Heavy Metal Accumulation, Tissue Injury, Oxidative Stress, and Inflammation in Dromedary Camels Living near Petroleum Industry Sites in Saudi Arabia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
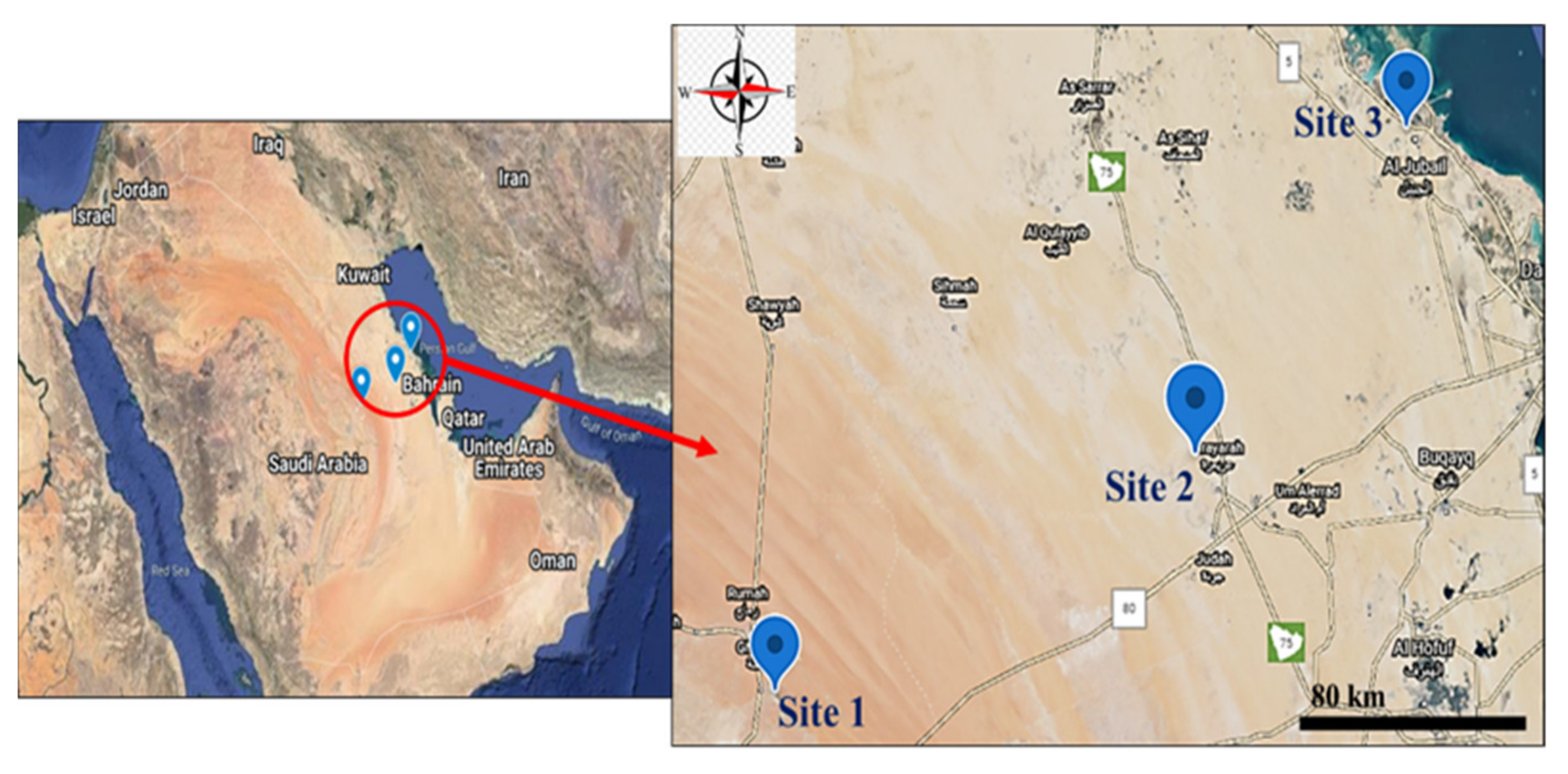
2.1. Collection of Samples
2.2. Determination of Soil and Tissue HMs
2.3. Determination of Aminotransferases, Creatininne, and Urea
2.4. Detremination of MDA and Antioxidants
2.5. Measurement of Pro-Inflammatory Cytokines
2.6. Histolopathological Examination
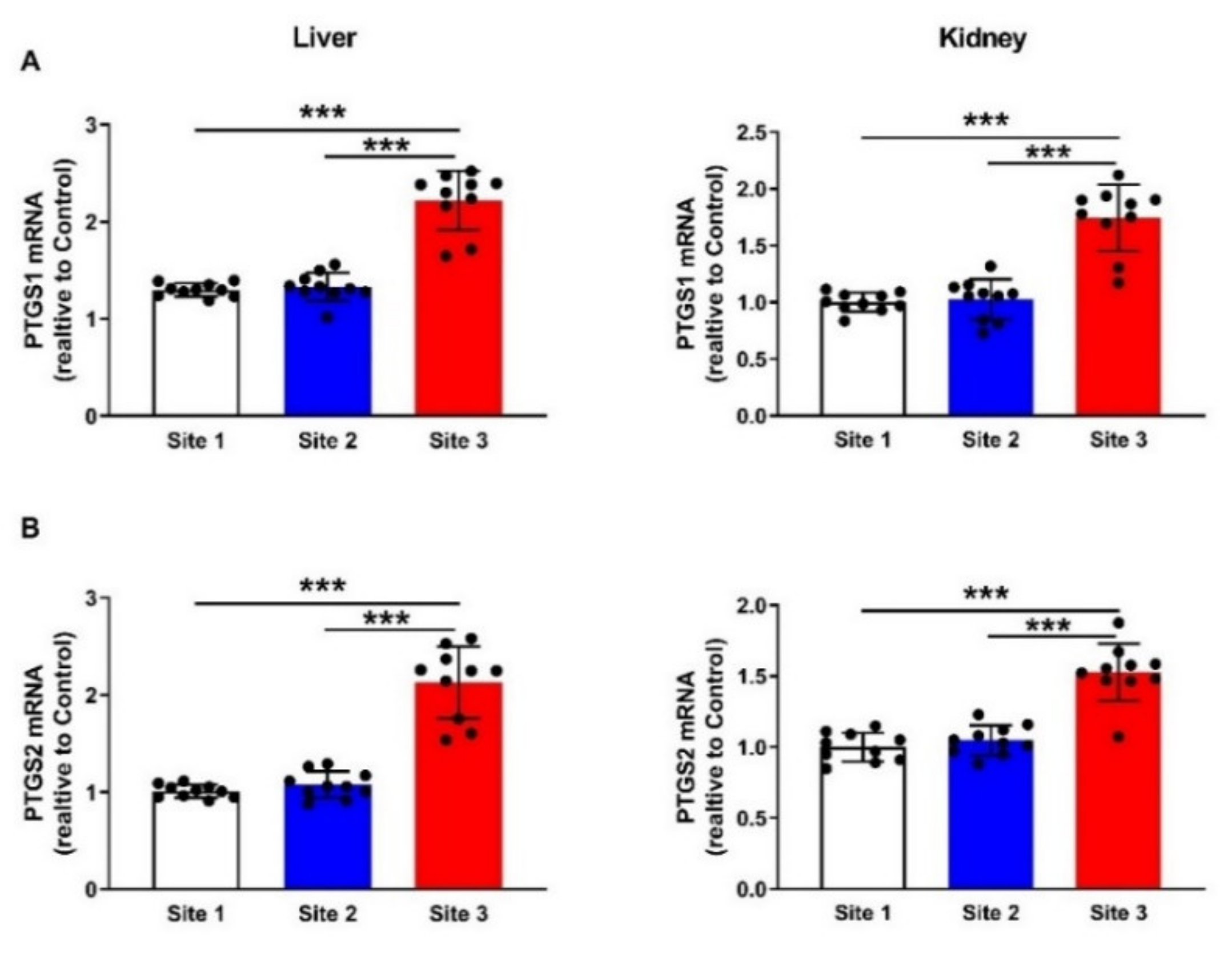
2.7. Gene Expression
2.8. Statistical Analysis
3. Results
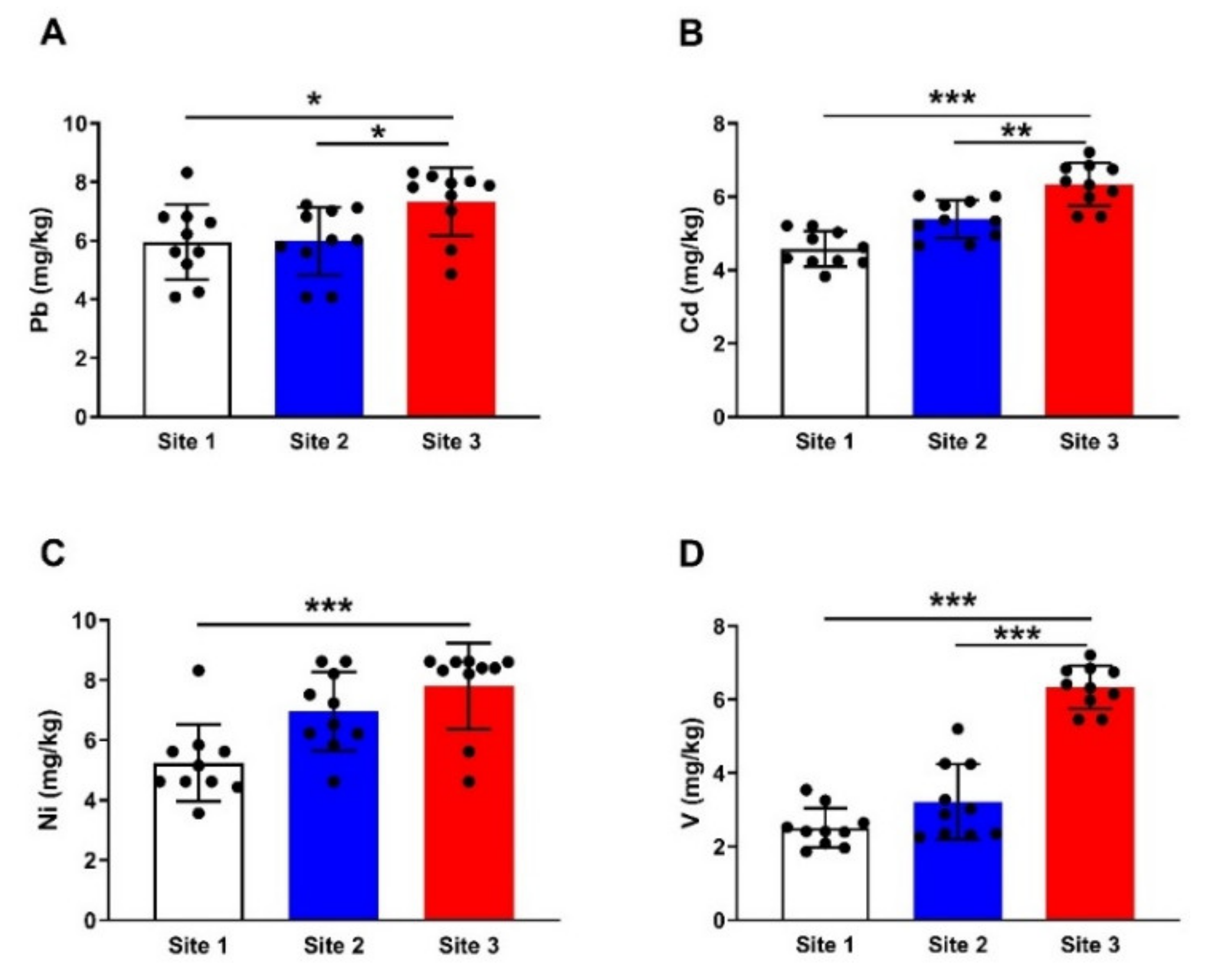
3.1. HMs in Soil Samples
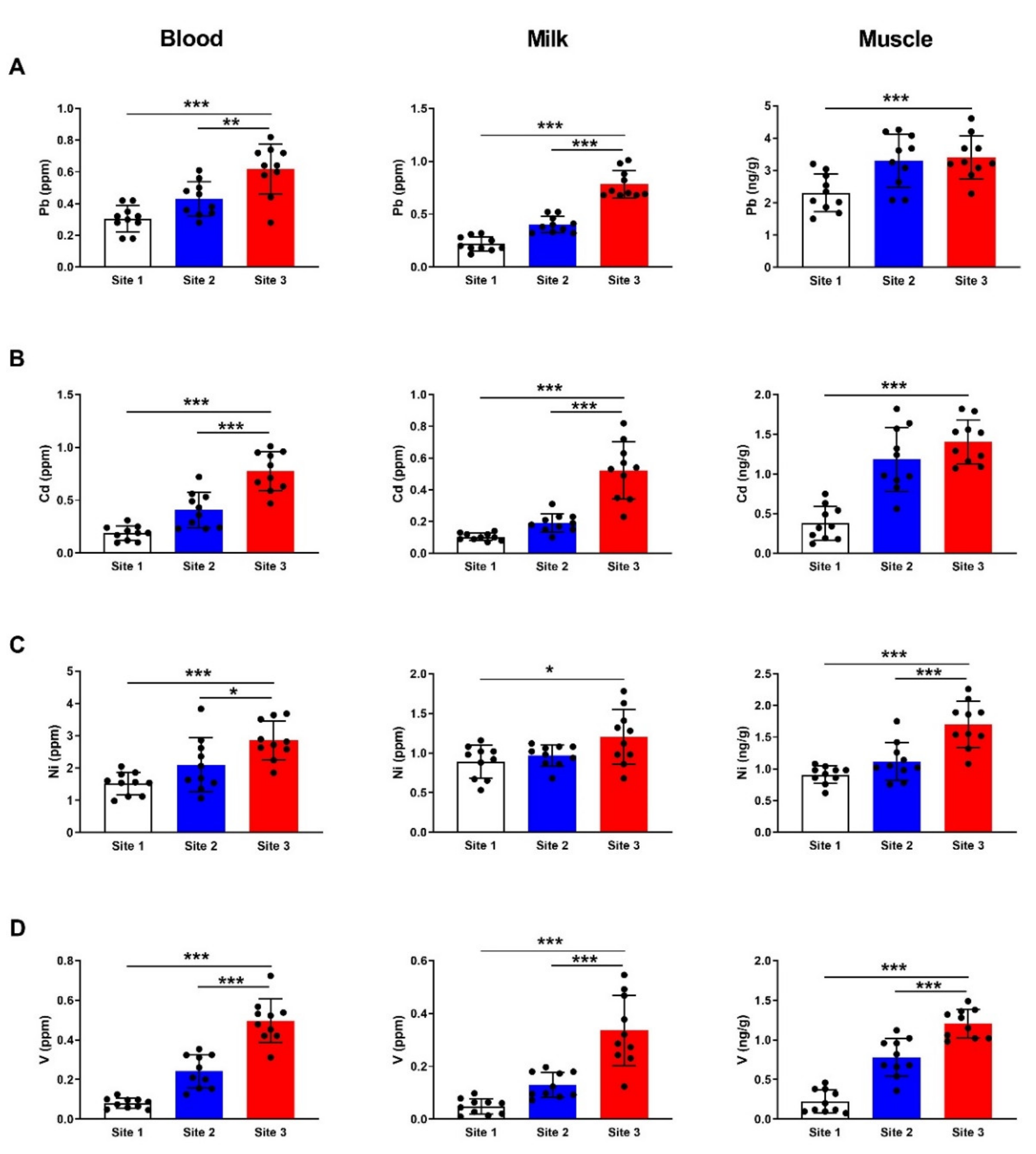
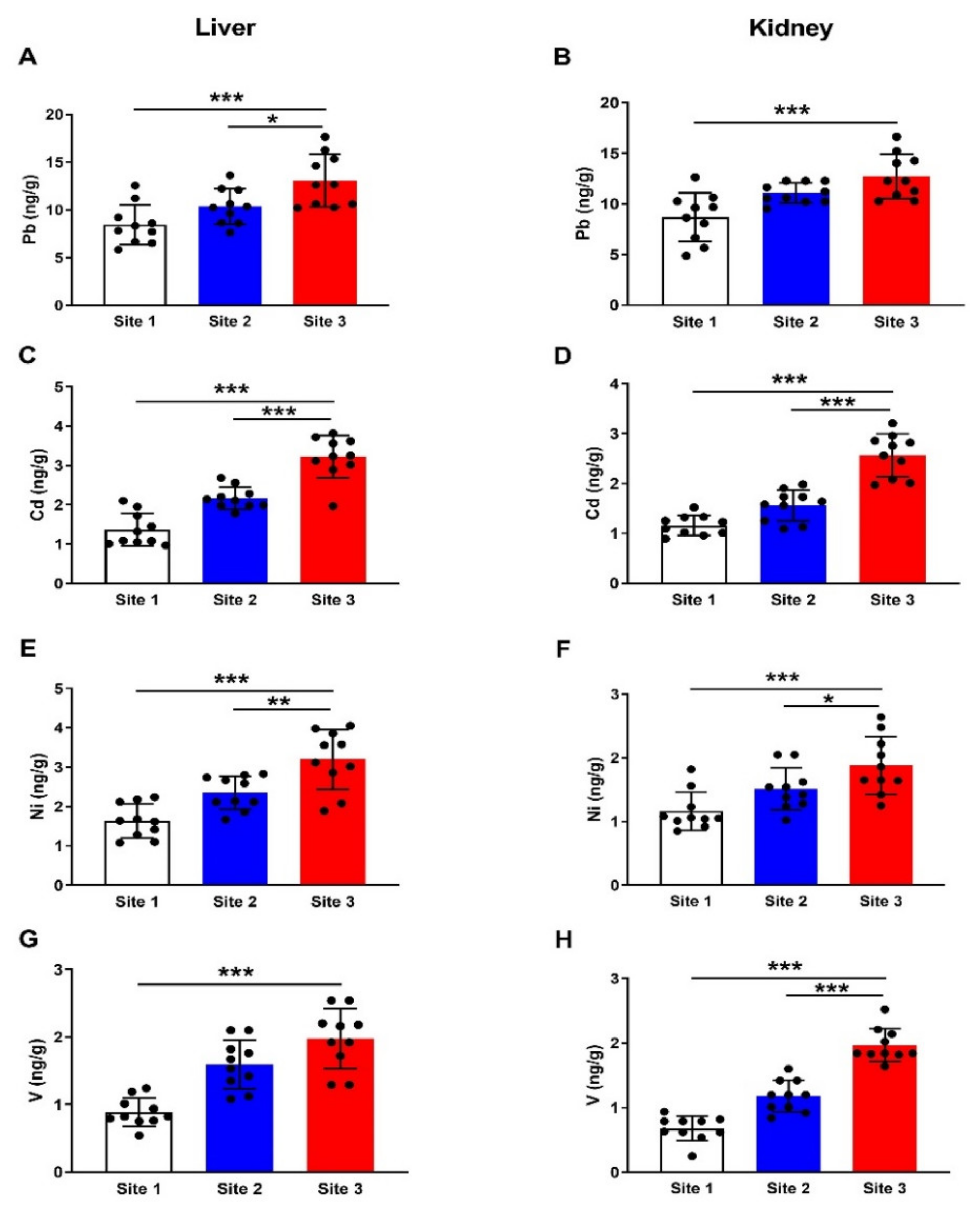
3.2. HMs in Different Tissues of Camels
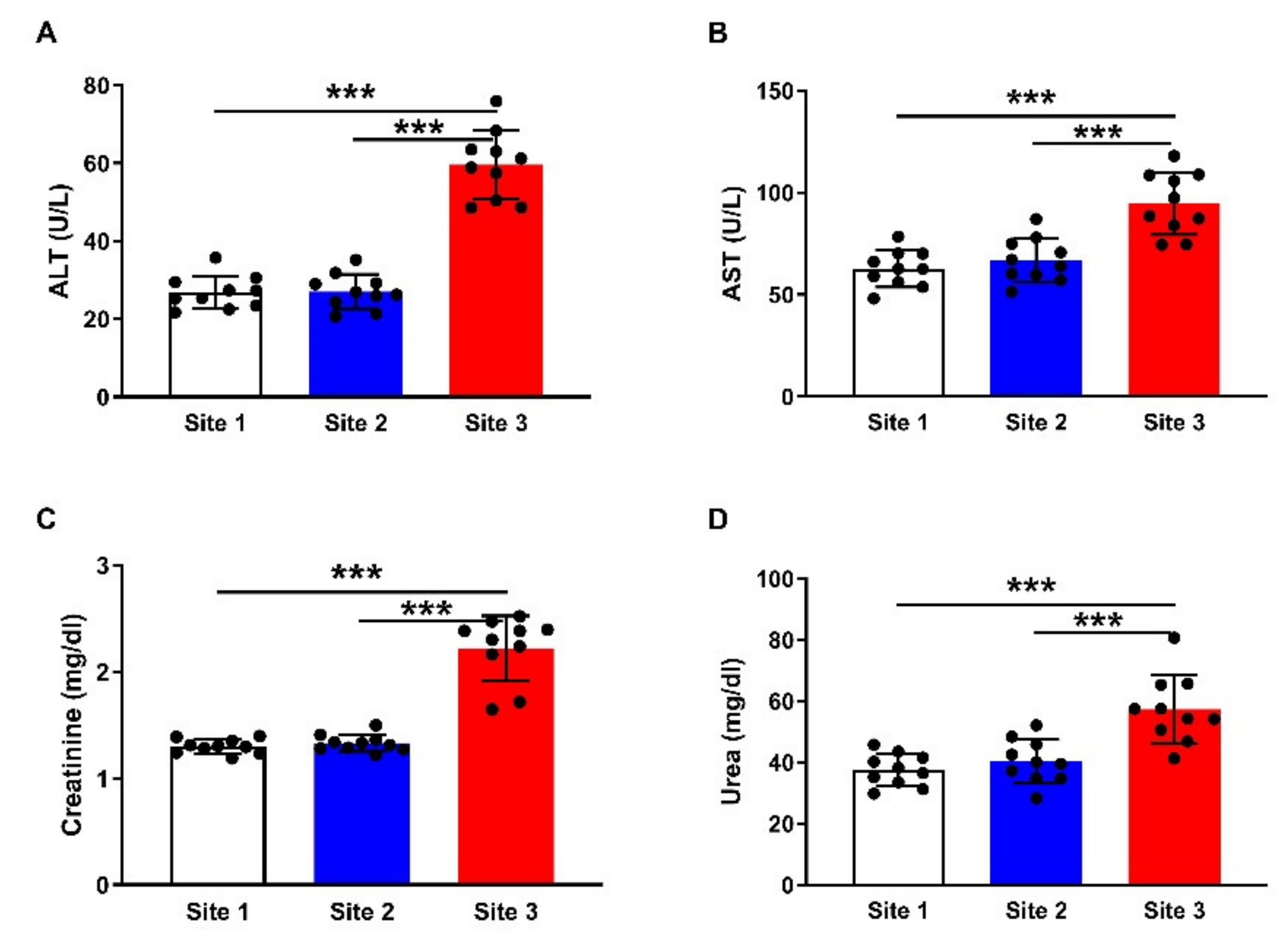
3.3. Liver and Kidney Function Markers
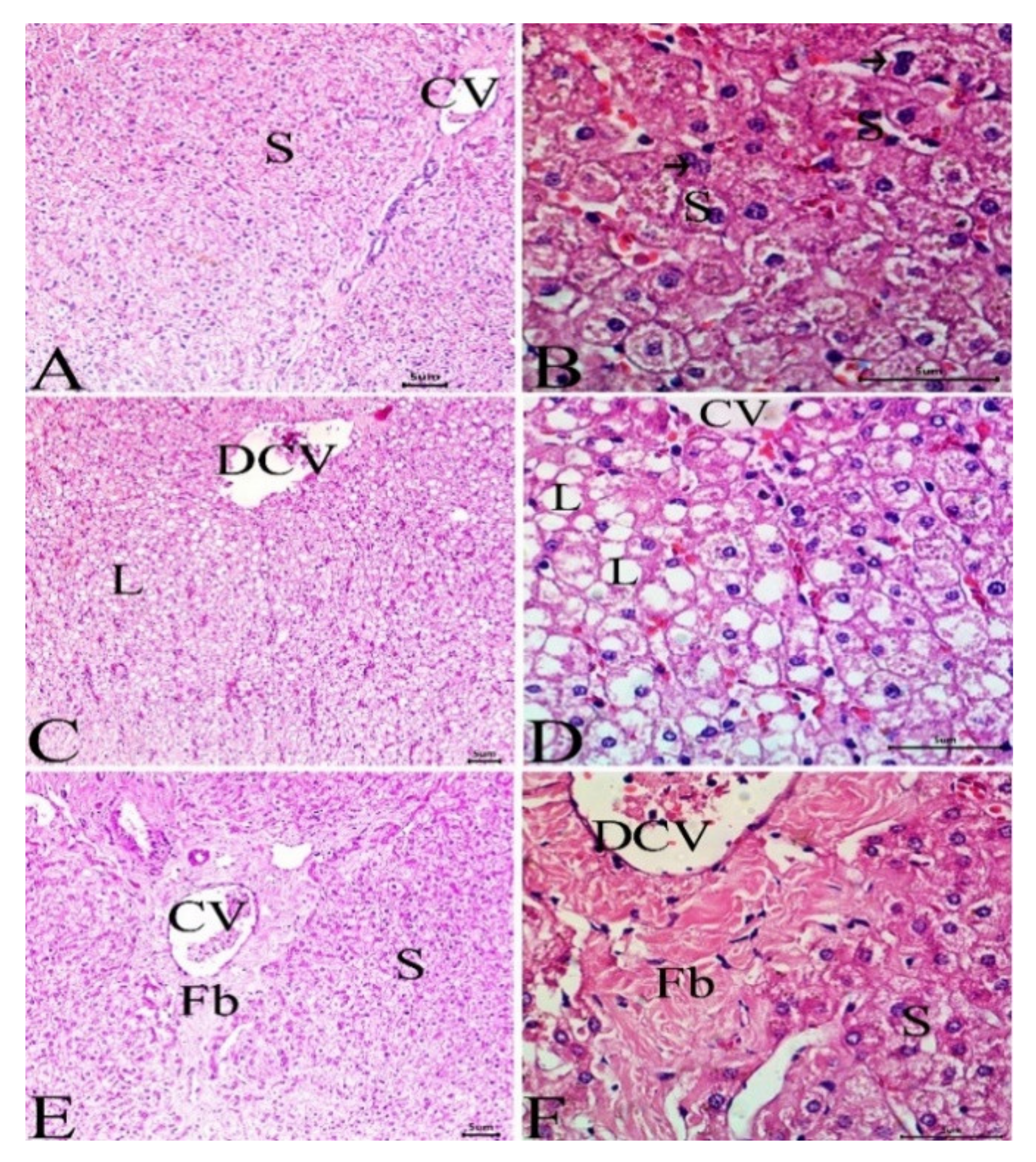
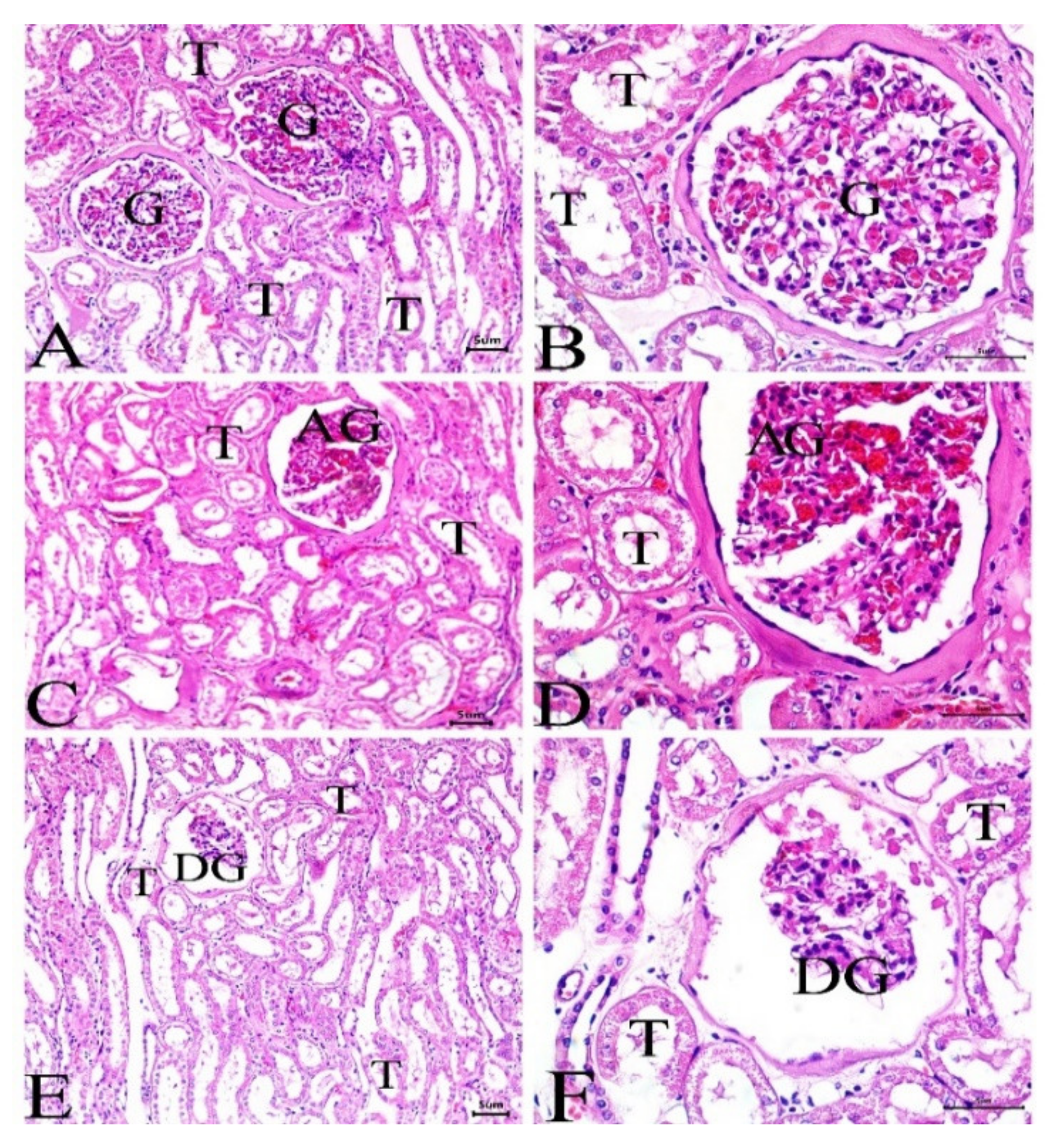
3.4. Histological Investigation
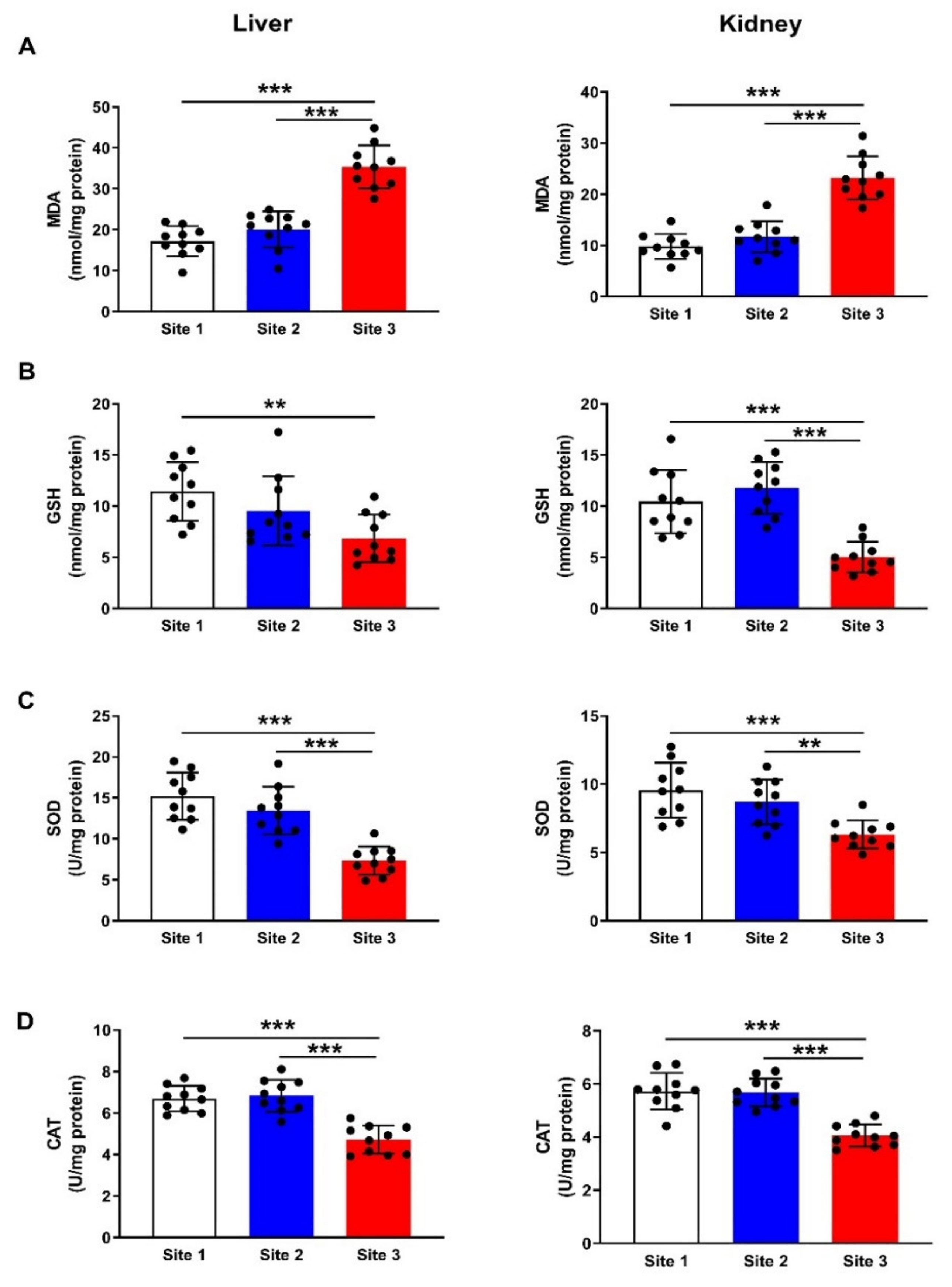
3.5. MDA and Antioxidants
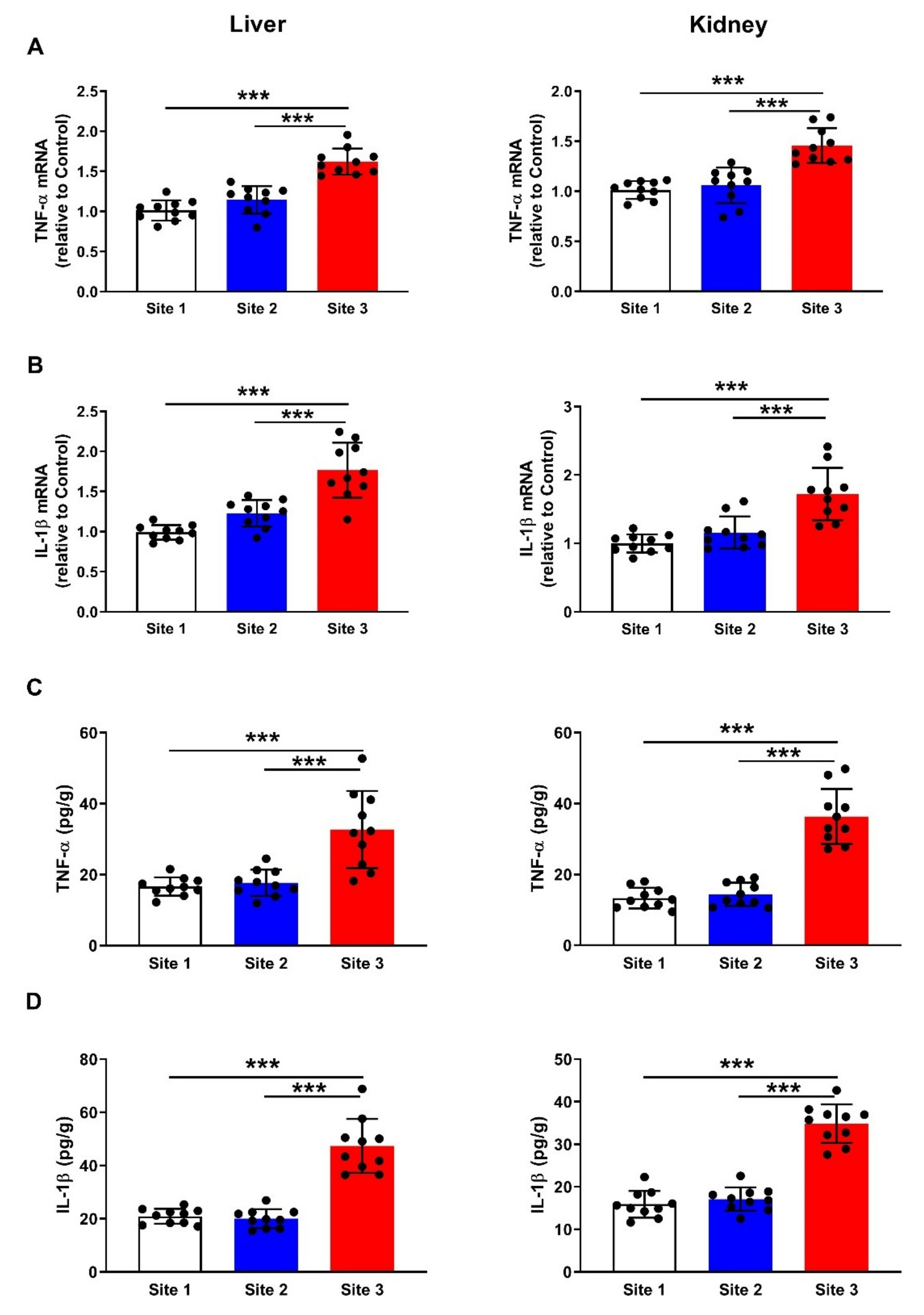
3.6. Inflammatory Cytokines
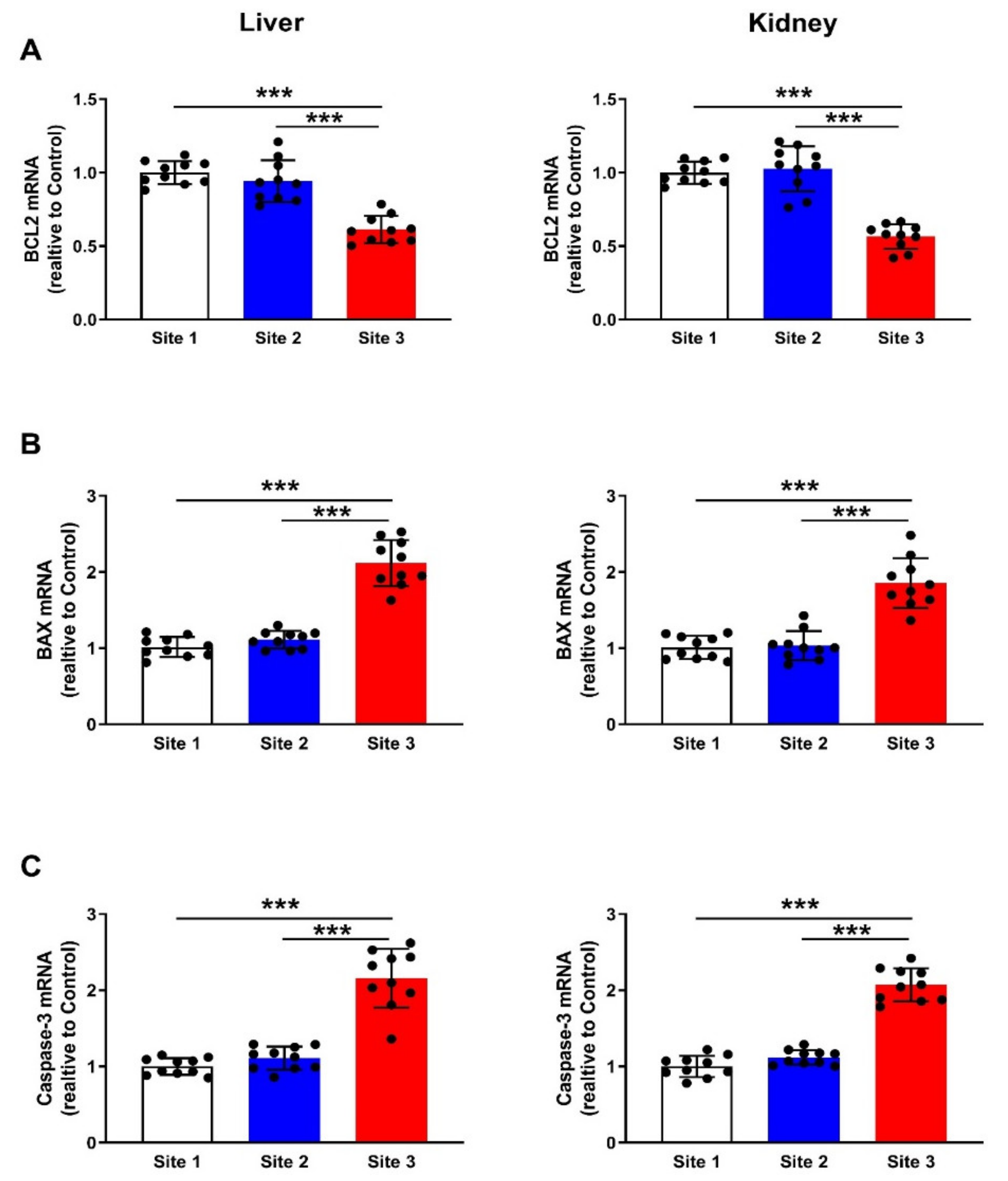
3.7. Apoptosis Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnston, J.E.; Lim, E.; Roh, H. Impact of upstream oil extraction and environmental public health: A review of the evidence. Sci. Total Environ. 2019, 657, 187–199. