Melatonin Rescues Dimethoate Exposure-Induced Meiotic and Developmental Defects of Porcine Oocytes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Dimethoate and Melatonin
2.2. Oocyte Maturation In Vitro
2.3. Oocyte Activation and In Vitro Culture
2.4. Immunofluorescence Staining
2.5. Determination of Mitochondrial Distribution
2.6. Evaluation of Cortical Granules Distribution
2.7. Determination of ROS Levels
2.8. Statistical Analysis
3. Results
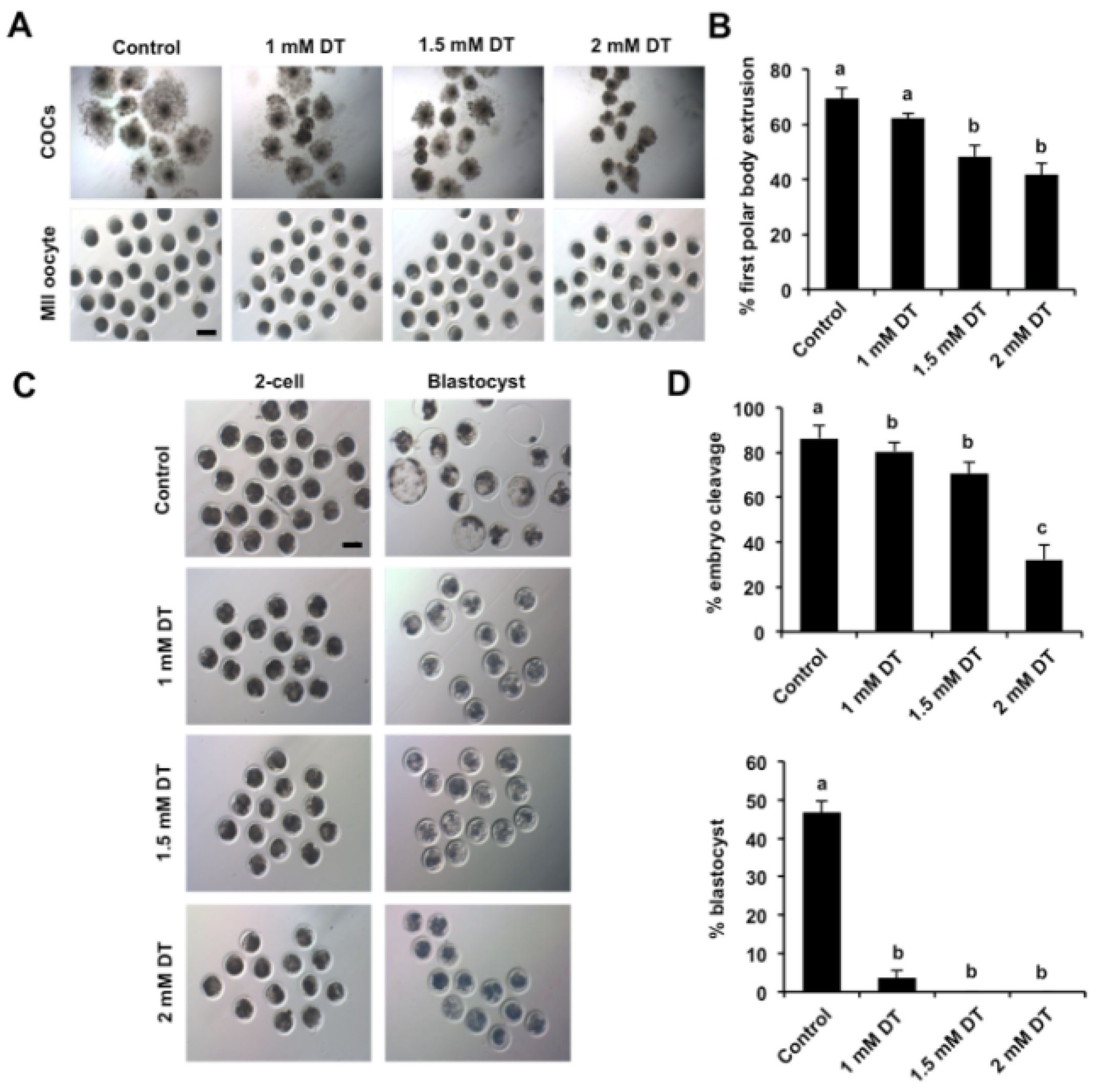
3.1. Dimethoate Exposure Impaired Oocyte Maturation and Early Embryonic Development
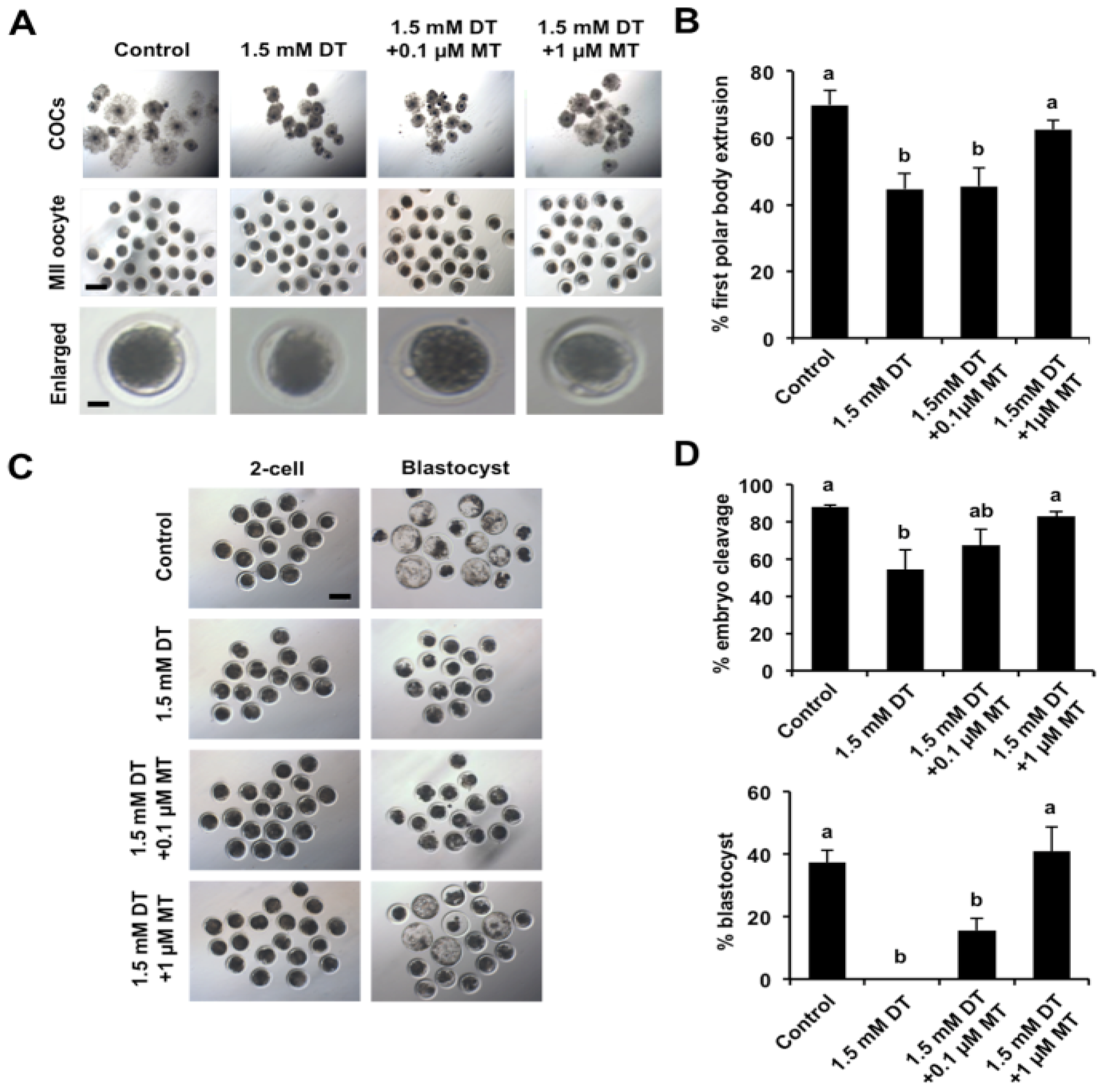
3.2. Melatonin Rescues Meiotic and Developmental Failures of DT-Exposed Oocytes
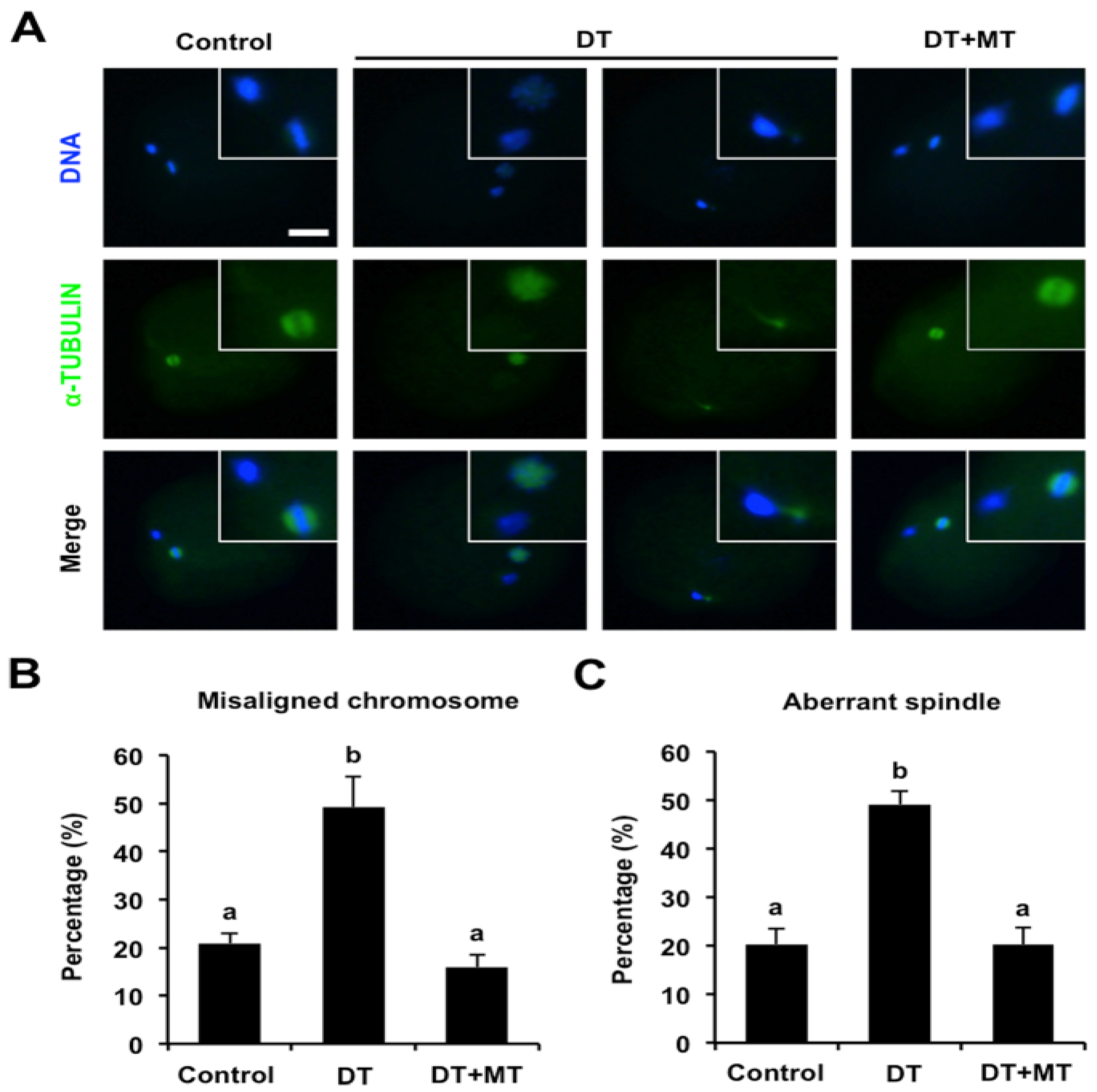
3.3. Melatonin Corrects Chromosome Misalignment and Spindle Disorganization in DT-Exposed Oocytes
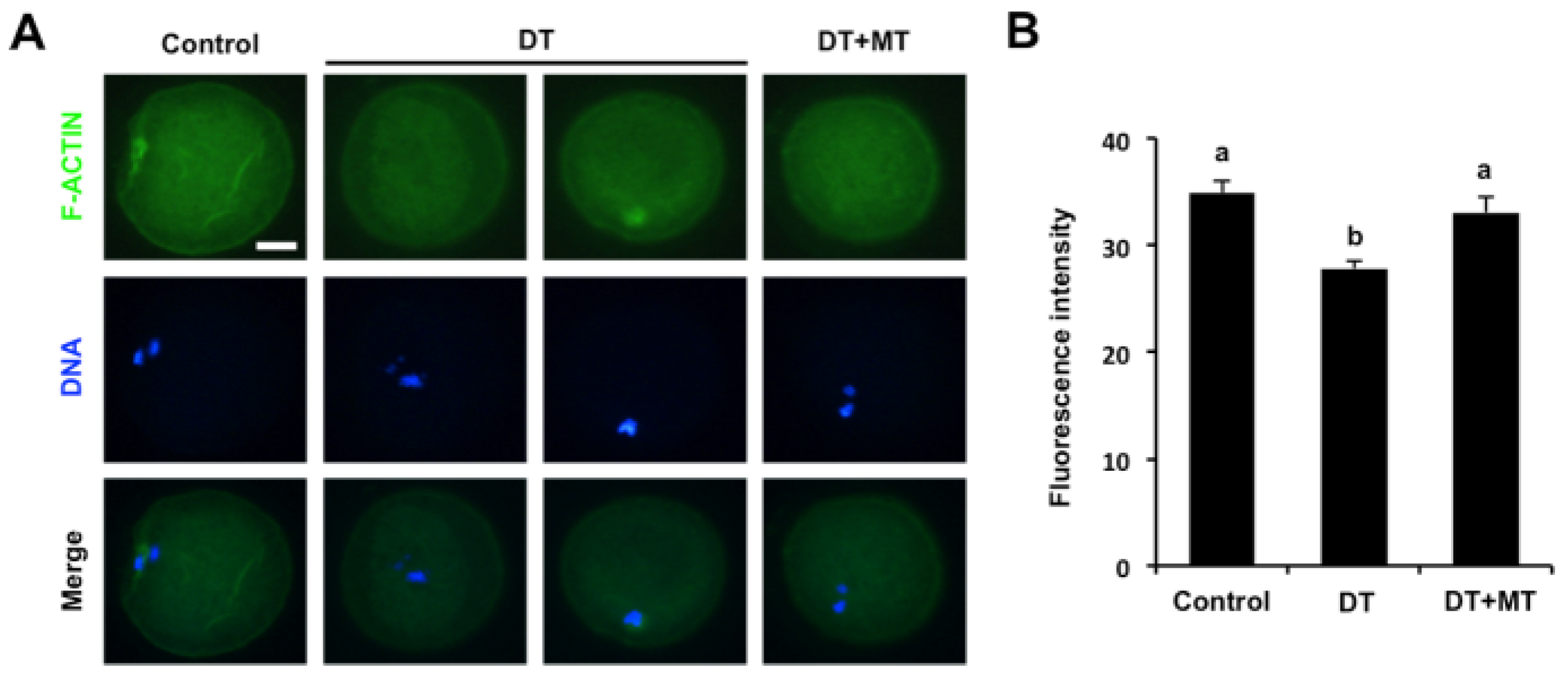
3.4. Melatonin Recovers the Assembly of Actin Filaments in DT-Exposed Oocytes
3.5. Melatonin Restores the Distribution of Mitochondria and Improves the Localization of Cortical Granules in DT-Exposed Oocytes
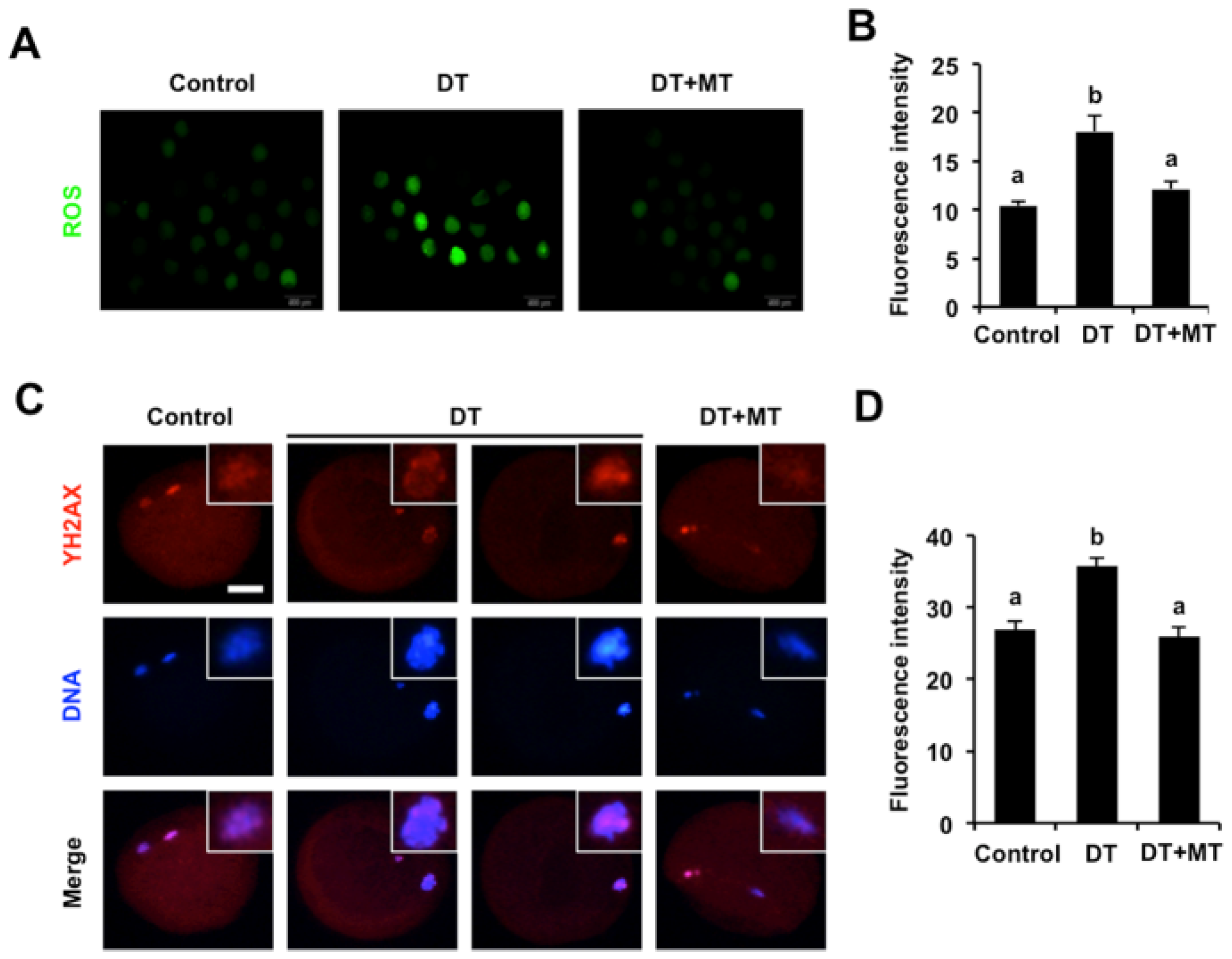
3.6. Melatonin Represses Oxidative Stress and DNA Damage of DT-Exposed Oocytes
3.7. Melatonin Rescues Excessive Autophagy Induced by Dimethoate Exposure in Porcine Oocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lim, W.; Song, G. Mechanisms of deleterious effects of some pesticide exposure on pigs. Pestic. Biochem. Physiol. 2021, 175, 104850. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of endocrine disrupting chemicals in pigs. Environ. Pollut. 2020, 263, 114505. [Google Scholar] [CrossRef] [PubMed]
