Fermentation of Soybean Meal with Lactobacillus acidophilus Allows Greater Inclusion of Vegetable Protein in the Diet and Can Reduce Vibrionacea in the Intestine of the South American Catfish (Rhamdia quelen)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
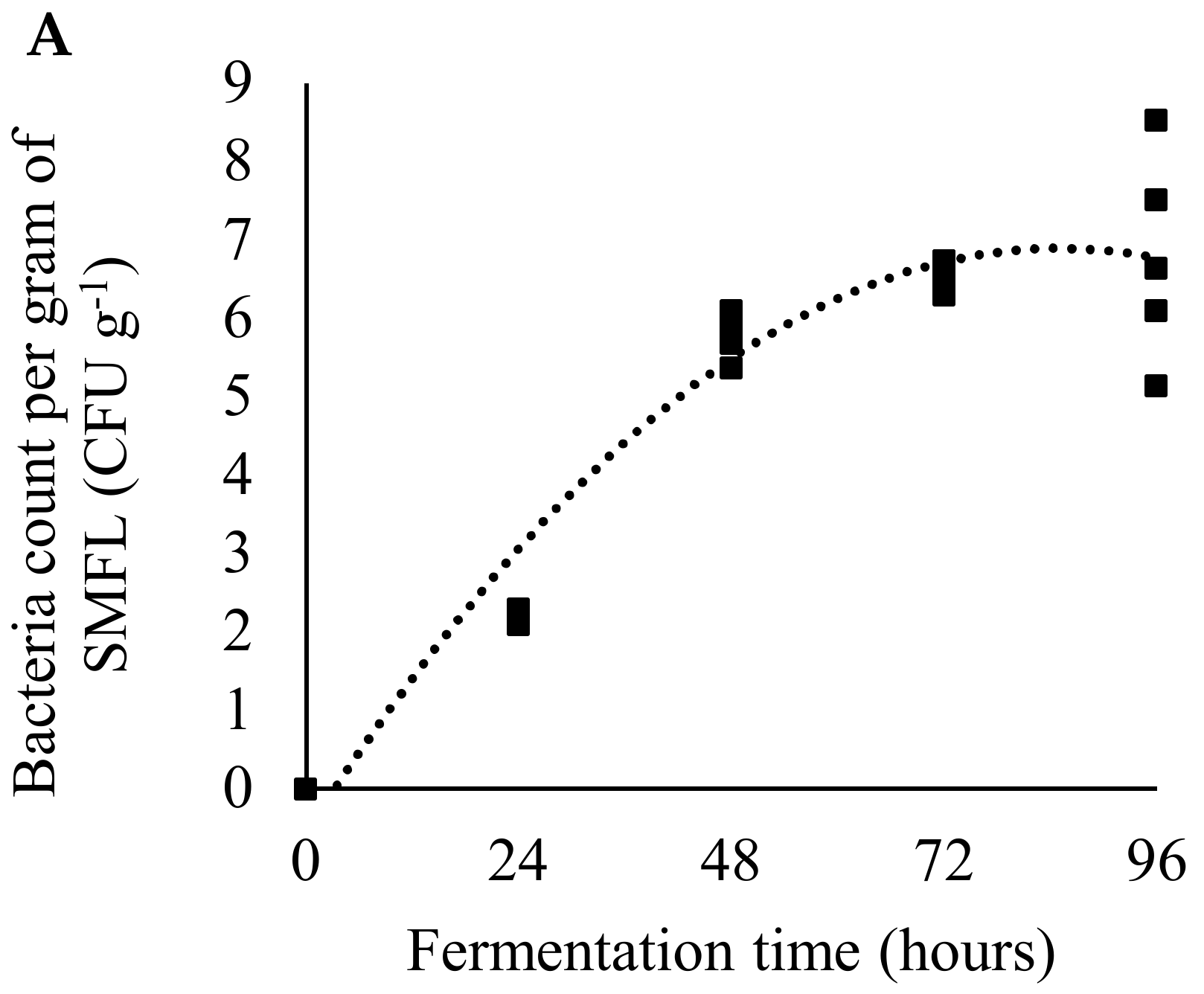
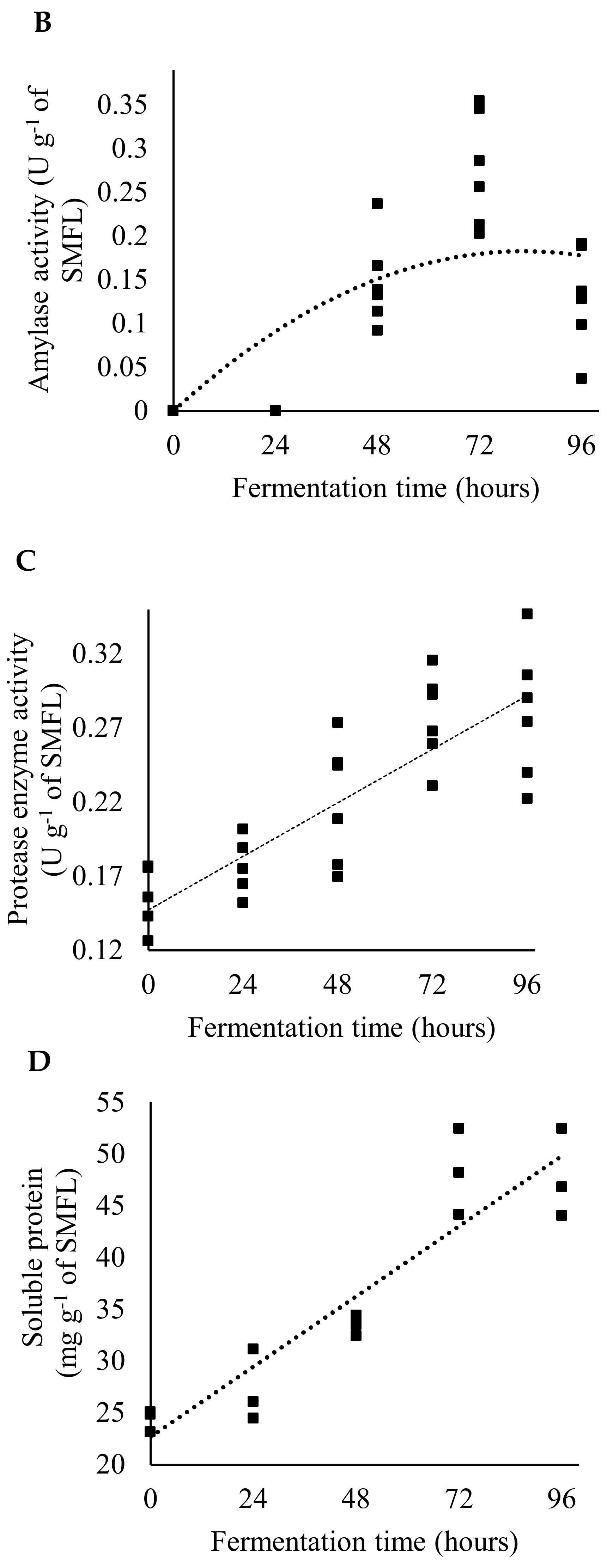
2.2. Production and Characterization of Fermented Soybean Meal
2.3. Experimental Diets
2.4. Animals and Facilities
2.5. Productive Performance and Sample Collection
2.6. Count of Total Heterotrophic, Lactic Acid and Vibrionaceae Bacteria
2.7. Enzyme Analysis
2.8. Intestinal Morphometry
2.9. Statistical Analysis
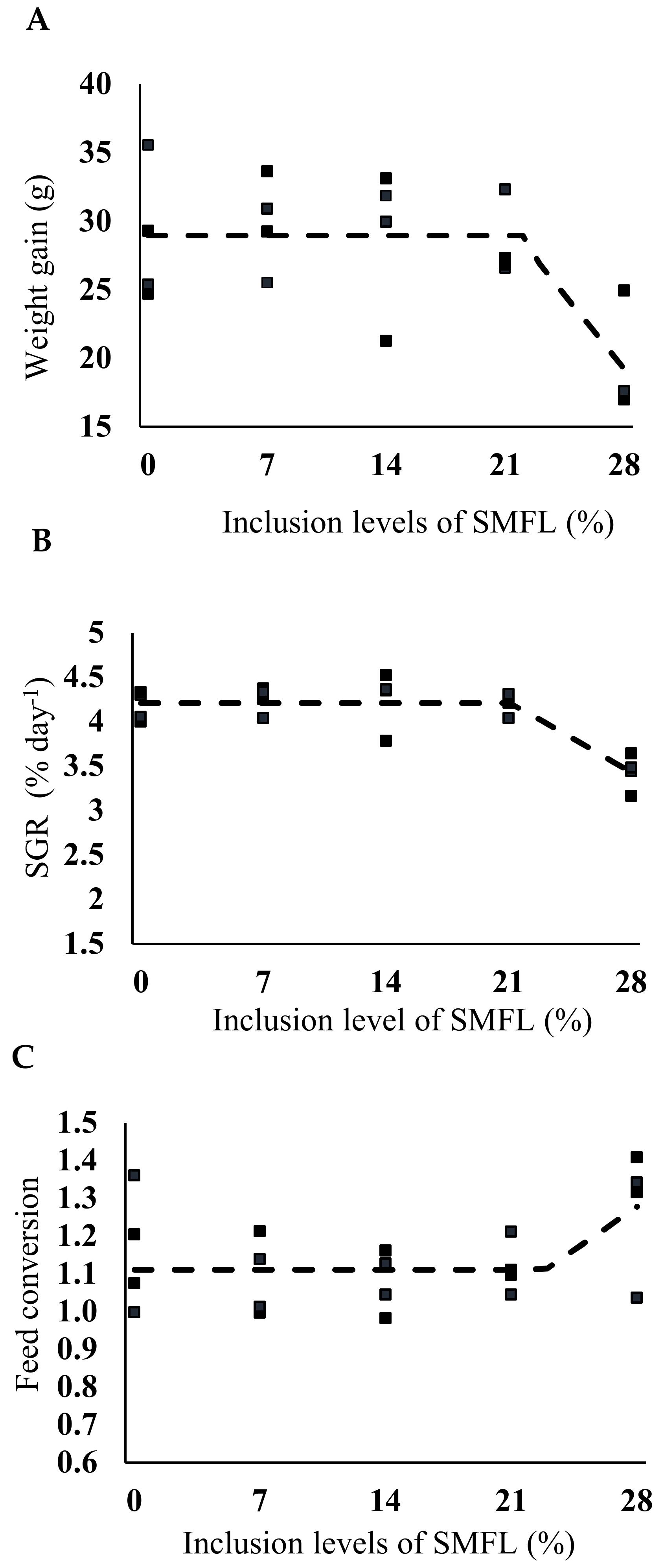
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yasothai, R. Antinutritional factors in soybean meal and its deactivation. Int. J. Environ. Sci. Technol. 2016, 5, 3793–3797. [Google Scholar]
- Iwashita, Y.; Yamamoto, T.; Furuita, H.; Sugita, T.; Suzuki, N. Influence of certain soybean antinutritional factors supplemented to a casein-based semipurified diet on intestinal and liver morphology in fingerling rainbow trout Oncorhynchus mykiss. Fish. Sci. 2008, 74, 1075–1082. [Google Scholar] [CrossRef]
- Liang, X.F.; Hu, L.; Dong, Y.C.; Wu, X.F.; Qin, Y.C.; Zheng, Y.H.; Shi, D.D.; Xue, M. Substitution of fish meal by fermented soybean meal affects the growth performance and flesh quality of Japanese seabass (Lateolabrax japonicus). Anim. Feed Sci. Technol. 2017, 229, 1–12. [Google Scholar] [CrossRef]
- Zheng, L.; Li, D.; Li, Z.L.; Kang, L.N.; Jiang, Y.Y.; Liu, X.Y.; Chi, Y.P.; Li, Y.Q.; Wang, J.H. Effects of Bacillus fermentation on the protein microstructure and anti-nutritional factors of soybean meal. Lett. Appl. Microbiol. 2017, 65, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Choi, D.G.; He, M.; Fang, H.; Wang, X.L.; Li, X.Q.; Leng, X.J. Replacement of fish meal with two fermented soybean meals in diets for rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2019, 26, 37–46. [Google Scholar] [CrossRef]
- Hutkins, R.W. (Ed.) Fermented vegetables. In Microbiology and Technology of Fermented Foods; Blackwell Publishing: Oxford, UK, 2006; pp. 223–259. [Google Scholar] [CrossRef]
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Role of fermentation in improving nutritional quality of soybean meal—A review. Asian-Australas. J. Anim. Sci. 2016, 29, 1523. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Esteban, M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2017, 10, 950–974. [Google Scholar] [CrossRef]
- Selle, K.M.; Klaenhammer, T.R.; Russel, W.M. Lactobacillus acidophilus . In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press Elsevier: Cambridge, MA, USA, 2014; Volume 1, pp. 412–417. [Google Scholar] [CrossRef]
- Gaenzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Ray, B. Probiotics of lactic acid bacteria: Science or myth? In Lactic Acid Bacteria: Current Advances in Metabolism, Genetics and Applications; Bozoglu, T.F., Ray, B., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 101–136. [Google Scholar]
- Gopal, P.K. Lactic Acid Bacteria: Lactobacillus spp.: Lactobacillus acidophilus. In Encyclopedia of Dairy Sciences, 2nd ed.; Gopal, P.K., Ed.; Academic Press: Palmerston North, New Zealand, 2011; pp. 91–95. [Google Scholar]
- Xu, C.; Liu, W.; Zhang, D.; Liu, J.; Xiaochuan, Z.; Zhang, C.; Yao, J.; Zhu, C.; Chi, C. Effects of partial fish meal replacement with two fermented soybean meals on the growth of and protein metabolism in the Chinese mitten crab (Eriocheir sinensis). Aquac. Rep. 2020, 17, 100328. [Google Scholar] [CrossRef]
- Xia, Y.; Lu, M.; Chen, G.; Cao, J.; Gao, F.; Wang, M.; Liu, Z.; Zhang, D.; Zhu, H.; Yi, M. Efects of dietary Lactobacillus rhamnosus JCM1136 and Lactococcus lactis subsp. lactis JCM5805 on the growth, intestinal microbiota, morphology, immune response and disease resistance of juvenile Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2018, 76, 368–379. [Google Scholar] [CrossRef]
- Jannathulla, R.; Rajaram, V.; Kalanjiam, R.; Kondusamy, A.; Moturi, M.D.; Dayal, J.S. Fishmeal availability in the scenarios of climate change: Inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquac. Res. 2019, 50, 3493–3506. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Lu, K.; Wang, L.; Song, K.; Mai, K.; Davis, D.A.; Zhang, C. Replacement of fish meal with Bacillus pumillus SE5 and Pseudozyma aphidis ZR1 fermented soybean meal in diets for Japanese seabass (Lateolabrax japonicus). Fish Shellfish Immunol. 2019, 84, 987–997. [Google Scholar] [CrossRef]
- Rui, X.; Wang, M.; Zhang, Y.; Chen, X.; Li, L.; Liu, Y.; Dong, M. Optimization of soy solid-state fermentation with selected lactic acid bacteria and the effect on the anti-nutritional components. J. Food Process. Preserv. 2016, 41, e13290. [Google Scholar] [CrossRef]
- Shi, C.; He, J.; Yu, J.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Chen, P. Solid state fermentation of rapeseed cake with Aspergillus niger for degrading glucosinolates and upgrading nutritional value. J. Anim. Sci. Biotechnol. 2015, 6, 13–19. [Google Scholar] [CrossRef]
- Sanjukta, S.; Rai, A.K. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci. Technol. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Kari, Z.A.; Kabir, M.A.; Mat, K.; Rusli, N.D.; Razab, M.K.A.A.; Ariff, N.S.N.A.; Wei, L.S. The possibility of replacing fish meal with fermented soy pulp on the growth performance, blood biochemistry, liver, and intestinal morphology of African catfish (Clarias gariepinus). Aquac. Rep. 2021, 21, 100–815. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Zhang, J.J.; Wang, L.; Sun, Y.; Zhang, C. Evaluation of Bacillus pumillus SE5 fermented soybean meal as a fish meal replacer in spotted seabass (Lateolabrax maculatus) feed. Aquaculture 2021, 531, 735975. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Liu, C.; Zhou, H.; Wang, X.; Mai, K.; He, G. Effects of dietary raw or Enterococcus faecium fermented soybean meal on growth, antioxidant status, intestinal microbiota, morphology, and inflammatory responses in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2020, 100, 261–271. [Google Scholar] [CrossRef]