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan-Gordo, C.; Orta-Martínez, M.; Kogevinas, M. Health effects of non-occupational exposure to oil extraction. Environ. Health A Glob. Access Sci. Source 2016, 15, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlgren, J.; Takhar, H.; Anderson-Mahoney, P.; Kotlerman, J.; Tarr, J.; Warshaw, R. Cluster of systemic lupus erythematosus (sle) associated with an oil field waste site: A cross sectional study. Environ. Health A Glob. Access Sci. Source 2007, 6, 8. [Google Scholar]
- Yermukhanova, L.S.; Zhexenova, A.N.; Izimbergenova, G.N.; Turebaev, M.N.; Bekbauova, A.U.; Zhumabekov, E.B.; Umbetov, M.U. Immunodeficiency states in persons residing in the oil-producing regions of kazakhstan. Res. J. Med. Sci. Res. J. Med. Sci. 2017, 11, 16–18. [Google Scholar]
- Dey, T.; Gogoi, K.; Unni, B.; Bharadwaz, M.; Kalita, M.; Ozah, D.; Kalita, M.; Kalita, J.; Baruah, P.K.; Bora, T. Role of environmental pollutants in liver physiology: Special references to peoples living in the oil drilling sites of assam. PLoS ONE 2015, 10, e0123370. [Google Scholar] [CrossRef] [Green Version]
- Paz-y-Miño, C.; López-Cortés, A.; Arévalo, M.; Sánchez, M.E. Monitoring of DNA damage in individuals exposed to petroleum hydrocarbons in ecuador. Ann. N. Y. Acad. Sci. 2008, 1140, 121–128. [Google Scholar] [CrossRef]
- Norouzirad, R.; González-Montaña, J.-R.; Martínez-Pastor, F.; Hosseini, H.; Shahrouzian, A.; Khabazkhoob, M.; Ali Malayeri, F.; Moallem Bandani, H.; Paknejad, M.; Foroughi-nia, B.; et al. Lead and cadmium levels in raw bovine milk and dietary risk assessment in areas near petroleum extraction industries. Sci. Total Environ. 2018, 635, 308–314. [Google Scholar] [CrossRef]