- Sabarwal, A.; Kumar, K.; Singh, R.P. Hazardous effects of chemical pesticides on human health-Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Fucic, A.; Duca, R.C.; Galea, K.S.; Maric, T.; Garcia, K.; Bloom, M.S.; Andersen, H.R.; Vena, J.E. Reproductive Health Risks Associated with Occupational and Environmental Exposure to Pesticides. Int. J. Environ. Res. Public Health 2021, 18, 6576. [Google Scholar] [CrossRef]
- Ye, M.; Beach, J.; Martin, J.W.; Senthilselvan, A. Pesticide exposures and respiratory health in general populations. J. Environ. Sci. 2017, 51, 361–370. [Google Scholar] [CrossRef]
- Van Scoy, A.; Pennell, A.; Zhang, X. Environmental Fate and Toxicology of Dimethoate. Rev. Environ. Contam. Toxicol. 2016, 237, 53–70. [Google Scholar]
- Antonious, G.F.; Ray, Z.M.; Rivers, L., Jr. Mobility of dimethoate residues from spring broccoli field. J. Environ. Sci Health B 2007, 42, 9–14. [Google Scholar] [CrossRef]
- Zafeiraki, E.; Kasiotis, K.M.; Nisianakis, P.; Manea-Karga, E.; Machera, K. Occurrence and human health risk assessment of mineral elements and pesticides residues in bee pollen. Food Chem. Toxicol. 2022, 161, 112826. [Google Scholar] [CrossRef]
- Arena, M.; Auteri, D.; Barmaz, S.; Brancato, A.; Brocca, D.; Bura, L.; Carrasco Cabrera, L.; Chiusolo, A.; Civitella, C.; Court Marques, D.; et al. Peer review of the pesticide risk assessment of the active substance dimethoate. EFSA J. 2018, 16, e05454. [Google Scholar]
- Sayim, F. Dimethoate-induced biochemical and histopathological changes in the liver of rats. Exp. Toxicol. Pathol. 2007, 59, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Amara, I.B.; Soudani, N.; Troudi, A.; Hakim, A.; Zeghal, K.M.; Boudawara, T.; Zeghal, N. Dimethoate induced oxidative damage and histopathological changes in lung of adult rats: Modulatory effects of selenium and/or vitamin E. Biomed. Environ. Sci. 2012, 25, 340–351. [Google Scholar] [PubMed]
