Simple Summary
Forage oat is an important feed resource in the world. Few studies on the application of different bacterial additives in forage oat silage have been found, which limits the utilization and promotion of oat silage in animal husbandry. In this study, we compared the fermentation quality and in vitro gas production of oat silage treated with four additives (Lactiplantibacillus plantarum F1,LP; Lacticaseibacillus rhamnosus XJJ01, LR; Lacticaseibacillus paracasei XJJ02, LC; and Propionibacterium acidipropionici 1.1161, PP). The results show that compared to the CK group (without additives), the LR group had a higher dry matter content, while the LP group showed an improvement in fermentation quality. At the same time, the bacterial community in the LR group was also different from that in other groups. The treatments of PP and LC had no significant effects on fermentation quality, but the in vitro gas production was significantly reduced in the treated oat silage. These results could help us to optimize the utilization of forage oat silage in balanced ruminant diets.
Abstract
Bacterial inoculants are considered as a good choice for successful ensiling, playing a key role in improving the silage quality. However, the potential of different bacteria, especially the propionic acid bacteria, in forage oat ensiling is yet to be explored. Therefore, the purpose of this study was to investigate the regulation effects of different bacterial additives on the fermentation quality of forage oat silage. Four additives (Lactiplantibacillus plantarum F1, LP; Lacticaseibacillus 0rhamnosus XJJ01, LR; Lacticaseibacillus paracasei XJJ02, LC; and Propionibacterium acidipropionici 1.1161, PP; without additives, CK) were inoculated in forage oat silage, and the fermentation quality and organic compounds were determined after 60 days of ensiling. Notably, LR showed higher dry matter preservation compared to other additives and CK. In addition, LP and LR showed strong lactic acid synthesis capacity, resulting in lower pH compared to other additives and CK. The treatments of PP and LC increased the bacterial diversity in silage, while the bacterial community in the LR group was different from that in other groups. In addition, the PP- and LC-treated oat silage showed significantly lower total in vitro gas production and a lower methane content. These results suggest that LP is more favorable for producing high-quality oat silage than LR, LC, or PP. Both the PP- and LC- treated oat silage may reduce rumen greenhouse gas emissions.
1. Introduction
Forage oat (Avena sativa) silage is considered a palatable, highly digestible and beneficial forage for ruminants in most parts of the world [1,2]. Large amounts of forage are consumed by ruminants, and the global trade of oat has been restricted due to the COVID-19 pandemic [3]. Meanwhile, oats have been widely cultivated in China, both as silage and hay, especially in northern China. In recent years, the research on oat silage has received continuous attention. In previous studies, low-temperature-tolerant lactic acid bacteria (LAB) and bacterial diversity in oat silage have been systematically studied [4,5]. However, different bacterial species in oats still need further exploration.
LAB plays an active role in silage processing and is widely used in various grass-based silages to reduce nutrient loss and extend the storage period. In addition, exogenous probiotics can normally change the type of fermentation in oat silage [6]. Therefore, adding exogenous bacteria or microbiota to silage has become an important way to regulate its anaerobic fermentation, and it also has a profound impact on the organic acid content and dry matter loss of oat silage [7]. Propionibacterium has been used as a microbial additive for forage oat to improve the silage quality [8]. In some cases, Propionibacterium provides better aerobic stability [9]. Silage inoculated with Propionibacterium can produce propionic acid in the anaerobic fermentation period to improve the aerobic stability [9]. However, there are still few reported studies on fermentation quality and in vitro gas production of oat silage treated with Propionibacterium.
Silage treated with lactic acid bacterial inoculants has been reported to increase ruminal microbial biomass when tested in vitro [10]. The rumen is one of the most powerful fiber-degrading fermentation systems known to date [11]. However, the degradation of fibers by a large number of microorganisms in the rumen produces organic acids, along with a large amount of methane and hydrogen. Methane (CH4) is a potent greenhouse gas and, along with carbon dioxide and nitrous oxide, CH4 emission from livestock production is a major contributor to global warming [12]. It is well-established that factors such as nutrient composition and degradability of ruminant diets greatly affect CH4 production [13]. In the in vitro experiment, Propionibacterium freudenreichii 53-W was found to exhibit the ability to reduce methane, but the relevant mechanism is not yet clear [14]. We hypothesize that silage treated with lactic acid bacteria or Propionibacterium can not only improve fermentation quality but also reduce methane emissions.
Therefore, this study aimed to investigate the effects of three different strains of LAB and one Propionibacterium strain on the fermentation quality of oat silage and in vitro gas production.