- Barwise, A.J.G. Role of nickel and vanadium in petroleum classification. Energy Fuels 1990, 4, 647–652. [Google Scholar] [CrossRef]
- Filby, R.H.; Branthaver, J.F. Metal Complexes in Fossil Fuels: Geochemistry, Characterization, and Processing; Chemical Society: Washington, DC, USA, 1987. [Google Scholar]
- Nesbitt, J.A.; Lindsay, M.B. Vanadium geochemistry of oil sands fluid petroleum coke. Environ. Sci. Technol. 2017, 51, 3102–3109. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Klein, E.M.; Vengosh, A. Global biogeochemical cycle of vanadium. Proc. Natl. Acad. Sci. USA 2017, 114, E11092–E11100. [Google Scholar] [CrossRef] [Green Version]
- Castellini, C.; Mourvaki, E.; Sartini, B.; Cardinali, R.; Moretti, E.; Collodel, G.; Fortaner, S.; Sabbioni, E.; Renieri, T. In vitro toxic effects of metal compounds on kinetic traits and ultrastructure of rabbit spermatozoa. Reprod. Toxicol. 2009, 27, 46–54. [Google Scholar] [CrossRef]
- Altamirano-Lozano, M.A.; Alvarez-Barrera, L.; Mateos-Nava, R.A.; Fortoul, T.I.; Rodriguez-Mercado, J.J. Potential for genotoxic and reprotoxic effects of vanadium compounds due to occupational and environmental exposures: An article based on a presentation at the 8th international symposium on vanadium chemistry, biological chemistry, and toxicology, Washington DC, august 15–18, 2012. J. Immunotoxicol. 2014, 11, 19–27. [Google Scholar]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Zaidi, A.; Wani, P.A.; Oves, M. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ. Chem. Lett. 2009, 7, 1–19. [Google Scholar] [CrossRef]