- Ben Amara, I.; Karray, A.; Hakim, A.; Ben Ali, Y.; Troudi, A.; Soudani, N.; Boudawara, T.; Zeghal, K.M.; Zeghal, N. Dimethoate induces kidney dysfunction, disrupts membrane-bound ATPases and confers cytotoxicity through DNA damage. Protective effects of vitamin E and selenium. Biol. Trace Elem. Res. 2013, 156, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Lu, X.; Zhang, W.; He, F. Biochemical changes in primary culture of skeletal muscle cells following dimethoate exposure. Toxicology 2002, 174, 79–85. [Google Scholar] [CrossRef]
- Ben Amara, I.; Soudani, N.; Hakim, A.; Bouaziz, H.; Troudi, A.; Zeghal, K.M.; Zeghal, N. Dimethoate-induced oxidative damage in erythrocytes of female adult rats: Possible protective effect of vitamin E and selenium supplemented to diet. Toxicol. Ind. Health 2012, 28, 222–237. [Google Scholar] [CrossRef]
- Martinez-Morcillo, S.; Perez-Lopez, M.; Soler-Rodriguez, F.; Gonzalez, A. The organophosphorus pesticide dimethoate decreases cell viability and induces changes in different biochemical parameters of rat pancreatic stellate cells. Toxicol. In Vitro 2019, 54, 89–97. [Google Scholar] [CrossRef]
- Aly, N.M.; El-Gendy, K.S. Effect of dimethoate on the immune system of female mice. J. Environ. Sci. Health B 2000, 35, 77–86. [Google Scholar] [CrossRef]
- Verma, R.; Mohanty, B. Early-life exposure to dimethoate-induced reproductive toxicity: Evaluation of effects on pituitary-testicular axis of mice. Toxicol. Sci. 2009, 112, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Ben Slima, A.; Ben Abdallah, F.; Keskes-Ammar, L.; Mallek, Z.; El Feki, A.; Gdoura, R. Embryonic exposure to dimethoate and/or deltamethrin impairs sexual development and programs reproductive success in adult male offspring mice. Andrologia 2012, 44 (Suppl. 1), 661–666. [Google Scholar] [CrossRef]