- Li, S.; Ding, G.; Song, F.; Sang, C.; Wang, A.; Chen, N. Comparison of dehulled, fermented and enzyme-treated soybean meal in diets for largemouth bass, Micropterus salmoides: Effects on growth performance, feed utilization, immune response and intestinal morphology. Anim. Feed Sci. Technol. 2020, 267, 114548. [Google Scholar] [CrossRef]
- He, M.; Yu, Y.; Li, X.; Poolsawat, L.; Yang, P.; Bian, Y.; Leng, X. An evaluation of replacing fish meal with fermented soybean meal in the diets of largemouth bass (Micropterus salmoides): Growth, nutrition utilization and intestinal histology. Aquac. Res. 2020, 51, 4302–4314. [Google Scholar] [CrossRef]
- Catalán, N.; Villasante, A.; Wacyk, J.; Ramírez, C.; Romero, J. Fermented soybean meal increases lactic acid bacteria in gut microbiota of Atlantic Salmon (Salmo salar). Probiotics Antimicrob. Proteins 2018, 10, 566–576. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; Khalafalla, M.M.; Khalid, A.; Asely, A.E.; Fadl, S.E.; Ahmed, H.A. Saccharomyces cerevisiae increases the acceptability of Nile tilapia (Oreochromis niloticus) to date palm seed meal. Aquac. Rep. 2020, 17, 100314. [Google Scholar] [CrossRef]
- Phulia, V.; Sardar, P.; Sahu, N.P.; Fawole, F.J.; Shamna, N.; Gupta, S. Substitution of soybean meal with fermented Jatropha kernel meal: Effect on growth performance, body composition, and metabolic enzyme activity of Labeo rohita. Fish Physiol. Biochem. 2017, 44, 475–487. [Google Scholar] [CrossRef]
- Sotoudeh, E.; Moghaddam, J.A.; Shahhosseini, G.; Aramli, M.S. Effect of dietary Gamma-irradiated and fermented soybean meal on the growth performance, body composition, and digestive enzymes activity of Caspian brown trout, Salmo trutta caspius, juvenile. J. World Aquac. Soc. 2016, 47, 830–842. [Google Scholar] [CrossRef]
- Shamna, N.; Sardar, P.; Sahu, N.P.; Pal, A.K.; Jain, K.K.; Phulia, V. Nutritional evaluation of fermented Jatropha protein concentrate in Labeo rohita fingerlings. Aquac. Nutr. 2015, 21, 33–42. [Google Scholar] [CrossRef]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Wu, P.; Golly, M.K.; Guo, Y.; Ma, H.; He, R.; Luo, X.; Zhu, J. Effect of partial replacement of soybean meal with high-temperature fermented soybean meal in antibiotic-growth-promoter-free diets on growth performance, organ weights, serum indexes, intestinal flora and histomorphology of broiler chickens. Anim. Feed Sci. Technol. 2020, 269, 114616. [Google Scholar] [CrossRef]