2. Materials and Methods
2.1. Additives and Ensiling
The four bacterial additives used in this study were Lactiplantibacillus plantarum F1 (isolated by Guo [15]), Lacticaseibacillus rhamnosus XJJ01, Lacticaseibacillus paracasei XJJ02 and Propionibacterium acidipropionici 1.1161. L. rhamnosus XJJ01 and L. paracasei XJJ02 (Accession Number: OK021555, OK021556) were isolated from cheese and stored in our laboratory. P. acidipropionici 1.1161 was purchased from Guangdong Microbial Culture Collection Center.
The oat forages were harvested in Kangbao County, Zhangjiakou City, Hebei Province, China (114°11′–114°56′ E, 41°25′–42°08′ N), which has an average altitude of 1450 m and an average annual temperature of 1.2 °C. On 3 October 2020, the fresh forage oat was harvested at the early milk stage and then directly chopped into segments at a theoretical length of 20 mm. Five hundred grams of fresh oat forages was stored in a cryogenic storage box and quickly brought back to the laboratory for raw materials analysis. The chemical composition of the oat forage is shown in Table 1. The control group was treated with no additives (CK), and the other four groups were treated with L. plantarum F1 (LP), L. rhamnosus XJJ01 (LR), L. paracasei XJJ02 (LC) and P. acidipropionici 1.1161 (PP) at 1.0 × 106 colony-forming units (CFU)/g of fresh matter (FM). Approximately 300 g of chopped oat was packed in polyethylene bags (18 × 26 cm), which were vacuum-sealed. A total of 15 bags (3 replicates) were prepared and equally distributed and stored in ambient temperature for 60 days.

Table 1.
Chemical composition and microbial population of oat by plate culture before ensiling.
2.2. Analysis of Chemical Composition and Fermentation Quality
The samples of fresh forages and silage were collected for analysis after sealing in polyethylene bags. About 200 g of each group of fresh material and silage sample was dried in an air-drying oven at 65 °C for 48 h to measure dry matter (DM) content and then milled by a hammer crusher before passing through a 0.425 mm screen for chemical composition determination and in vitro ruminal fermentation. Crude protein (CP) and EE (ether extract) were analyzed according to AOAC [15]. The content of neutral detergent fiber (aNDF) and acid detergent fiber (ADF) was assayed using the method of Van Soest [16], quantifying aNDF while adding heat-stable α-amylase to the Ankom A2000i system (ANKOM Technology, Macedon, NY, USA). Water-soluble carbohydrate (WSC) content was assayed using the anthrone method [17].
The silage samples (20 g) were mixed with 180 mL of sterilized water, stored at 4 °C for 24 h and then serially diluted from 10−1 to 10−5. Then, 20 μL of each dilution at 10−1, 10−3 and 10−5 was spread onto the corresponding agar separately. The microbial populations were measured as described in our previously study [4]. The 10−1 diluted samples were filtered through a 0.22 μm filter, and the pH value was measured by a pH meter (PHS- 3C, INESA Scientific Instrument, Shanghai, China). The filtrate was used to determine the ammonia nitrogen (NH3-N) and organic acids. The NH3-N content was determined by the phenol hypochloric acid colorimetry method [18]. Lactic acids (LA), AA (acetic acids), PA (propionic acids) and BA (butyric acids) were determined via high-performance liquid chromatography (HPLC) as described in our previously study [4]. The fermentation quality of the oat silage was evaluated by Flieg’s point index, which was calculated as follows [19,20]:
Flieg’s point = 220 + [(2 × %DM) × 15] − 40 × pH
2.3. Analysis of Microbial Community
Ten-gram samples were immediately put into 90 mL of sterile water for collecting microorganisms and treated with a shaker at 160 rpm for 2 h (4 °C). Then, they were filtered through two layers of sterile gauze and rinsed for several times with sterile water to recover residual microorganisms. The filtrates were centrifuged at 10,000× g, 4 °C, for 15 min. Total bacterial DNA was extracted from each sample of the oat silage according to the method described in a previous study [16].
The total DNA was amplified with the bacterial 16 S rDNA primers targeting the V3-V4 regions of 338F (ACTCCTACGGGAGGCAGCAG) and 806R (GGACTACHVGGGTWTCTAAT). The PCR conditions, sequencing and analyzing details conformed to the protocol described by Guo [15]. The diversity index and microbial community of the oat silage were calculated via bioinformatics cloud analytics platform (https://cloud.majorbio.com/. accessed on 15 January 2022).