- Jallouli, M.; Dhouib Iel, B.; Dhouib, H.; Gharbi, N.; El Fazaa, S. Effects of dimethoate in male mice reproductive parameters. Regul. Toxicol. Pharmacol. 2015, 73, 853–858. [Google Scholar] [CrossRef]
- Bakir, E.; Sariozkan, S.; Endirlik, B.U.; Kilic, A.B.; Yay, A.H.; Tan, F.C.; Eken, A.; Turk, G. Cherry laurel fruit extract counters dimethoate-induced reproductive impairment and testicular apoptosis. Arch. Ind. Hyg. Toxicol. 2020, 71, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.P.; Webster, D.R.; Stocco, D.M. Dimethoate inhibits steroidogenesis by disrupting transcription of the steroidogenic acute regulatory (StAR) gene. J. Endocrinol. 2000, 167, 253–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Wang, Y.; Zhu, Q.; Li, X.; Huang, T.; Li, H.; Zhao, J.; Ge, R.S. Dimethoate blocks pubertal differentiation of Leydig cells in rats. Chemosphere 2020, 241, 125036. [Google Scholar] [CrossRef] [PubMed]
- Mahadevaswami, M.P.; Kaliwal, B.B. Effect of dimethoate administration schedules on compensatory ovarian hypertrophy, follicular dynamics, and estrous cycle in hemicastrated mice. J. Basic Clin. Physiol. Pharmacol. 2002, 13, 225–248. [Google Scholar] [CrossRef] [PubMed]
- Mahadevaswami, M.P.; Kaliwal, B.B. Evaluation of dimethoate toxicity on pregnancy in albino mice. J. Basic Clin. Physiol. Pharmacol. 2004, 15, 211–221. [Google Scholar] [CrossRef]
