Efficacy Evaluation of a Combined Hemorrhagic Septicemia–Mastitis Vaccine in Dairy Cows and Buffaloes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin and Selection of the Vaccinal Isolates
2.2. Preparation of HS–Mastitis Combined Vaccine
2.3. Preliminary Control-Testing of the Combined Vaccine
2.4. Experimental Design
2.5. Evaluation Parameters
2.6. Statistical Analysis
3. Results
3.1. Serum Antibody Titers against Selected Pathogens
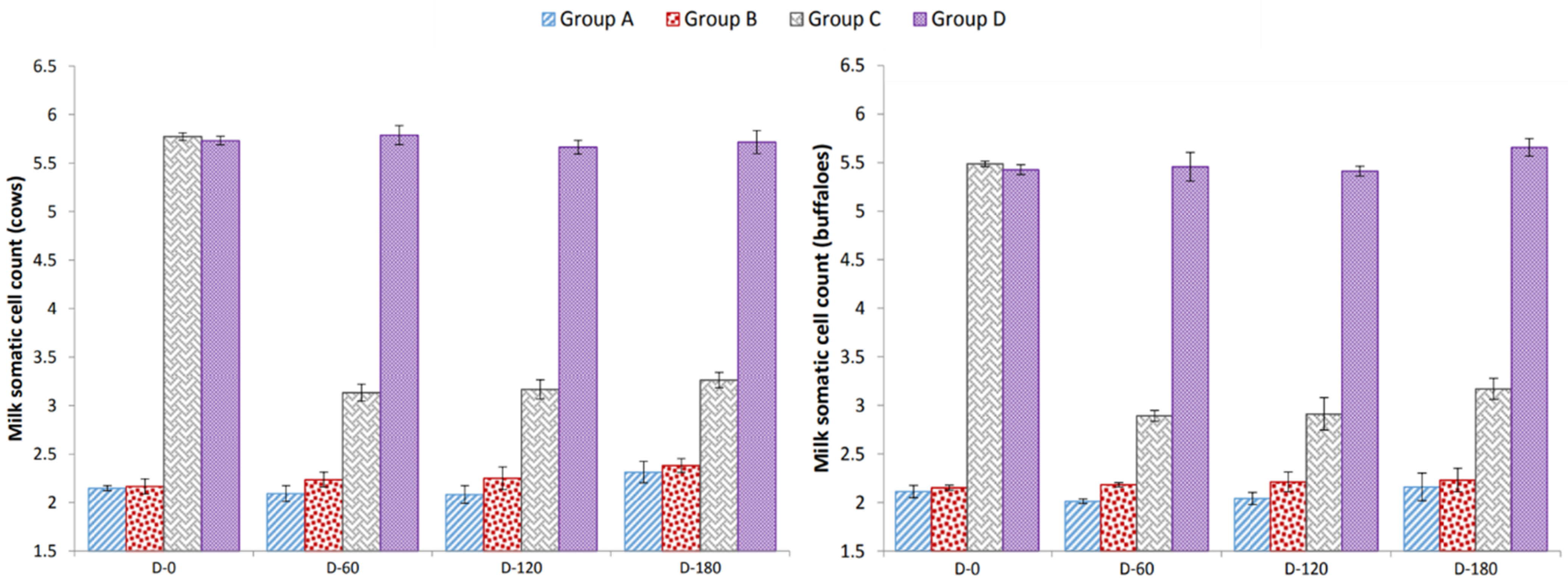
3.2. Milk Somatic Cell Count
3.3. Severity of Mastitis in Vaccinated and Unvaccinated Animals
3.4. Effect of Vaccination on Milk Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghafar, A.; McGill, D.; Stevenson, M.A.; Badar, M.; Kumbher, A.; Warriach, H.M.; Gasser, R.B.; Jabbar, A. A Participatory Investigation of Bovine Health and Production Issues in Pakistan. Front. Vet. Sci. 2020, 7, 248. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, P.; Goudar, A.L.; Suresh, K.P.; Roy, P. Global and countrywide prevalence of subclinical and clinical mastitis in dairy cattle and buffaloes by systematic review and meta-analysis. Res. Vet. Sci. 2021, 136, 561–586. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Usman, T.; Niaz, K. A Review on Bovine Mastitis with Special Focus on Cd4 as a Potential Candidate Gene for Mastitis Resistance—A Review. Ann. Anim. Sci. 2020, 20, 735–755. [Google Scholar] [CrossRef]
- Shivachandra, S.; Viswas, K.; Kumar, A. A review of hemorrhagic septicemia in cattle and buffalo. Anim. Health Res. Rev. 2011, 12, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Almoheer, R.; Abd Wahid, M.E.; Zakaria, H.A.; Jonet, M.A.B.; Al-shaibani, M.M.; Al-Gheethi, A.; Addis, S.N.K. Spatial, Temporal, and Demographic Patterns in the Prevalence of Hemorrhagic Septicemia in 41 Countries in 2005–2019: A Systematic Analysis with Special Focus on the Potential Development of a New-Generation Vaccine. Vaccines 2022, 10, 315. [Google Scholar] [CrossRef]
- Ahmad, T.; Muhammad, G. Evaluation of Staphylococcus aureus and Streptococcus agalactiae aluminium hydroxide adjuvanted mastitis vaccine in rabbits. Pak. J. Agric. Sci. 2008, 45, 353–361. [Google Scholar]
- Ashraf, A.; Imran, M. Causes, types, etiological agents, prevalence, diagnosis, treatment, prevention, effects on human health and future aspects of bovine mastitis. Anim. Health Res. Rev. 2020, 21, 36–49. [Google Scholar] [CrossRef]
- Sharif, A.; Muhammad, G. Mastitis control in dairy animals. Pak. Vet. J. 2009, 29, 145–148. [Google Scholar]