2.4. Gas Production of Oat Silages
The ruminal fluid was obtained from three ruminally fistulated small-tail Han sheep (corn–soybean meal diet with alfalfa hay) before morning feeding. It was then immediately taken to the laboratory, filtered through four layers of gauze, kept at 39 °C in a water bath and blended with buffer solution (1:2, v/v) under a continuous flush of CO2 [17]. Two hundred milligrams of the samples were put into a 100 mL glass syringe with 30 mL of the mixed solution added in. After the addition of the liquid, it was placed in an artificial rumen incubator in a 39 °C water bath. There were three replicates for each sample, and three blank controls were set up. Then, the amount of gas generated after 0 h, 2 h, 4 h, 6 h, 8 h, 12 h, 24 h, 36 h, 48 h, 60 h and 72 h was recorded, and finally, the gas was collected and its composition was determined by gas chromatography. The temperature of both the thermal conductivity detectors and the oven was 100 °C. Standard gas, CH4, was used at a concentration of 25.0%; CO2 at 65.0%; H2 at 2.03% and O2 at 2.00%. The balance gas was nitrogen, and carrier gas was high-purity argon. The flow rate was 30 mL/min. The pressure was 0.5 MPa; and the volume of each injection was 1 mL.
2.5. Statistical Analysis
All the data were analyzed using one-way analysis of variance (ANOVA) to determine the variables of different inoculant treatments, and post hoc analysis was performed using Duncan’s multiple range test with SPSS 24.0 software (IBM Corp., Armonk, NY, USA). After gas production at different time points was calculated, the in vitro fermentation parameters, such as the potential gas production (B) and gas production rate constant (c), were calculated via SAS 9.3 software (SAS Institute Inc., Cary, NC, USA) NLIN program. Statistically significant difference was set at p < 0.05 and p < 0.01.
3. Results
3.1. Effects of Inoculants on the Chemical Composition and Fermentation Quality
Table 2 shows the chemical composition and microbial population by plate culture after 60 d of ensiling. Though the four inoculant treatments did not show significant differences in the contents of CP, EE, NDF or ADF, significant effects on the contents of DM and WSC were observed. The LR-treated oat silage had a higher DM content than the other inoculants and CK (p < 0.01). The LC-treated oat silage showed a higher WSC content than CK and PP, but there was no significant difference among LP, LC and LR treatments. The amount of LAB was higher in the LR-treated silage than the other inoculants and CK (p < 0.05).

Table 2.
Chemical composition and microbial population of the oat silage by plate culture.
The fermentation quality of the oat silage is shown in Table 3. LP had a lower pH than PP, LC and CK (p < 0.05), while the pH of LR was intermediate and not different from either LP or CK. (p > 0.05). In addition, LP had a lower NH3-N content than all the other treatments (p < 0.05). LR had a higher LA content than PP, LC and CK (p < 0.05). In addition, there were no significant differences in LA content among CK, LP, PP or LC (p > 0.05). Both LP and LC had a lower BA content than CK and LC (p < 0.05) but no significant difference from PP. Overall, LP had a higher Flieg’s point than CK, PP and LR.

Table 3.
Fermentation quality of the oat silage.
3.2. Bacterial Community Analysis of the Oat Silage
As shown in Table 4, compared to CK, there was no significant difference among any of the treatments (p > 0.05), but the Shannon index was higher in the oat silage in PP and LC than in other inoculants (p < 0.05). None of the treatments had significant effects on the Simpson, ACE or Chao indices. In addition, all coverage values were higher than 0.999, which indicates that the sequencing depth was sufficient to reveal the bacterial diversity of the oat silage.

Table 4.
Estimation of bacterial community diversity of the oat silage.
The bacterial community based on genus-level classification is shown in Figure 1A. The dominant genera included Kosakonia (0.4851) and Lactobacillus (0.1548) in the CK group. The main genera in LP group were Kosakonia (0.6670) and Lactobacillus (0.1475). In addition, the dominant genera in PP group were Kosakonia (0.2785) and Lactobacillus (0.2069). Kosakonia (0.2963) and Lactobacillus (0.1689) were the dominant genera in LR. Kosakonia (0.4359) and Lactobacillus (0.2573) were also the dominant genera in the LC group. Principle component analysis (PCA) revealed that components 1 and 2 could explain 63.94% and 19.91% of the total variation, respectively. LR was different from the other treatment groups, and LP and CK were similar but different from the other groups (Figure 1B). It also indicates that LR was located outside the gathering point of all the groups.