- Chachaj, R.; Sembratowicz, I.; Krauze, M.; Ognik, K. The effect of partial replacement of soybean meal with fermented soybean meal on chicken performance and immune status. J. Anim. Feed Sci. 2019, 28, 263–271. [Google Scholar] [CrossRef]
- Feng, J.; Liu, X.; Xu, Z.R.; Lu, Y.P.; Liu, Y.Y. The effect of Aspergillus oryzae fermented soybean meal on growth performance, digestibility of dietary components and activities of intestinal enzymes in weaned piglets. Anim. Feed Sci. Technol. 2007, 134, 295–303. [Google Scholar] [CrossRef]
- Gomes, L.D.C.; Golombieski, J.I.; Gomes, A.R.C.; Baldisserotto, B. Biologia do jundiá Rhamdia quelen (TELEOSTEI, PIMELODIDAE). Cienc. Rural 2000, 30, 179–185. [Google Scholar] [CrossRef]
- Oliveira Filho, P.R.C.D.; Fracalossi, D.M. Coeficientes de digestibilidade aparente de ingredientes para juvenis de jundiá. Rev. Bras. Zootec. 2006, 35, 1581–1587. [Google Scholar] [CrossRef]
- Lazzari, R.; Baldisserotto, B. Nitrogen and phosphorus waste in fish farming. Bol. Inst. Pesca 2008, 34, 591–600. [Google Scholar]
- Lazzari, R.; Radünz Neto, J.; Pedron, F.D.A.; Loro, V.L.; Pretto, A.; Gioda, C.R. Protein sources and digestive enzyme activities in jundiá (Rhamdia quelen). Sci. Agric. 2010, 67, 259–266. [Google Scholar] [CrossRef]
- Azarm, H.M.; Lee, S. Effects of partial substitution of dietary fish meal by fermented soybean meal on growth performance, amino acid and biochemical parameters of juvenile black sea bream Acanthopagrus schlegeli. Aquac. Res. 2014, 45, 994–1003. [Google Scholar] [CrossRef]
- Métais, P.; Bieth, J. Determination of alpha-amylase by a microtechnic. Ann. Biol. Clin. 1968, 26, 133–142. [Google Scholar]
- García-Careño, F.L.; Haard, N.F. Characterization of proteinase classes in langostilla (Pleuroncodes planipes) and crayfish (Pacifastacus astacus) extracts. J. Food Biochem. 1993, 17, 97–113. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists—AOAC. Official Methods of Analysis, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000; p. 389. [Google Scholar]
- White, J.A.; Hart, R.J.; Fry, J.C. An evaluation of the Waters Pico-Tag system for the amino-acid analysis of food materials. J. Autom. Chem. 1986, 8, 170–177. [Google Scholar] [CrossRef]
- Lane, R.H. Cereal Foods. In Methods of Analysis of AOAC International, 16th ed.; Association of Official Agricultural Chemists: Arlington, TX, USA, 1995; Volume 2, p. 143. [Google Scholar]
- Rostagno, H.S.; Albino, L.F.T.; Hannas, M.I.; Donzele, J.L.; Sakomura, N.K.; Perazzo, F.G.; Saraiva, A.; Teixeira, M.L.; Rodrigues, P.B.; Oliveira, R.F.; et al. Tabelas Brasileiras Para Aves e Suínos: Composição de Alimentos e Exigências Nutricionais, 4th ed.; UFV-Departamento de Zootecnia: Viçosa, Brazil, 2017. [Google Scholar]
- Meyer, G.; Fracalossi, D.M. Protein requirement of jundiá fingerlings, Rhamdia. Aquaculture 2004, 240, 331–343. [Google Scholar] [CrossRef]
