Estimation of the Maternal Investment of Sea Turtles by Automatic Identification of Nesting Behavior and Number of Eggs Laid from a Tri-Axial Accelerometer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
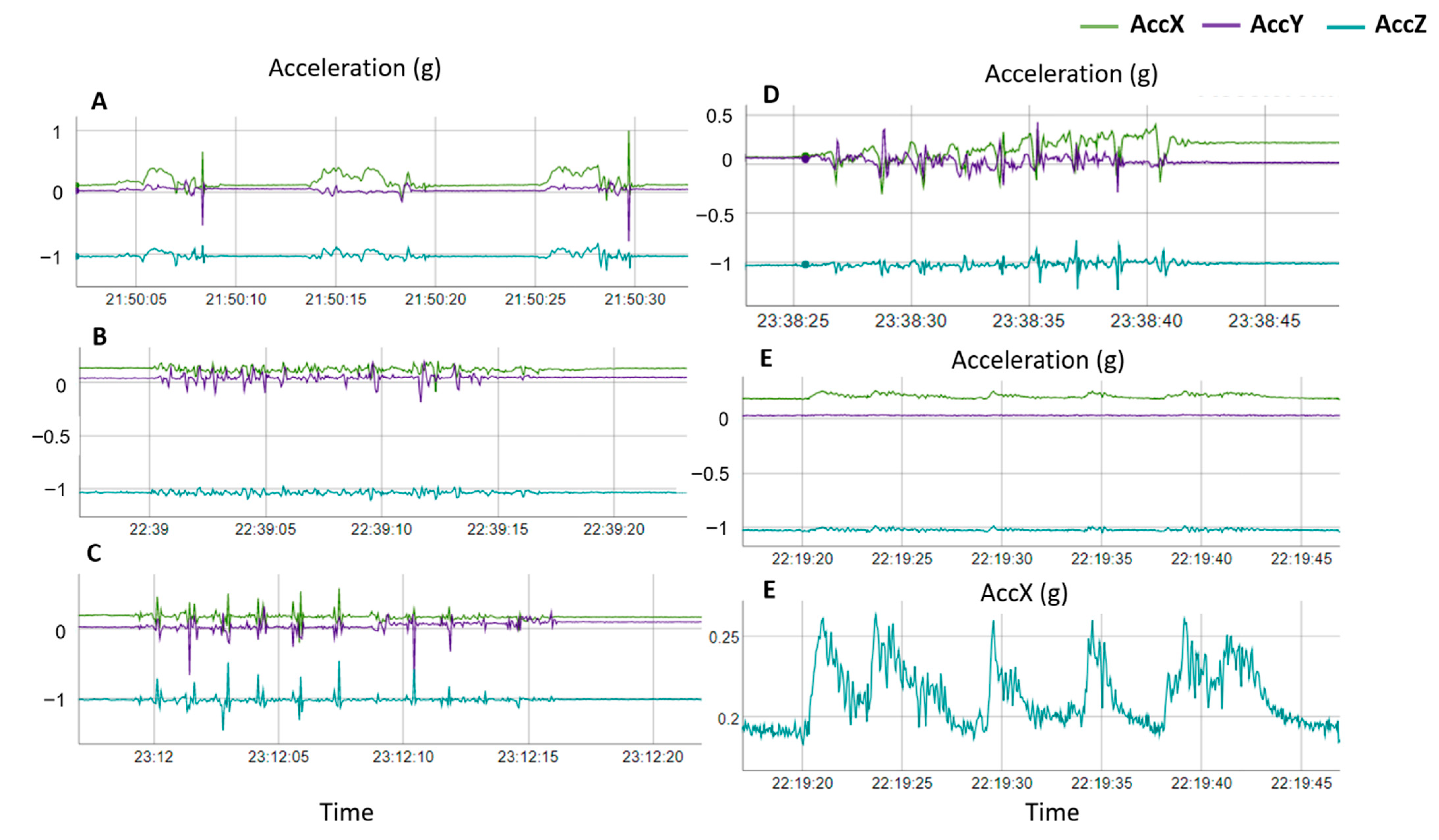
2.1. Data Collection
2.2. Labelling of Nesting Behaviors
2.3. Automatic Behavioral Identification through Deep Learning
2.4. Estimation of Laid Eggs
2.4.1. Cutting off the Egg Laying Period
- Binarize the behaviors sequence: label “1” is assigned to the behavior Egg laying while all the others are labelled as “0” (Figure 3a);
- Perform a convolution of the binarized sequence with a Gaussian mask whose standard deviation is empirically chosen. The convolved signal is represented in blue as the ‘Smoothed density’ (Figure 3b);
- Choose a minimal threshold (threshold = 0.7), and extract the acceleration values associated to the part of the convolved signal which is greater than it (Figure 3b).