- Miedico, O.; Iammarino, M.; Paglia, G.; Tarallo, M.; Mangiacotti, M.; Chiaravalle, A.E. Environmental monitoring of the area surrounding oil wells in val d’agri (italy): Element accumulation in bovine and ovine organs. Environ. Monit. Assess. 2016, 188, 338. [Google Scholar] [CrossRef]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [Green Version]
- Rzymski, P.; Niedzielski, P.; Klimaszyk, P.; Poniedzialek, B. Bioaccumulation of selected metals in bivalves (unionidae) and phragmites australis inhabiting a municipal water reservoir. Environ. Monit. Assess. 2014, 186, 3199–3212. [Google Scholar] [CrossRef] [Green Version]
- Rzymski, P.; Tomczyk, K.; Rzymski, P.; Poniedzialek, B.; Opala, T.; Wilczak, M. Impact of heavy metals on the female reproductive system. Ann. Agric. Environ. Med. AAEM 2015, 22, 259–264. [Google Scholar] [CrossRef]
- Markiewicz-Górka, I.; Januszewska, L.; Michalak, A.; Prokopowicz, A.; Januszewska, E.; Pawlas, N.; Pawlas, K. Effects of chronic exposure to lead, cadmium, and manganese mixtures on oxidative stress in rat liver and heart. Arh. Za Hig. Rada I Toksikol. 2015, 66, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Ajarem, J.S.; Hegazy, A.K.; Allam, G.A.; Allam, A.A.; Maodaa, S.N.; Mahmoud, A.M. Effect of visnagin on altered steroidogenesis and spermatogenesis, and testicular injury induced by the heavy metal lead. Comb. Chem. High Throughput Screen. 