- Chaudhry, H.R.; Khan, S.A.; Jamil, T. Bacteriology of Sub-Clinical Mastitis in the Dairy Buffaloes Maintained at Private Farms of Yazman, Distt. Bahawalpur. Biologia (Pakistan) 2013, 59, 259–262. [Google Scholar]
- Shakoor, A.; Athar, M.; Muhammad, G.; Rahman, S.; Butt, A.; Hussain, I.; Ahmad, R. Effect of different Staphylococcus aureus mastitis vaccines on the milk yield, fat, protein and somatic cell count in buffaloes. Pak. Vet. J. 2006, 26, 67–72. [Google Scholar]
- Ali, M.; Ahmad, M.; Muhammad, K.; Anjum, A. Prevalence of sub clinical mastitis in dairy buffaloes of Punjab, Pakistan. J. Anim. Plant Sci. 2011, 21, 477–480. [Google Scholar]
- Oliver, S.P.; National Mastitis Council. Microbiological Procedures for the Diagnosis of Bovine Udder Infection and Determination of Milk Quality; NMC: Verona, WI, USA, 2004. [Google Scholar]
- Qudratullah; Muhammad, G.; Saqib, M.; Bilal, M.Q. Isolation, characterization, virulence and immunogenicity testing of field isolates of Pasteurella multocida, Staphylococcus aureus, and Streptococcus agalactiae in laboratory settings. Acta Trop. 2017, 172, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Qudratullah. Laboratory and Field Evaluation of a Locally Prepared Montanide® Adjuvanted Combined Hemorrhagic Septicemia-Mastitis Vaccine; University of Agriculture: Faisalabad, Pakistan, 2015. [Google Scholar]
- Qudratullah; Muhammad, G.; Saqib, M.; Bilal, M.Q. Effect of Montanide® adjuvanted combined hemorrhagic septicemia-mastitis vaccine on incidence and prevalence of P. multocida, S. aureus and Str. agalactiae in cows and buffaloes. Transylv. Rev. 2016, XXIV, 1376–1380. [Google Scholar]
- Muhammad, G.; Naureen, A.; Asi, M.N.; Saqib, M.; Fazalur, R. Evaluation of a 3% surf solution (surf field mastitis test) for the diagnosis of subclinical bovine and bubaline mastitis. Trop. Anim. Health Prod. 2010, 42, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.; Saxena, H.M. Estimation of titers of antibody against Pasteurella multocida in cattle vaccinated with haemorrhagic septicemia alum precipitated vaccine. Vet. World 2014, 7, 224–228. [Google Scholar] [CrossRef]
- Yousaf, A.; Muhammad, G.; ur Rahman, S.; Siddique, M.; Masood, M. Effect of montanide adjuvanted staphylococcus aureus bacterin-toxiod on prevalence and incidence of mastitis in cows. Pak. J. Agri. Sci. 2009, 46, 119–123. [Google Scholar]
- Schalm, O.W.; Carroll, E.J.; Jain, N.C. Bovine Mastitis; Lea & Febiger: Philadelphia, PA, USA, 1971. [Google Scholar]
- Singh, M. Post milking teat dip effect on somatic cell count, milk production and composition in cows and buffaloes. Asian-Australas. J. Anim. Sci. 2002, 15, 1517–1522. [Google Scholar]