- Martinelli, S.G.; Radünz Neto, J.; Silva, L.P.D.; Bergamin, G.T.; Maschio, D.; Della Flora, M.A.L.; Nunes, L.M.C.; Possani, G. Densidade de estocagem e frequência alimentar no cultivo de jundiá em tanques-rede. Pesq. Agropecu. Bras. 2013, 48, 871–877. [Google Scholar] [CrossRef][Green Version]
- Camargo, S.G.O.; Pouey, J.L.O.F.; Vaz, B.S. Efeito da salinidade nos parâmetros hematológicos do jundiá (Rhamdia quelen–Quoy & Gaimard, 1824). R. Bras. Agrociênc. 2006, 12, 453–460. [Google Scholar]
- Baldisseroto, B.; Radünz Neto, J. Criação de Jundiá; UFSM: Santa Maria, Brazil, 2004; pp. 73–94. [Google Scholar]
- Jatobá, A.; Vieira, F.D.N.; Buglione Neto, C.; Silva, B.C.; Mouriño, J.L.P.; Jerônimo, G.T.; Martins, M.L. Lactic-acid bacteria isolated from the intestinal tract of Nile tilapia utilized as probiotic. Pesq. Agropecu. Bras. 2008, 43, 1201–1207. [Google Scholar] [CrossRef]
- Brabcová, J.; Prchalová, D.; Demianová, Z.; Bučánková, A.; Vogel, H.; Valterová, I.; Pichová, I.; Zarevúcka, M. Characterization of neutral lipase BT-1 isolated from the labial gland of Bombus terrestris males. PLoS ONE 2013, 8, e80066. [Google Scholar] [CrossRef]
- Pastore, G.A.M.; Costa, V.S.S.R.; Koblitz, M.G.B. Purificação parcial e caracterização bioquímica de lipase extracelular produzida por nova linhagem de Rhizopus sp. Food Sci. Technol. 2003, 23, 135–140. [Google Scholar] [CrossRef]
- Behmer, O.A.; Tolosa, E.M.C. Manual de Técnicas Para Histología Normal e Patológica, 2nd ed.; Manole: São Paulo, Brazil, 2003; p. 331. [Google Scholar]
- Zhang, S.; Shi, Y.; Zhang, S.; Shang, W.; Gao, X.; Wang, H. Whole soybean as probiotic lactic acid bacteria carrier food in solid-state fermentation. Food Control 2014, 41, 1–6. [Google Scholar] [CrossRef]
- Chisti, Y. Fermentation (Industrial) Basic Considerations. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 751–761. [Google Scholar] [CrossRef]
- Hong, K.J.; Lee, C.H.; Kim, S.W. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J. Med. Food 2004, 7, 430–436. [Google Scholar] [CrossRef]
- Ha, N.; Jesus, G.F.A.; Gonçalves, A.F.N.; Oliveira, N.S.; Sugai, J.K.; Pessatti, M.L.; Fabregat, T.E.H.P. Sardine (Sardinella spp.) protein hydrolysate as growth promoter in South American catfish (Rhamdia quelen) feeding: Productive performance, digestive enzymes activity, morphometry and intestinal microbiology. Aquaculture 2019, 500, 99–106. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef]
- Uczay, J.; Battisti, E.K.; Lazzari, R.; Pessatti, M.L.; Schneider, T.L.S.; Hermes, L.B.; Fabregat, T.E.H.P. Fish meal replaced by hydrolysed soybean meal in diets increases growth and improves the antioxidant defense system of silver catfish (Rhamdia quelen). Aquac. Res. 2019, 50, 1438–1447. [Google Scholar] [CrossRef]
- Wosniak, B.; Melim, E.W.H.; Ha, N.; Uczay, J.; Pilatti, C.; Pessatti, M.L.; Fabregat, T.E.H.P. Effect of diets containing different types of sardine waste (Sardinella sp.) protein hydrolysate on the performance and intestinal morphometry of silver catfish juveniles (Rhamdia quelen). Lat. Am. J. Aquat. Res. 2016, 44, 957–966. [Google Scholar] [CrossRef]