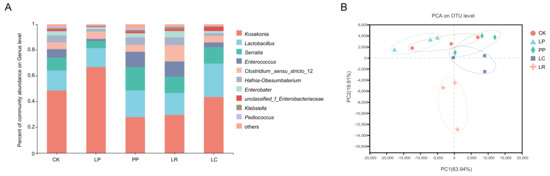
Figure 1.
The relative abundance of microbial community at the genus level (A) and principal component analysis (PCA) based on operational taxonomic units (OTU) level of the oat silage after 60 days of ensiling (B). LP, L. plantarum F1; LR, L. rhamnosus XJJ01; LC, L. paracasei XJJ02; L. paracasei; PP, P. acidipropionici 1.1161.
3.3. Gas Production and In Vitro Fermentation of the Oat Silage
The effects of the inoculant treatments on gas production were largely significant within 48 h of fermentation, which indicates lower gas production in PP, LC and LR than that in LP and CK (Table 5, Figure S1). However, the rate of gas production showed no significant difference, as shown in Table 6. The theoretical maximum of gas production of in vitro fermentation of the oat silage predicted by the model was close to the actual gas production. There was no significant difference between the treatments in methane and carbon dioxide production (p > 0.05). In addition, the effects of the treatments on pH and AN were largely insignificant.

Table 5.
Cumulative gas production (mL/200 mg DM) of the oat silage within 72 h of in vitro fermentation.

Table 6.
In vitro fermentation characteristics and greenhouse gas production (72 h) of the oat silage.
4. Discussion
The forage oat industry has been well-developed domestically, and the research on oat silage and lactic acid bacteria has attracted widespread attention. Propionibacterium widely exists in dairy products and silage. However, studies on the application of propionic acid bacteria in oat silage are relatively rare. Therefore, this study investigated the different effects of four additives including propionibacterium and common lactic acid bacteria on the fermentation quality and microbial composition of oat silages.
Higher dry matter was detected in the LR group compared to the other additives and CK, suggesting that LR has the potential to reduce silage dry matter losses. This supports the hypothesis that the substance metabolism of three lactic acid bacteria and propionibacterium in oat silages is different. WSC also plays a key role in the energy metabolism of microorganisms during the ensiling process. This study found that LR, LP and LC all had a better lactate synthesis capacity compared to PP and CK, which is consist with the result of the previous study [18].
The ensiling process reduces the pH through microbial anaerobic fermentation and provides a stable environment that could inhibit harmful microorganisms. As the DM content increases, the bacterial activity is restricted by low moisture. Usually, due to the lactic acid synthesis by lactic acid bacteria, the final pH of well-fermented and preserved silages could be reduced to 4.0 or lower [19,20]. In this study, the LP group had the lowest pH, which is consistent with the results of the previous studies [21,22]. LR could also reduce pH, which is consistent with Chen’s report [23]. However, unlike lactic acid, propionic acid contributed less to pH reduction [24], which may explain the higher pH in the PP group. Ammonia N is another component which is important for assessing fermentation quality. Palatability, intake and N utilization decline as the NH3-N content increases in silages. Fast acidification in the initial days of ensiling is essential to controlling the reproduction of clostridium bacteria, which may lead to hydrolysis of proteins and amino acids and produce large amounts of ammonia nitrogen [25]. Unlike PP, LC and CK, LP and LR could reduce the NH3-N content in oat, which is consist with the results of the previous studies [21,26]. Therefore, the comparison between different treatments clearly showed the benefits of inoculation under favorable ensiling conditions. This is likely to exacerbate the differences among treatments under the high DM content of the oat silage. Lactic acid is an important product in silage fermentation that reduces pH to achieve a stable state conducive to storage. Compared to PP, LC and CK, LR significantly increased the LA content, which might be attributed to the high amount of lactic acid bacteria and the WSC content, resulting in rapid production of lactic acid. Britt reported that the lower LA content in silages might be attributed to the addition of propionic acid [27]. In contrast, in this study, the PP-treated silage did not reduce the LA content probably due to the amount of lactic acid bacteria. Thus, propionic acid and P. acidipropionici may have different functions in oat silage. Flieg’s point, which is based on pH and DM content, has been widely used to assess the fermentation quality of silages [28]. Although additive treatments improved the fermentation quality of oat silage, only LP had a significantly increased Flieg’s point. Flieg’s point was above 50 for the treatments, which represented a moderate quality [29].