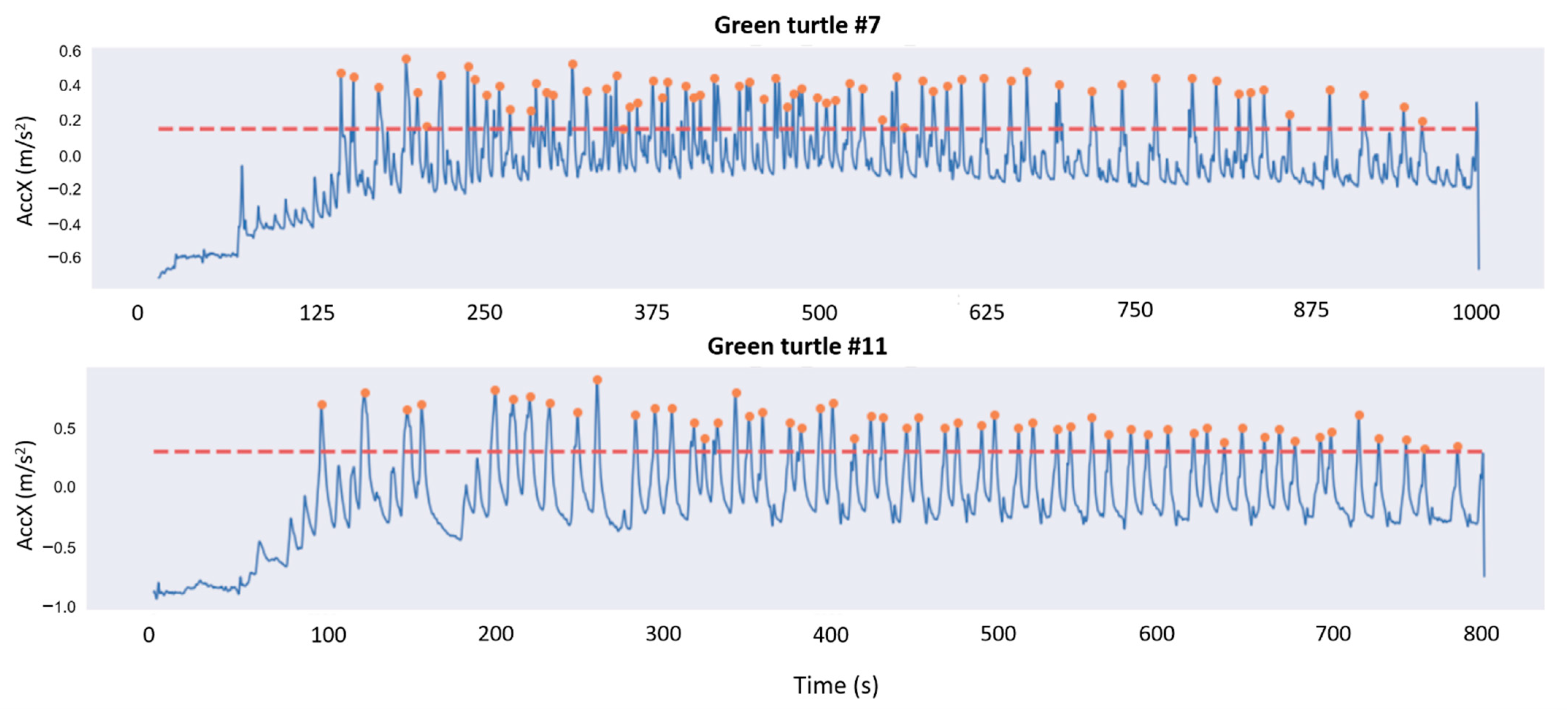
2.4.2. Peak Detection