- Seong, M.; Lee, S.; Lee, S.; Song, Y.; Bae, J.; Chang, K.; Bai, S.C. The effects of different levels of dietary fermented plant-based protein concentrate on growth, hematology and non-specific immune responses in juvenile olive flounder, Paralichthys olivaceus. Aquaculture 2018, 483, 196–202. [Google Scholar] [CrossRef]
- Lee, S.M.; Azarm, H.M.; Chang, K.H. Effects of dietary inclusion of fermented soybean meal on growth, body composition, antioxidant enzyme activity and disease resistance of rockfish (Sebastes schlegeli). Aquaculture 2016, 459, 110–116. [Google Scholar] [CrossRef]
- Shiu, Y.L.; Hsieh, S.L.; Guei, W.C.; Tsai, Y.T.; Chiu, C.H.; Liu, C.H. Using Bacillus subtilis E20-fermented soybean meal as replacement for fish meal in the diet of orange-spotted grouper (Epinephelus coioides, H amilton). Aquac. Res. 2013, 46, 1403–1416. [Google Scholar] [CrossRef]
- Siddik, M.A.; Howieson, J.; Partridge, G.J.; Fotedar, R.; Gholipourkanani, H. Dietary tuna hydrolysate modulates growth performance, immune response, intestinal morphology and resistance to Streptococcus iniae in juvenile barramundi, Lates calcarifer. Sci. Rep. 2018, 8, 15942. [Google Scholar] [CrossRef]
- Wei, Y.; Liang, M.; Mai, K.; Zheng, K.; Xu, H. The effect of ultrafiltered fish protein hydrolysate levels on the liver and muscle metabolic profile of juvenile turbot (Scophthalmus maximus L.) by 1H NMR-based metabolomics studies. Aquac. Res. 2016, 48, 3515–3527. [Google Scholar] [CrossRef]
- Xu, H.; Mu, Y.; Zhang, Y.; Li, J.; Liang, M.; Zheng, K.; Wei, Y. Graded levels of fish protein hydrolysate in diets rich in plants for turbot (Scophthalmus maximus): Effects on growth performance and lipid accumulation. Aquaculture 2016, 454, 140–147. [Google Scholar] [CrossRef]
- Khosravi, S.; Bui, H.T.D.; Rahimnejad, S.; Herault, M.; Fournier, V.; Kim, S.S.; Lee, K.J. Dietary supplementation of marine protein hydrolysates in fish-meal based diets for red sea bream (Pagrus major) and olive flounder (Paralichthys olivaceus). Aquaculture 2015, 435, 371–376. [Google Scholar] [CrossRef]
- Wei, Y.; Liang, M.; Xu, H. Fish protein hydrolysate affected amino acid absorption and related gene expressions of IGF-1/AKT pathways in turbot (Scophthalmus maximus). Aquac. Nutr. 2020, 26, 145–155. [Google Scholar] [CrossRef]
- Gominho-Rosa, M.; Rodrigues, A.P.O.; Mattioni, B.; de Francisco, A.; Moraes, G.; Fracalossi, D.M. Comparison between the omnivorous jundiá catfish (Rhamdia quelen) and Nile tilapia (Oreochromis niloticus) on the utilization of dietary starch sources: Digestibility, enzyme activity and starch microstructure. Aquaculture 2015, 435, 92–99. [Google Scholar] [CrossRef]
- Vivas, M.; Rubio, V.C.; Sánchez-Vázquez, F.J.; Mena, C.; García, B.G.; Madrid, J.A. Dietary self-selection in sharpsnout seabream (Diplodus puntazzo) fed paired macronutrient feeds and challenged with protein dilution. Aquaculture 2006, 251, 430–437. [Google Scholar] [CrossRef]