Coverage of all treatments exceeded 0.999, indicating that the sequencing data were sufficient to reflect the profile of bacterial community. In general, the stable state of aerobic and acidic environments inhibits the growth of most microorganisms, leading to low ecological diversity. PP and LC treatments led to the significant increase in the Shannon index of the oat silage, suggesting that PP and LC had a highly diverse bacterial community. Due to different treatments, index calculation methods and the number of observed OTUs, only the Shannon index showed differences. The Simpson index also indicated that PP had high bacterial community diversity. The ACE and Chao indices indicated that all treatments had the same richness of oat silage. In addition, the ACE and Chao indices may be less susceptible to inoculant treatment in oat silage, which is consist with the result of Jia’s previous study [8].
To the best of our knowledge, forage sources affect the bacterial community of silages [30]. Apparently, Kosakonia has been observed by many researchers in barley silage [31], stylo silage [32], ricestraw silage [33] and cornstalk silage [34]. Kosakonia is a genus of enterobacteria in the family of Enterobacteriaceae. To date, it has been identified in many studies, but no adverse effects on the ensiling process have been found. Most importantly, Kosakonia is considered to have the ability to reduce ammonia nitrogen [32]. Unlike LP, which may have a tendency to increase the abundance of kosakonia in oat silage, the addition of vanillic acid could reduce the abundance of kosakonia in stylo silage [35]. In addition, tannic acid could lead to the higher abundance of kosakonia in stylo silage [36]. Kosakonia is positively related to many volatile chemicals in silage [37]. In addition, the different abundances of Clostridium sensu stricto 12 could be related to the inhibitory ability of different strain treatments and soil contamination of the plants during harvest [38]. Despite the increasing use of high-throughput sequencing in the field of ensiling in recent years, efforts in analyzing certain unknown microorganisms, especially non-cultivable bacteria, are still expected [39]. It is difficult to draw a definitive conclusion from the relative abundance of microbial communities, so we tried to obtain more intuitive characteristics through PCA analysis. Lacticaseibacillus rhamnosus was thought to inhibit ethanol fermentation and enterobacter activity in grass silage [40]. In this study, we hypothesized that LR and other additives had slightly different metabolic patterns and expression of functional genes in the fermentation process. Moving forward, increasing studies are expected to consider not only how relative abundance of bacterial communities in the silage varieties, but also the functional genes, present.
In vitro gas production is not only applied to determine the nutritive values of silage, but is also used to predict the digestion status of ruminants [41]. In vitro gas production is highly dependent on the availability of soluble fractions, which favors ruminal fermentation at an earlier fermentation stage [42]. In this study, LP increased gas production within the first 48 h, and this might be because LP treatments could provide more available substrates for microbial degradation at the initial rumen liquid fermentation stages. However, PP and LC treatments may consume the key substrates in the ensiling process, which is good for gas production in in vitro fermentation. Intriguingly, gas production within 72 h had no significant difference, which may possibly be explained by different bacterial inoculants preferring slowly/rapidly degradable DM fractions in oat silages. Similar to Chen’s reports [41], there was no significant difference in the gas production rate constant between oat silage treated with LP and PP, which is possibly due to the rumen liquid source (ruminant species) or forage characters. Therefore, further studies should pay more attention to those different bacterial inoculants, whereas their internal biological mechanisms need to be clarified. Methane (CH4) is the third most important greenhouse gas, following water vapor and CO2, contributing to climate change [43]. Of the 16.5 billion tons of greenhouse gas emissions from global total agri-food systems in 2019, 7.2 billion tons came from the farm gate according to the new analysis [44]. The emission of methane will also cause energy loss (from 2% to 12% of gross energy (GE)) [45]. Therefore, significant research investments are needed to reduce the carbon footprint of ruminants, improve rumen fermentation and clarify the proton transfer strategy [46]. Although the in vitro gas production (both methane and carbon dioxide) of oat silage fermented with PP and LC showed no significant difference from other groups, both reduced the emissions of methane and carbon dioxide. We hypothesized that both PP and LC might have positive effects on rumen methane proton transfer. Both oat grain and whole plants contain several types of antioxidants, such as α-tocopherol [47] and polyphenols [48]. Propionibacterium acidipropionici 1.1161. and Lacticaseibacillus paracasei treatments may protect the antioxidants in oat ensiling processing [49], thereby regulating the metabolism of rumen methane bacteria and reducing gas production. Moreover, those additives may provide a new prospect for oat silage fermentation and digestion and utilization of ruminants.