- Silva, M.S.; De Souza, D.V.; Alpire, M.E.S.; Malinverni, A.C.M.; Da Silva, R.C.B.; Viana, M.B.; Oshima, C.T.F.; Ribeiro, D.A. Dimethoate induces genotoxicity as a result of oxidative stress: In vivo and in vitro studies. Environ. Sci. Pollut. Res. Int. 2021, 28, 43274–43286. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Fink, R. The protective role of antioxidants in the defence against ROS/RNS-mediated environmental pollution. Oxid. Med. Cell. Longev. 2014, 2014, 671539. [Google Scholar] [CrossRef] [Green Version]
- Limon-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. 2009, 674, 137–147. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Asada, H.; Yamagata, Y.; et al. Melatonin as a free radical scavenger in the ovarian follicle. Endocr. J. 2013, 60, EJ12-0263. [Google Scholar] [CrossRef] [Green Version]
- Lemos, A.J.; Peixoto, C.A.; Teixeira, A.A.; Luna, R.L.; Rocha, S.W.; Santos, H.M.; Silva, A.K.; Nunes, A.K.; Wanderley-Teixeira, V. Effect of the combination of metformin hydrochloride and melatonin on oxidative stress before and during pregnancy, and biochemical and histopathological analysis of the livers of rats after treatment for polycystic ovary syndrome. Toxicol. Appl. Pharmacol. 2014, 280, 159–168. [Google Scholar] [CrossRef]
- Yong, W.; Ma, H.; Na, M.; Gao, T.; Zhang, Y.; Hao, L.; Yu, H.; Yang, H.; Deng, X. Roles of melatonin in the field of reproductive medicine. Biomed. Pharmacother. 2021, 144, 112001. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Zhang, Y.; Wan, X.; Pan, M.H.; Xu, Y.; Sun, S.C. Melatonin ameliorates ochratoxin A-induced oxidative stress and apoptosis in porcine oocytes. Environ. Pollut. 2020, 256, 113374. [Google Scholar] [CrossRef] [PubMed]