- Cheng, A.C.; Lin, H.L.; Shiu, Y.L.; Tyan, Y.C.; Liu, C.H. Isolation and characterization of antimicrobial peptides derived from Bacillus subtilis E20-fermented soybean meal and its use for preventing vibrio infection in shrimp aquaculture. Fish Shellfish Immunol. 2017, 67, 270–279. [Google Scholar] [CrossRef]
- Choudhury, T.G.; Kamilya, D. Paraprobiotics: An aquaculture perspective. Rev. Aquac. 2019, 11, 1258–1270. [Google Scholar] [CrossRef]
- Chai, T.T.; Tan, Y.N.; Ee, K.Y.; Xiao, J.; Wong, F.C. Seeds, fermented foods, and agricultural by-products as sources of plant-derived antibacterial peptides. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. S1), S162–S177. [Google Scholar] [CrossRef]
- Ranjan, A.; Sahu, N.P.; Deo, A.D.; Kumar, H.S.; Kumar, S.; Jain, K.K. Comparative evaluation of fermented and non-fermented de-oiled rice bran with or without exogenous enzymes supplementation in the diet of Labeo rohita (Hamilton, 1822). Fish Physiol. Biochem. 2018, 44, 1037–1049. [Google Scholar] [CrossRef]
- Liu, H.; Jin, J.; Zhu, X.; Han, D.; Yang, Y.; Xie, S. Effect of substitution of dietary fish meal by soybean meal on different sizes of gibel carp (Carassius auratus gibelio): Digestive enzyme gene expressions and activities, and intestinal and hepatic histology. Aquac. Nutr. 2017, 23, 129–147. [Google Scholar] [CrossRef]
- Zhang, C.; Rahimnejad, S.; Wang, Y.R.; Lu, K.; Song, K.; Wang, L.; Mai, K. Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus): Effects on growth, digestive enzymes activity, gut histology, and expression of gut inflammatory and transporter genes. Aquaculture 2018, 483, 173–182. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; He, R.; Xu, W.; Mai, K.; He, G. Effects of soybean meal fermentation by Lactobacillus plantarum P8 on growth, immune responses, and intestinal morphology in juvenile turbot (Scophthalmus maximus L.). Aquaculture 2016, 464, 87–94. [Google Scholar] [CrossRef]

| Soybean Meal * | SMFL | |
|---|---|---|
| Proximal composition (%) | ||
| Crude protein | 44.82 | 43.99 |
| Ethereal extract | 5.23 | 4.19 |
| Dry matter | 88.35 | 90.64 |
| Mineral matter | 7.42 | 7.75 |
| pH | 6.64 | 6.47 |
| Essential amino acids (%) | ||
| Arginine | 3.35 | 3.47 |
| Histidine | 1.20 | 1.31 |
| Isoleucine | 2.13 | 2.00 |
| Leucine | 3.51 | 3.84 |
| Lysine | 2.87 | 3.14 |
| Methionine | 0.61 | 0.60 |
| Phenylalanine | 2.34 | 2.73 |
| Valine | 2.22 | 2.38 |
| Threonine | 1.78 | 1.98 |
| Nonessential amino acids (%) | ||
| Proline | 2.31 | 2.63 |
| Cystine | 0.67 | 0.72 |
| Alanine | 2.03 | 2.33 |
| Aspartic acid | 3.22 | 5.86 |
| Glutamic acid | 4.44 | 9.12 |
| Glycine | 1.97 | 2.15 |
| Serine | 2.46 | 2.70 |
| Tyrosine | 1.66 | 1.74 |
| Sum of amino acids (%) | 38.77 | 48.70 |
| Experimental Diets | |||||