2021, 24, 758–766. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, K.; Bao, R.; Li, J.; Tang, Y.; Teng, X. Th1/th2 imbalance and heat shock protein mediated inflammatory damage triggered by manganese via activating nf-κb pathway in chicken nervous system in vivo and in vitro. Environ. Sci. Pollut. Res. Int. 2021, 28, 44361–44373. [Google Scholar] [CrossRef]
- Chen, X.; Bi, M.; Yang, J.; Cai, J.; Zhang, H.; Zhu, Y.; Zheng, Y.; Liu, Q.; Shi, G.; Zhang, Z. Cadmium exposure triggers oxidative stress, necroptosis, th1/th2 imbalance and promotes inflammation through the tnf-α/nf-κb pathway in swine small intestine. J. Hazard. Mater. 2022, 421, 126704. [Google Scholar] [CrossRef]
- Alhusaini, A.; Fadda, L.; Hasan, I.H.; Ali, H.M.; El Orabi, N.F.; Badr, A.M.; Zakaria, E.; Alenazi, A.M.; Mahmoud, A.M. Arctium lappa root extract prevents lead-induced liver injury by attenuating oxidative stress and inflammation, and activating akt/gsk-3β signaling. Antioxidants 2019, 8, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhusaini, A.; Fadda, L.; Hasan, I.H.; Zakaria, E.; Alenazi, A.M.; Mahmoud, A.M. Curcumin ameliorates lead-induced hepatotoxicity by suppressing oxidative stress and inflammation, and modulating akt/gsk-3β signaling pathway. Biomolecules 2019, 9, 703. [Google Scholar] [CrossRef] [Green Version]