- Ali, L. Epidemiology of Mastitis in Dairy Buffalo and Cow in Tehsil Samundri of District Faisalabad; University of Agriculture Faisalabad: Faisalabad, Pakistan, 2009. [Google Scholar]
- Rainard, P.; Gilbert, F.B.; Martins, R.P.; Germon, P.; Foucras, G. Progress towards the Elusive Mastitis Vaccines. Vaccines 2022, 10, 296. [Google Scholar] [CrossRef]
- Muneer, R.; Hussain, M.; Zahoor, A. Efficacy of oil based haemorrhagic septicaemia vaccine: A field trial. Int. J. Agric. Biol. 2005, 7, 571–573. [Google Scholar]
- Athar, M.; Muhammad, G.; Shakoor, A.; Saqib, M.; Ahmad, R.; Naureen, A. A preliminary study on the effect of inactivated polyvalent mastitis vaccines on milk quantity and quality in dairy buffaloes. Pak. Vet. J. 2007, 27, 85–91. [Google Scholar]
- Ashfaq, M.; Muhammad, G.; Shamsheer-ul-Haq, A.R. Effects of Livestock Diseases on Dairy Production and In-Comes in District Faisalabad, Punjab, Pakistan; Working Paper; Pakistan Strategy Support Program-International Food Policy Research Institute: Islamabad, Pakistan, 2014. [Google Scholar]
- Owens, W.; Watts, J.; Boddie, R.; Nickerson, S. Antibiotic treatment of mastitis: Comparison of intramammary and intramammary plus intramuscular therapies. J. Dairy Sci. 1988, 71, 3143–3147. [Google Scholar] [CrossRef]
- Hoedemaker, M.; Korff, B.; Edler, B.; Emmert, M.; Bleckmann, E. Dynamics of Staphylococcus aureus infections during vaccination with an autogenous bacterin in dairy cattle. J. Vet. Med. Ser. B 2001, 48, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.L. Serological response of sheep to live and killed Staphylococcus aureus vaccines. Vaccine 1987, 5, 275–278. [Google Scholar] [CrossRef]
- Tollersrud, T.; Nørstebø, P.E.; Engvik, J.P.; Andersen, S.R.; Reitan, L.J.; Lund, A. Antibody Responses in Sheep Vaccinated against Staphylococcus aureus Mastitis: A Comparison of Two Experimental Vaccines Containing Different Adjuvants. Vet. Res. Commun. 2002, 26, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Klimka, A.; Michels, L.; Glowalla, E.; Tosetti, B.; Krönke, M.; Krut, O. Montanide ISA 71 VG is Advantageous to Freund’s Adjuvant in Immunization against S. aureus Infection of Mice. Scand. J. Immunol. 2015, 81, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.K.; Bayry, J.; Ramakrishna, C.; Hugar, B.; Misra, L.D.; Natarajan, C. Immune responses of goats against foot-and-mouth disease quadrivalent vaccine: Comparison of double oil emulsion and aluminium hydroxide gel vaccines in eliciting immunity. Vaccine 2002, 20, 2781–2789. [Google Scholar] [CrossRef]