5. Conclusions
The ability of L. plantarum F1 to improve the quality of oat silages was stronger than that of P. acidipropionici 1.1161, even L. rhamnosus XJJ01 and L. paracasei XJJ02. At the same time, L. plantarum F1 reduced the bacterial community diversity in the ensiling process and caused the oat silage to produce more gas at an earlier in vitro fermentation stage. Compared to other additives, L. paracasei XJJ02 and P. acidipropionici 1.1161 may result in less greenhouse gas production in rumens. Consequently, this finding could help us to choose more suitable silage additives for different feeding strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12091122/s1, Figure S1: Dynamic gas production from oat silage during 72 h of in vitro fermentation.
Author Contributions
Conceptualization, Y.L. and F.Y.; methodology, K.N. and L.G.; software, Y.X.; validation, Y.X., J.X. and D.J.; formal analysis, J.X.; investigation, J.X.; resources, F.C. and X.L.; data curation, C.G., Y.X. and Y.C.; writing—original draft preparation, Y.X. and J.X.; writing—review and editing, K.N.; visualization, F.Y.; supervision, F.Y.; project administration, F.Y.; funding acquisition, F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Modern Agro-industry Technology Research System of China, under grant number CARS-07-E-3, and the National Key Research & Development Program of China (grant no. 2021YFD1300300).
Institutional Review Board Statement
The experiment was conducted in accordance with the Chinese Guidelines for Animal Welfare and Experimental Protocol and approved by the Animal Care and Use Committee of China Agricultural University (ID:AW22601202-5-1).
Data Availability Statement
The data presented in this study are available in this article (and Supplementary Material).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nicholson, I.A. The effect of stage of maturity on the yield and chemical composition of oats for haymaking. J. Agric. Sci. 1957, 49, 129–140. [Google Scholar] [CrossRef]
- Suttie, J.M.; Fao, R.; Reynolds, S.G. Fodder Oats: A World Overview; FAO: Rome, Italy, 2004. [Google Scholar]
- Xiong, Y.; Guo, C.; Wang, L.; Chen, F.; Dong, X.; Li, X.; Ni, K.; Yang, F. Effects of Paper Mulberry Silage on the Growth Performance, Rumen Microbiota and Muscle Fatty Acid Composition in Hu Lambs. Fermentation 2021, 7, 286. [Google Scholar] [CrossRef]
- Li, X.; Chen, F.; Wang, X.; Sun, L.; Guo, L.; Xiong, Y.; Wang, Y.; Zhou, H.; Jia, S.; Yang, F.; et al. Impacts of Low Temperature and Ensiling Period on the Bacterial Community of Oat Silage by SMRT. Microorganisms 2021, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bai, S.; You, M.; Xiao, B.; Li, P.; Cai, Y. Effect of a low temperature tolerant lactic acid bacteria inoculant on the fermentation quality and bacterial community of oat round bale silage. Anim. Feed. Sci. Technol. 2020, 269, 114669. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, J.; Dong, Z.; Li, J.; Kaka, N.A.; Shao, T. Sequencing and microbiota transplantation to determine the role of microbiota on the fermentation type of oat silage. Bioresour. Technol. 2020, 309, 123371. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [Green Version]
- Jia, T.; Yun, Y.; Yu, Z. Propionic Acid and Sodium Benzoate Affected Biogenic Amine Formation, Microbial Community, and Quality of Oat Silage. Front. Microbiol. 2021, 12, 750920. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Monteiro, H.F.; Paula, E.M.; Muck, R.E.; Broderick, G.A.; Faciola, A.P. Effects of lactic acid bacteria in a silage inoculant on ruminal nutrient digestibility, nitrogen metabolism, and lactation performance of high-producing dairy cows. J. Dairy Sci. 2021, 104, 8826–8834. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Kelly, W.J.; Janssen, P.H.; Attwood, G.T. Rumen microbial (meta)genomics and its application to ruminant production. Animal 2013, 7, 184–201. [Google Scholar] [CrossRef] [Green Version]
- Moss, A.R.; Jouany, J.-P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. De Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef] [Green Version]