- Sarawi, W.S.; Alhusaini, A.M.; Fadda, L.M.; Alomar, H.A.; Albaker, A.B.; Aljrboa, A.S.; Alotaibi, A.M.; Hasan, I.H.; Mahmoud, A.M. Curcumin and nano-curcumin mitigate copper neurotoxicity by modulating oxidative stress, inflammation, and akt/gsk-3β signaling. Molecules 2021, 26, 5591. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.F.; Al-Joufi, F.; Abou Seif, H.S.; Alzoghaibi, M.A.; Djouhri, L.; Ahmeda, A.F.; Mahmoud, A.M. Umbelliferone inhibits spermatogenic defects and testicular injury in lead-intoxicated rats by suppressing oxidative stress and inflammation, and improving nrf2/ho-1 signaling. Drug Des. Dev. Ther. 2020, 14, 4003–4019. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Sun, Q.; Liu, Y.; Tang, Y.; Teng, X. Nlrp3 inflammasome is involved in the mechanism of mitigative effect of selenium on lead-induced inflammatory damage in chicken kidneys. Environ. Sci. Pollut. Res. Int. 2021, 28, 10898–10908. [Google Scholar] [CrossRef] [PubMed]
- Adegbesan, B.O.; Adenuga, G.A. Effect of lead exposure on liver lipid peroxidative and antioxidant defense systems of protein-undernourished rats. Biol. Trace Elem. Res. 2007, 116, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hao, M.; Liu, C.; Liu, R. Cadmium induced apoptosis in mouse primary hepatocytes: The role of oxidative stress-mediated erk pathway activation and the involvement of histone h3 phosphorylation. RSC Adv. 2015, 5, 31798–31806. [Google Scholar] [CrossRef]
- Kachhawa, K.; Varma, M.; Kachhawa, P.; Agrawal, D.; Shaikh, M.; Kumar, S. Study of dyslipidemia and antioxidant status in chronic kidney disease patients at a hospital in south east asia. J. Health Res. Rev. 2016, 3, 28–30. [Google Scholar] [CrossRef]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Sinha, A.K.J.A.b. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Philadelphia, PA, USA, 2008. [Google Scholar]