| Groups | Experimental Treatment | Dairy Species and Number (n) of Animals | HS–Mastitis Status at the Time of Initiation of Trial |
|---|---|---|---|
| A | Vaccination twice at 21-day interval | Buffalo (n = 30) Cow (n = 10) | Milk culture − ve for S. aureus and Str. agalactiae, SFMT − ve; no history of vaccination against HS during the last six months, no or low antibody titers against P. multocida |
| B (control) | No vaccination | Buffalo (n = 15) Cow (n = 15) | Milk culture − ve for S. aureus and Str. agalactiae, SFMT − ve; no history of vaccination against HS during the last six months, no or low antibody titers against P. multocida |
| C (Sub-clinically mastitic buffaloes and cows) | Vaccination twice at 21-day interval | Buffalo (n = 15) Cow (n = 15) | Milk culture + ve for S. aureus/Str. agalactiae, SFMT + ve in one or more quarter but no clinical signs of mastitis |
| D (Sub-clinically mastitic buffaloes and cows) Infected control | No vaccination | Buffalo (n = 10) Cow (n = 10) | Milk culture + ve for S. aureus/Str. agalactiae, SFMT + ve in one or more quarter but no clinical signs of mastitis |
| Type of Antibody Determination | Sampling Day | Group A | Group B | Group C | Group D |
|---|---|---|---|---|---|
| anti-P. multocida | 0 | 0.47 ± 0.006 b | 0.35 ± 0.005 d | 0.59 ± 0.004 a | 0.41 ± 0.007 c |
| 60 | 2.55 ± 0.027 a | 0.31 ± 0.011 d | 1.63 ± 0.006 b | 0.36 ± 0.006 c | |
| 120 | 2.13 ± 0.013 a | 0.31 ± 0.015 c | 0.74 ± 0.005 b | 0.29 ± 0.006 d | |
| 180 | 1.31 ± 0.009 a | 0.20 ± 0.005 d | 0.70 ± 0.005 b | 0.30 ± 0.004 c | |
| anti-S. aureus | 0 | 0.38 ± 0.007 b | 0.36 ± 0.005 c | 1.42 ± 0.010 a | 0.31 ± 0.007 d |
| 60 | 2.56 ± 0.015 a | 0.41 ± 0.006 c | 1.73 ± 0.006 b | 0.39 ± 0.007 d | |
| 120 | 2.14 ± 0.011 a | 0.32 ± 0.007 c | 0.84 ± 0.007 b | 0.21 ± 0.006 d | |
| 180 | 1.32 ± 0.008 a | 0.30 ± 0.007 c | 0.80 ± 0.006 b | 0.30 ± 0.006 c | |
| anti-Str. agalactiae | 0 | 0.37 ± 0.007 b | 0.27 ± 0.005 c | 0.51 ± 0.004 a | 0.21 ± 0.006 d |
| 60 | 1.86 ± 0.009 a | 0.32 ± 0.006 b | 1.87 ± 0.008 a | 0.29 ± 0.007 c | |
| 120 | 1.88 ± 0.005 a | 0.49 ± 0.006 c | 1.40 ± 0.006 b | 0.19 ± 0.005 d | |
| 180 | 0.56 ± 0.005 b | 0.45 ± 0.005 c | 0.80 ± 0.005 a | 0.31 ± 0.006 d |
| Animal Groups | Number of Animals | Mean ± SEM * of the Group Quarter Mastitis Severity Scores ** | Distribution of Quarters as per Their Mastitis Severity Scores |
|---|---|---|---|
| Group A = vaccinated (SFMT and culture − ve) | cows (n = 10) | 0.125 ± 0.0639 | 3 quarters with score = 1 1 quarter with score = 2 |
| buffaloes (n = 30) | 0.025 ± 0.0186 | 1 quarter with score = 1 1 quarter with score = 2 | |
