhang. 2022. "Influence of Condensed and Hydrolysable Tannins on the Bacterial Community, Protein Degradation, and Fermentation Quality of Alfalfa Silage" Animals 12, no. 7: 831. https://doi.org/10.3390/ani12070831
APA StyleKe, W., Zhang, H., Li, S., Xue, Y., Wang, Y., Dong, W., Cai, Y., & Zhang, G. (2022). Influence of Condensed and Hydrolysable Tannins on the Bacterial Community, Protein Degradation, and Fermentation Quality of Alfalfa Silage. Animals, 12(7), 831. https://doi.org/10.3390/ani12070831