- Gholami, K.; Loh, S.Y.; Salleh, N.; Lam, S.K.; Hoe, S.Z. Selection of suitable endogenous reference genes for qpcr in kidney and hypothalamus of rats under testosterone influence. PLoS ONE 2017, 12, e0176368. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D.J.m. Analysis of relative gene expression data using real-time quantitative pcr and 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Almalki, A.M.; Ajarem, J.; Altoom, N.; Al-Otaibi, F.S.; Maodaa, S.N.; Allam, A.A.; Mahmoud, A.M. Effects of mining activities on gerbillus nanus in saudi arabia: A biochemical and histological study. Animals 2019, 9, 664. [Google Scholar] [CrossRef] [Green Version]
- Almalki, A.; Ajarem, J.; Allam, A.; El-Serehy, H.; Maodaa, S.; Mahmoud, A.M. Use of spilopelia senegalensis as a biomonitor of heavy metal contamination from mining activities in riyadh (saudi arabia). Animals 2019, 9, 1046. [Google Scholar] [CrossRef] [Green Version]
- Al-Otaibi, F.S.; Ajarem, J.S.; Abdel-Maksoud, M.A.; Maodaa, S.; Allam, A.A.; Al-Basher, G.I.; Mahmoud, A.M. Stone quarrying induces organ dysfunction and oxidative stress in meriones libycus. Toxicol. Ind. Health 2018, 34, 679–692. [Google Scholar] [CrossRef]
- World Health Organization. Lead Poisoning and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health (accessed on 14 December 2021).