- Asghari, M.H.; Moloudizargari, M.; Bahadar, H.; Abdollahi, M. A review of the protective effect of melatonin in pesticide-induced toxicity. Expert Opin. Drug Metab. Toxicol. 2017, 13, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Y.; Zhang, P.; Wang, J.J.; Liu, J.C.; Li, L.; Shen, W.; Zhai, Q.Y. Melatonin alleviates meiotic defects in fetal mouse oocytes induced by Di (2-ethylhexyl) phthalate in vitro. Aging 2018, 10, 4175–4187. [Google Scholar] [CrossRef]
- Kechiche, S.; Venditti, M.; Knani, L.; Jablonska, K.; Dziegiel, P.; Messaoudi, I.; Reiter, R.J.; Minucci, S. First evidence of the protective role of melatonin in counteracting cadmium toxicity in the rat ovary via the mTOR pathway. Environ. Pollut. 2021, 270, 116056. [Google Scholar] [CrossRef]
- Lan, M.; Han, J.; Pan, M.H.; Wan, X.; Pan, Z.N.; Sun, S.C. Melatonin protects against defects induced by deoxynivalenol during mouse oocyte maturation. J. Pineal Res. 2018, 65, e12477. [Google Scholar] [CrossRef]
- Pang, Y.W.; Jiang, X.L.; Wang, Y.C.; Wang, Y.Y.; Hao, H.S.; Zhao, S.J.; Du, W.H.; Zhao, X.M.; Wang, L.; Zhu, H.B. Melatonin protects against paraquat-induced damage during in vitro maturation of bovine oocytes. J. Pineal Res. 2019, 66, e12532. [Google Scholar] [CrossRef]
- Cheng, L.; Qin, Y.; Hu, X.; Ren, L.; Zhang, C.; Wang, X.; Wang, W.; Zhang, Z.; Hao, J.; Guo, M.; et al. Melatonin protects in vitro matured porcine oocytes from toxicity of Aflatoxin B1. J. Pineal Res. 2019, 66, e12543. [Google Scholar] [CrossRef]
- Schatten, H.; Sun, Q.Y. Centrosome dynamics during mammalian oocyte maturation with a focus on meiotic spindle formation. Mol. Reprod. Dev. 2011, 78, 757–768. [Google Scholar] [CrossRef]