|---|---|---|---|---|---|
| Ingredients (%) | SMFL 0% | SMFL 7% | SMFL 14% | SMFL 21% | SMFL 28% |
| Soybean meal | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 |
| Corn | 9.5 | 9.0 | 8.5 | 8.5 | 8.5 |
| Wheat flour | 12.0 | 12.0 | 12.0 | 12.0 | 12.0 |
| Fishmeal | 46.5 | 40.5 | 34.5 | 28.0 | 22.0 |
| SMFL | 0.0 | 7.0 | 14.0 | 21.0 | 28.0 |
| Soybean oil | 6.0 | 5.5 | 5.0 | 4.5 | 3.5 |
| Premix 1 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Centesimal composition | |||||
| Crude protein (%) | 39.19 | 38.74 | 38.43 | 37.93 | 37.45 |
| Gross energy (kcal kg−1) | 4298.93 | 4314.77 | 4205.53 | 4219.67 | 4333.41 |
| Ethereal extract (%) | 11.05 | 10.38 | 9.71 | 7.09 | 7.87 |
| Mineral matter (%) | 14.62 | 12.86 | 11.57 | 10.27 | 9.63 |
| Dry matter (%) | 91.21 | 91.38 | 91.50 | 91.79 | 92.30 |
| Essential amino acids (%) | |||||
| Lysine | 3.38 | 3.22 | 3.08 | 2.93 | 2.88 |
| Methionine | 1.00 | 0.92 | 0.85 | 0.77 | 0.70 |
| Arginine | 2.18 | 2.26 | 2.36 | 2.44 | 2.48 |
| Threonine | 1.83 | 1.82 | 1.77 | 1.73 | 1.71 |
| Leucine | 3.51 | 3.45 | 3.41 | 3.36 | 3.32 |
| Isoleucine | 2.46 | 2.37 | 2.28 | 2.19 | 2.12 |
| Valine | 2.51 | 2.43 | 2.36 | 2.27 | 2.22 |
| Histidine | 1.08 | 1.07 | 1.06 | 1.05 | 1.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, N.S.; Ha, N.; da Cunha, L.; Cipriani, L.A.; Neto, A.T.; Skoronski, E.; Gisbert, E.; Perez Fabregat, T.E.H. Fermentation of Soybean Meal with Lactobacillus acidophilus Allows Greater Inclusion of Vegetable Protein in the Diet and Can Reduce Vibrionacea in the Intestine of the South American Catfish (Rhamdia quelen). Animals 2022, 12, 690. https://doi.org/10.3390/ani12060690
de Oliveira NS, Ha N, da Cunha L, Cipriani LA, Neto AT, Skoronski E, Gisbert E, Perez Fabregat TEH. Fermentation of Soybean Meal with Lactobacillus acidophilus Allows Greater Inclusion of Vegetable Protein in the Diet and Can Reduce Vibrionacea in the Intestine of the South American Catfish (Rhamdia quelen). Animals. 2022; 12(6):690. https://doi.org/10.3390/ani12060690
Chicago/Turabian Stylede Oliveira, Nandara Soares, Natalia Ha, Larissa da Cunha, Luiz Augusto Cipriani, André Thaler Neto, Everton Skoronski, Enric Gisbert, and Thiago El Hadi Perez Fabregat. 2022. "Fermentation of Soybean Meal with Lactobacillus acidophilus Allows Greater Inclusion of Vegetable Protein in the Diet and Can Reduce Vibrionacea in the Intestine of the South American Catfish (Rhamdia quelen)" Animals 12, no. 6: 690. https://doi.org/10.3390/ani12060690
APA Stylede Oliveira, N. S., Ha, N., da Cunha, L., Cipriani, L. A., Neto, A. T., Skoronski, E., Gisbert, E., & Perez Fabregat, T. E. H. (2022). Fermentation of Soybean Meal with Lactobacillus acidophilus Allows Greater Inclusion of Vegetable Protein in the Diet and Can Reduce Vibrionacea in the Intestine of the South American Catfish (Rhamdia quelen). Animals, 12(6), 690. https://doi.org/10.3390/ani12060690