- Hristov, A.N.; Oh, J.; Firkins, J.L.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.P.S.; Adesogan, A.T.; Yang, W.; Lee, C.; et al. Special Topics—Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options1. J. Anim. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeyanathan, J.; Martin, C.; Eugène, M.; Ferlay, A.; Popova, M.; Morgavi, D.P. Bacterial direct-fed microbials fail to reduce methane emissions in primiparous lactating dairy cows. J. Anim. Sci. Biotechnol. 2019, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yao, D.; Li, D.; Lin, Y.; Bureenok, S.; Ni, K.; Yang, F. Effects of Lactic Acid Bacteria Isolated From Rumen Fluid and Feces of Dairy Cows on Fermentation Quality, Microbial Community, and in vitro Digestibility of Alfalfa Silage. Front. Microbiol. 2019, 10, 2998. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Minh, T.T.; Tu, T.T.M.; Tsuruta, T.; Pang, H.; Nishino, N. Comparative microbiota assessment of wilted Italian ryegrass, whole crop corn, and wilted alfalfa silage using denaturing gradient gel electrophoresis and next-generation sequencing. Appl. Microbiol. Biotechnol. 2017, 101, 1385–1394. [Google Scholar] [CrossRef]
- Li, D.-x.; Ni, K.-k.; Zhang, Y.-c.; Lin, Y.-l.; Yang, F.-y. Influence of lactic acid bacteria, cellulase, cellulase-producing Bacillus pumilus and their combinations on alfalfa silage quality. J. Integr. Agric. 2018, 17, 2768–2782. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage; Chalcombe Publications: Marlow, UK, 1991; p. 340. [Google Scholar]
- Ni, K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.; Pan, Y.; Tao, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef]
- Jia, T.; Wang, B.; Yu, Z.; Wu, Z. The effects of stage of maturity and lactic acid bacteria inoculants on the ensiling characteristics, aerobic stability and in vitro digestibility of whole-crop oat silages. Grassl. Sci. 2020, 67, 55–62. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Dong, Z.; Chen, L.; Shao, T. Effect of microbial inoculants on the fermentation characteristics, nutritive value, and in vitro digestibility of various forages. Anim. Sci. J. 2019, 90, 178–188. [Google Scholar] [CrossRef]
- Chen, M.M.; Liu, Q.H.; Xin, G.R.; Zhang, J.G. Characteristics of lactic acid bacteria isolates and their inoculating effects on the silage fermentation at high temperature. Lett. Appl. Microbiol. 2013, 56, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Heron, S.; Edwards, R.A.; Mcdonald, P. Changes in the nitrogenous components of gamma-irradiated and inoculated ensiled ryegrass. J. Sci. Food Agric. 1986, 37, 979–985. [Google Scholar] [CrossRef]
- Salimei, E.; Capilongo, V.; Simoni, A.; Peiretti, P.G.; Maglieri, C.; Romano, C.A.; Mannina, L.; Coppola, R.; Sorrentino, E. Lactobacillus rhamnosus as additive for maize and sorghum ensiling. J. Agric. Food Chem. 2007, 55, 9600–9607. [Google Scholar] [CrossRef]
- Britt, D.G.; Huber, J.T.; Rogers, A.L. Fungal Growth and Acid Production During Fermentation and Refermentation of Organic Acid Treated Corn Silages1. J. Dairy Sci. 1975, 58, 532–539. [Google Scholar] [CrossRef]
- Flieg, O. A key for the evaluation of silage samples. Futterb. Giirfutterbereitung 1938, 1, 112–128. [Google Scholar]
- Chen, S.; Zhao, J.; Dong, D.; Hu, J.; Huang, G.; Sun, F.; Yu, C.; Shao, T. Effect of citric acid residue and short-chain fatty acids on fermentation quality and aerobic stability of lucerne ensiled with lactic acid bacteria inoculants. J. Appl. Microbiol. 2021, 132, 189–198. [Google Scholar] [CrossRef]
- Dunière, L.; Sindou, J.; Chaucheyras-Durand, F.; Chevallier, I.; Thévenot-Sergentet, D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed. Sci. Technol. 2013, 182, 1–15. [Google Scholar] [CrossRef]
- Sun, L.; Na, N.; Li, X.; Li, Z.; Wang, C.; Wu, X.; Xiao, Y.; Yin, G.; Liu, S.; Liu, Z. Impact of Packing Density on the Bacterial Community, Fermentation, and In Vitro Digestibility of Whole-Crop Barley Silage. Agriculture 2021, 11, 672. [Google Scholar] [CrossRef]