- Yu, Y.; Cui, Y.; Niedernhofer, L.J.; Wang, Y. Occurrence, Biological Consequences, and Human Health Relevance of Oxidative Stress-Induced DNA Damage. Chem. Res. Toxicol. 2016, 29, 2008–2039. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Li, Y.; Zhang, W.; Qian, H.; Zhang, Z.; Xu, K. Alginic acid induces oxidative stress-mediated hormone secretion disorder, apoptosis and autophagy in mouse granulosa cells and ovaries. Toxicology 2022, 467, 153099. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Franciosi, F. Acquisition of oocyte competence to develop as an embryo: Integrated nuclear and cytoplasmic events. Hum. Reprod. Update 2018, 24, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Kidder, G.M.; Vanderhyden, B.C. Bidirectional communication between oocytes and follicle cells: Ensuring oocyte developmental competence. Can. J. Physiol. Pharmacol. 2010, 88, 399–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunet, S.; Maro, B. Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: Integrating time and space. Reproduction 2005, 130, 801–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severson, A.F.; von Dassow, G.; Bowerman, B. Oocyte Meiotic Spindle Assembly and Function. Curr. Top. Dev. Biol. 2016, 116, 65–98. [Google Scholar] [PubMed]
- Duan, X.; Sun, S.C. Actin cytoskeleton dynamics in mammalian oocyte meiosis. Biol. Reprod. 2019, 100, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Trebichalska, Z.; Kyjovska, D.; Kloudova, S.; Otevrel, P.; Hampl, A.; Holubcova, Z. Cytoplasmic maturation in human oocytes: An ultrastructural study dagger. Biol. Reprod. 2021, 104, 106–116. [Google Scholar] [CrossRef]
- Dumollard, R.; Duchen, M.; Carroll, J. The role of mitochondrial function in the oocyte and embryo. Curr. Top. Dev. Biol. 2007, 77, 21–49. [Google Scholar]
- Berg, L.K.; Wessel, G.M. Cortical granules of the sea urchin translocate early in oocyte maturation. Development 1997, 124, 1845–1850. [Google Scholar] [CrossRef]