| Group B = control (SFMT and culture − ve) | cows (n = 15) | 0.20 ± 0.0744 | 5 quarters with score = 1 2 quarters with score = 2 1 quarter with score = 1 |
| buffaloes (n = 15) | 0.12 ± 0.0481 | 5 quarters with score = 1 1 quarter with score = 2 | |
| Group C = vaccinated (SFMT and culture + ve) | cows (n = 15) | 0.05 ± 0.0402 | 2 quarters with score = 1 1 quarter with score = 2 |
| buffaloes (n = 15) | 0.033 ± 0.0234 | 2 quarters with score = 1 | |
| Group D = control (SFMT and culture + ve) | cows (n = 10) | 0.20 ± 0.0859 | 6 quarters with score = 1 2 quarters with score = 2 |
| buffaloes (n = 10) | 0.15 ± 0.0706 | 5 quarters with score = 1 1 quarter with score = 2 |
| Animal Groups | Number of Animals | Milk Yield (Mean ± SE; L/24 h) | |||
|---|---|---|---|---|---|
| Day 0 | Day 60 | Day 120 | Day 180 | ||
| A (Healthy vaccinated) | Cows (n = 10) | 7.89 ± 0.15 | 8.17 ± 0.12↑ *** | 7.95 ± 0.17↓ | 5.45 ± 0.16↓ *** |
| Buffaloes (n = 30) | 9.13 ± 0.05 | 9.71 ± 0.06↑ ** | 9.52 ± 0.03↓ | 6.22 ± 0.10↓ *** | |
| B (Healthy control) | Cows (n = 15) | 7.87 ± 0.19 | 7.72 ± 0.11↓ * | 7.67 ± 0.20↓ | 4.49 ± 0.22↓ *** |
| Buffaloes (n = 15) | 9.10 ± 0.18 | 9.13 ± 0.09↑ | 8.57 ± 0.31↓ | 5.15 ± 0.36↓ *** | |
| C (Infected vaccinated) | Cows (n = 15) | 5.21 ± 0.13 | 6.13 ± 0.19↑ ** | 6.01 ± 0.14↓ | 4.76 ± 0.25↓ * |
| Buffaloes (n = 15) | 6.11 ± 0.25 | 7.10 ± 0.18↑ *** | 6.43 ± 0.28↓ | 4.72 ± 0.23↓ *** | |
| D (Infected control) | Cows (n = 10) | 5.18 ± 0.12 | 5.10 ± 0.08↓ | 4.95 ± 0.17↓ | 3.32 ± 0.11↓ *** |
| Buffaloes (n = 10) | 6.17 ± 0.14 | 5.86 ± 0.13↓ *** | 5.27 ± 0.04↓ | 3.79 ± 0.12↓ *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qudratullah; Muhammad, G.; Jamil, T.; Rashid, I.; Ullah, Q.; Saqib, M. Efficacy Evaluation of a Combined Hemorrhagic Septicemia–Mastitis Vaccine in Dairy Cows and Buffaloes. Animals 2022, 12, 706. https://doi.org/10.3390/ani12060706
Qudratullah, Muhammad G, Jamil T, Rashid I, Ullah Q, Saqib M. Efficacy Evaluation of a Combined Hemorrhagic Septicemia–Mastitis Vaccine in Dairy Cows and Buffaloes. Animals. 2022; 12(6):706. https://doi.org/10.3390/ani12060706
Chicago/Turabian StyleQudratullah, Ghulam Muhammad, Tariq Jamil, Imaad Rashid, Qudrat Ullah, and Muhammad Saqib. 2022. "Efficacy Evaluation of a Combined Hemorrhagic Septicemia–Mastitis Vaccine in Dairy Cows and Buffaloes" Animals 12, no. 6: 706. https://doi.org/10.3390/ani12060706
APA StyleQudratullah, Muhammad, G., Jamil, T., Rashid, I., Ullah, Q., & Saqib, M. (2022). Efficacy Evaluation of a Combined Hemorrhagic Septicemia–Mastitis Vaccine in Dairy Cows and Buffaloes. Animals, 12(6), 706. https://doi.org/10.3390/ani12060706