- Mudipalli, A. Lead hepatotoxicity & potential health effects. Indian J. Med. Res. 2007, 126, 518–527. [Google Scholar]
- Jarup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Thevenod, F.; Lee, W.K. Toxicology of cadmium and its damage to mammalian organs. Met. Ions Life Sci. 2013, 11, 415–490. [Google Scholar]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Cienc. Saude Coletiva 2011, 16, 2587–2602. [Google Scholar] [CrossRef]
- Brown, P. Qualitative methods in environmental health research. Environ. Health Perspect. 2003, 111, 1789–1798. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Gu, Z.; Gu, X.; Tan, X.; Wang, S.; Zhang, R.; Li, R.; Sun, M.; Gui, C.; Li, S.; et al. Nano-selenium attenuates mitochondrial-associated apoptosis via the pi3k/akt pathway in nickel-induced hepatotoxicity in vivo and in vitro. Environ. Toxicol. 2022, 37, 101–119. [Google Scholar] [CrossRef]
- Elangovan, P.; Ramakrishnan, R.; Amudha, K.; Jalaludeen, A.M.; Sagaran, G.K.; Babu, F.R.; Pari, L. Beneficial protective effect of troxerutin on nickel-induced renal dysfunction in wistar rats. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 1–14. [Google Scholar] [CrossRef]
- El-Nekeety, A.A.; El-Kady, A.A.; Soliman, M.S.; Hassan, N.S.; Abdel-Wahhab, M.A. Protective effect of aquilegia vulgaris (L.) against lead acetate-induced oxidative stress in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2009, 47, 2209–2215. [Google Scholar] [CrossRef]
- Jia, Q.; Ha, X.; Yang, Z.; Hui, L.; Yang, X. Oxidative stress: A possible mechanism for lead-induced apoptosis and nephrotoxicity. Toxicol. Mech. Methods 2012, 22, 705–710. [Google Scholar] [CrossRef]
- El-Tantawy, W.H. Antioxidant effects of spirulina supplement against lead acetate-induced hepatic injury in rats. J. Tradit. Complementary Med. 2016, 6, 327–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flora, S.J.S.; Flora, G.; Saxena, G. Environmental occurrence, health effects and management of lead poisoning. In Lead Chemistry, Analytical Aspects, Environmental Impacts and Health Effects; Cascas, S.B., Sordo, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 158–228. [Google Scholar]
- Castagnetto, J.M.; Hennessy, S.W.; Roberts, V.A.; Getzoff, E.D.; Tainer, J.A.; Pique, M.E. Mdb: The metalloprotein database and browser at the scripps research institute. Nucleic Acids Res. 2002, 30, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, J.; Leonard, S.S.; Rao, K.M. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic. Biol. Med. 2004, 36, 1434–1443. [Google Scholar] [CrossRef]
- Akomolafe, R.O.; Imafidon, C.E.; Olukiran, O.S.; Oladele, A.A.; Ajayi, A.O. Livolin forte ameliorates cadmium-induced kidney injury in wistar rats. Serb. J. Exp. Clin. Res. 2016, 17, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Niture, S.; Lin, M.; Qi, Q.; Moore, J.T.; Levine, K.E.; Fernando, R.A.; Kumar, D. Role of autophagy in cadmium-induced hepatotoxicity and liver diseases. J. Toxicol. 2021, 2021, 9564297. [Google Scholar] [CrossRef]
- Kong, L.; Gao, X.; Zhu, J.; Cheng, K.; Tang, M. Mechanisms involved in reproductive toxicity caused by nickel nanoparticle in female rats. Environ. Toxicol. 2016, 31, 1674–1683. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, G.H.; Yoon, C.; Kim, D.W. Comparison of distribution and toxicity following repeated oral dosing of different vanadium oxide nanoparticles in mice. Environ. Res. 2016, 150, 154–165. [Google Scholar] [CrossRef]
- Xiong, Z.; Xing, C.; Xu, T.; Yang, Y.; Liu, G.; Hu, G.; Cao, H.; Zhang, C.; Guo, X.; Yang, F. Vanadium induces oxidative stress and mitochondrial quality control disorder in the heart of ducks. Front. Vet. Sci. 2021, 8, 756534. [Google Scholar] [CrossRef]
- Heidari, R.; Ahmadi, A.; Mohammadi, H.; Ommati, M.M.; Azarpira, N.; Niknahad, H. Mitochondrial dysfunction and oxidative stress are involved in the mechanism of methotrexate-induced renal injury and electrolytes imbalance. Biomed. Pharmacother. 2018, 107, 834–840. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, C.Y.; Xu, H.; Sun, Y.; Hu, F.F.; Bian, J.C.; Liu, X.Z.; Gu, J.H.; Liu, Z.P. Cadmium-induced apoptosis in primary rat cerebral cortical neurons culture is mediated by a calcium signaling pathway. PLoS ONE 2013, 8, e64330. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; Zhao, S.; Chen, J.; Yang, J.; Wang, T.; Zou, H.; Wang, Y.; Gu, J.; Liu, X.; et al. Cadmium-induced apoptosis in neuronal cells is mediated by fas/fasl-mediated mitochondrial apoptotic signaling pathway. Sci. Rep. 2018, 8, 8837. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Milner, J.N.; Howard, C.B.; Tchounwou, P.B. Basic apoptotic mechanisms of lead toxicity in human leukemia (hl-60) cells. Int. J. Environ. Res. Public Health 2010, 7, 2008–2017. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Zhang, K.; Mao, X.; Ding, X.; Zeng, Q.; Bai, S.; Xuan, Y.; Peng, H. Vanadate oxidative and apoptotic effects are mediated by the mapk-nrf2 pathway in layer oviduct magnum epithelial cells. Met. Integr. Biometal Sci. 2017, 9, 1562–1575. [Google Scholar] [CrossRef]
- Markopoulou, S.; Kontargiris, E.; Batsi, C.; Tzavaras, T.; Trougakos, I.; Boothman, D.A.; Gonos, E.S.; Kolettas, E. Vanadium-induced apoptosis of hacat cells is mediated by c-fos and involves nuclear accumulation of clusterin. FEBS J. 2009, 276, 3784–3799. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Wang, X.; Zhao, L.; Wu, B.; Chen, K.; Deng, J. Nickel chloride-induced apoptosis via mitochondria- and fas-mediated caspase-dependent pathways in broiler chickens. Oncotarget 2016, 7, 79747–79760. [Google Scholar] [CrossRef] [Green Version]
| Gene | Primers (5′-3′) | GenBank Accession Number | Product Size (bp) |
|---|---|---|---|
| PTGS1 | F: CGTGGAGTTCAACCAGCTCTA R: AGGTGTTGAAGAGGAACTGCT | XM_010997991.1 | 101 |
| PTGS2 | F: CTGGTCTGATGATGTACGCC R: ACAACCGCTCATCATCCCAT | XM_010992100.1 | 99 |
| TNF-a | F: CCAGCTCATGAACCCTCTGG R: GGGTATTGGCAAACCGCTTC | NM_001319880.1 | 134 |
| IL-1B | F: TGAACCCGCCAGTGAAATGA R: GACGCAGCACTTCATCTGTT | XM_010984994.1 | 94 |
| BAX | F: CACCAAGGTGCCTGAACTGA R: CGTGGGTGTCCCAAAGTAGG | XM_031459056.1 | 130 |
| BCL2 | F: GTTTGAACTGAGGTACCGGC R: CCCATCCCGGAAGAGTTCAT | XM_010993888.1 | 115 |
| CASP3 | F: GCTTCTTCAGAGGGGACAGT R: TCGGCAGGCCTGAATAATGA | XM_010974562.1 | 71 |
| PPIA | F: ACCACCAGACCATTCCTTCT R: TATGGAACCCCGAAAACTGC | XM_010987886.1 | 109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajarem, J.S.; Hegazy, A.K.; Allam, G.A.; Allam, A.A.; Maodaa, S.N.; Mahmoud, A.M. Heavy Metal Accumulation, Tissue Injury, Oxidative Stress, and Inflammation in Dromedary Camels Living near Petroleum Industry Sites in Saudi Arabia. Animals 2022, 12, 707. https://doi.org/10.3390/ani12060707
Ajarem JS, Hegazy AK, Allam GA, Allam AA, Maodaa SN, Mahmoud AM. Heavy Metal Accumulation, Tissue Injury, Oxidative Stress, and Inflammation in Dromedary Camels Living near Petroleum Industry Sites in Saudi Arabia. Animals. 2022; 12(6):707. https://doi.org/10.3390/ani12060707
Chicago/Turabian StyleAjarem, Jamaan S., Ahmad K. Hegazy, Gamal A. Allam, Ahmed A. Allam, Saleh N. Maodaa, and Ayman M. Mahmoud. 2022. "Heavy Metal Accumulation, Tissue Injury, Oxidative Stress, and Inflammation in Dromedary Camels Living near Petroleum Industry Sites in Saudi Arabia" Animals 12, no. 6: 707. https://doi.org/10.3390/ani12060707
APA StyleAjarem, J. S., Hegazy, A. K., Allam, G. A., Allam, A. A., Maodaa, S. N., & Mahmoud, A. M. (2022). Heavy Metal Accumulation, Tissue Injury, Oxidative Stress, and Inflammation in Dromedary Camels Living near Petroleum Industry Sites in Saudi Arabia. Animals, 12(6), 707. https://doi.org/10.3390/ani12060707