- Cheeseman, L.P.; Boulanger, J.; Bond, L.M.; Schuh, M. Two pathways regulate cortical granule translocation to prevent polyspermy in mouse oocytes. Nat. Commun. 2016, 7, 13726. [Google Scholar] [CrossRef]
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common Feature of Pesticides: Oxidative Stress-The Role of Oxidative Stress in Pesticide-Induced Toxicity. Oxid. Med. Cell. Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Levine, B. Autosis and autophagic cell death: The dark side of autophagy. Cell Death Differ. 2015, 22, 367–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roohbakhsh, A.; Shamsizadeh, A.; Hayes, A.W.; Reiter, R.J.; Karimi, G. Melatonin as an endogenous regulator of diseases: The role of autophagy. Pharmacol. Res. 2018, 133, 265–276. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Qi, X.; Ding, C.; Liu, X.; Lei, Y.; Li, S.; Cao, Z. Melatonin Rescues Dimethoate Exposure-Induced Meiotic and Developmental Defects of Porcine Oocytes. Animals 2022, 12, 832. https://doi.org/10.3390/ani12070832
Jiang Q, Qi X, Ding C, Liu X, Lei Y, Li S, Cao Z. Melatonin Rescues Dimethoate Exposure-Induced Meiotic and Developmental Defects of Porcine Oocytes. Animals. 2022; 12(7):832. https://doi.org/10.3390/ani12070832
Chicago/Turabian StyleJiang, Qi, Xin Qi, Chi Ding, Xingyu Liu, Yuanyuan Lei, Siying Li, and Zubing Cao. 2022. "Melatonin Rescues Dimethoate Exposure-Induced Meiotic and Developmental Defects of Porcine Oocytes" Animals 12, no. 7: 832. https://doi.org/10.3390/ani12070832
APA StyleJiang, Q., Qi, X., Ding, C., Liu, X., Lei, Y., Li, S., & Cao, Z. (2022). Melatonin Rescues Dimethoate Exposure-Induced Meiotic and Developmental Defects of Porcine Oocytes. Animals, 12(7), 832. https://doi.org/10.3390/ani12070832