- He, L.; Chen, N.; Lv, H.; Wang, C.; Zhou, W.; Chen, X.; Zhang, Q. Gallic acid influencing fermentation quality, nitrogen distribution and bacterial community of high-moisture mulberry leaves and stylo silage. Bioresour. Technol. 2020, 295, 122255. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, P.; Zou, X.; Chen, X.; Zhang, Q. Influence of Pyroligneous Acid on Fermentation Parameters, CO2 Production and Bacterial Communities of Rice Straw and Stylo Silage. Front. Microbiol. 2021, 12, 701434. [Google Scholar] [CrossRef]
- He, L.; Wang, C.; Xing, Y.; Zhou, W.; Zhang, Q. Ensiling characteristics, proteolysis and bacterial community of high-moisture corn stalk and stylo silage prepared with Bauhinia variegate flower. Bioresource Technol. 2019, 296, 122336. [Google Scholar] [CrossRef]
- He, L.; Li, S.; Wang, C.; Chen, X.; Zhang, Q. Effects of Vanillic Acid on Dynamic Fermentation Parameter, Nitrogen Distribution, Bacterial Community, and Enzymatic Hydrolysis of Stylo Silage. Front. Microbiol. 2021, 12, 690801. [Google Scholar] [CrossRef]
- Wang, C.; Pian, R.; Chen, X.; Lv, H.; Zhou, W.; Zhang, Q. Beneficial Effects of Tannic Acid on the Quality of Bacterial Communities Present in High-Moisture Mulberry Leaf and Stylo Silage. Front. Microbiol. 2020, 11, 586412. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, X.; Zheng, M.; Chen, D.; Chen, X. Altering microbial communities: A possible way of lactic acid bacteria inoculants changing smell of silage. Anim. Feed. Sci. Technol. 2021, 279, 114998. [Google Scholar] [CrossRef]
- Muraro, G.B.; de Almeida Carvalho-Estrada, P.; de Oliveira Pasetti, M.H.; Santos, M.C.; Nussio, L.G. Bacterial dynamics of sugarcane silage in the tropics. Environ. Microbiol. 2021, 23, 5979–5991. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Dubourg, G.; Million, M.; Cadoret, F.; Bilen, M.; Fenollar, F.; Levasseur, A.; Rolain, J.-M.; Fournier, P.-E.; Raoult, D. Culturing the human microbiota and culturomics. Nat. Rev. Microbiol. 2018, 16, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Nishino, N. Bacterial and fungal communities of wilted Italian ryegrass silage inoculated with and without Lactobacillus rhamnosus or Lactobacillus buchneri. Lett. Appl. Microbiol. 2011, 52, 314–321. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, X.J.; Li, J.F.; Dong, Z.H.; Wang, S.R.; Guo, G.; Shao, T. Effects of applying lactic acid bacteria and propionic acid on fermentation quality, aerobic stability and in vitro gas production of forage-based total mixed ration silage in Tibet. Anim. Prod. Sci. 2019, 59, 376. [Google Scholar] [CrossRef]
- Nagadi, S.; Herrero, M.; Jessop, N.S. The influence of diet of the donor animal on the initial bacterial concentration of ruminal fluid and in vitro gas production degradability parameters. Anim. Feed. Sci. Technol. 2000, 87, 231–239. [Google Scholar] [CrossRef]
- Fant, P.; Ramin, M.; Jaakkola, S.; Grimberg, Å.; Carlsson, A.S.; Huhtanen, P. Effects of different barley and oat varieties on methane production, digestibility, and fermentation pattern in vitro. J. Dairy Sci. 2020, 103, 1404–1415. [Google Scholar] [CrossRef]
- UN-News. FAO Analysis Reveals Carbon Footprint of Agri-Food Supply Chain. Available online: https://news.un.org/en/story/2021/11/1105172New (accessed on 15 January 2022).
- Li, Z.; Lei, X.; Chen, X.; Yin, Q.; Shen, J.; Yao, J. Long-term and combined effects of N-[2-(nitrooxy)ethyl]-3-pyridinecarboxamide and fumaric acid on methane production, rumen fermentation, and lactation performance in dairy goats. J. Anim. Sci. Biotechnol. 2021, 12, 125. [Google Scholar] [CrossRef]
- Hook, S.E.; Wright, A.G.; Mcbride, B.W. Methanogens: Methane Producers of the Rumen and Mitigation Strategies. Archaea 2010, 2010, 945785. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.H.; Wu, J.X.; Dong, Z.H.; Wang, S.R.; Shao, T. Effects of overnight wilting and additives on the fatty acid profile, α-tocopherol and β-carotene of whole plant oat silages. Anim. Feed. Sci. Technol. 2020, 260, 114370. [Google Scholar] [CrossRef]
- Peterson, D.M. Oat Antioxidants. J. Cereal Sci. 2001, 33, 115–129. [Google Scholar] [CrossRef]
- Wang, X.; Cao, X.; Liu, H.; Guo, L.; Lin, Y.; Liu, X.; Xiong, Y.; Ni, K.; Yang, F. Effects of Lactic Acid Bacteria on Microbial Metabolic Functions of Paper Mulberry Silage: A Biolog Eco Microplates Approach. Front. Microbiol. 2021, 12, 689174. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).