2.4.3. Estimation of the Number of Eggs
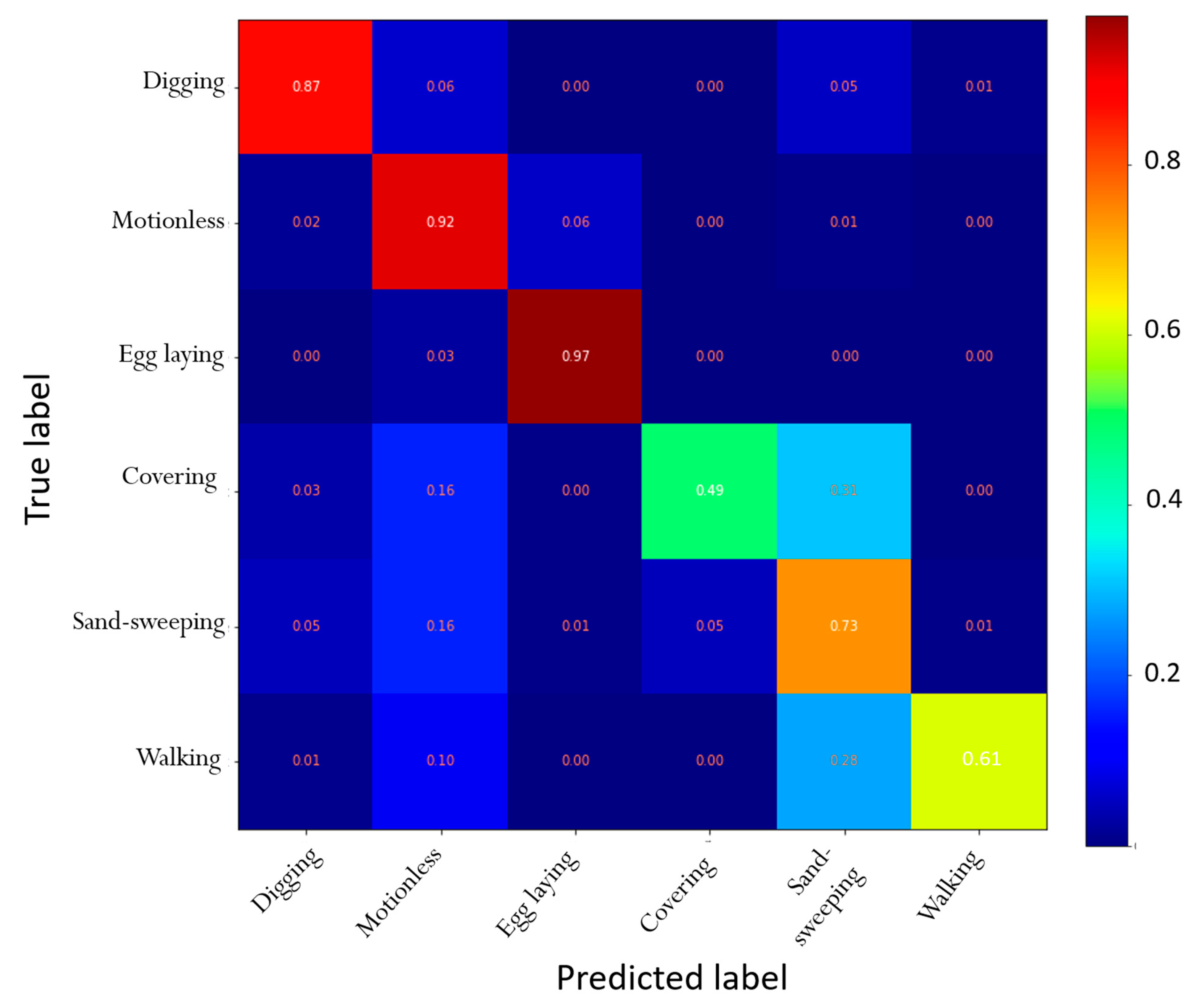
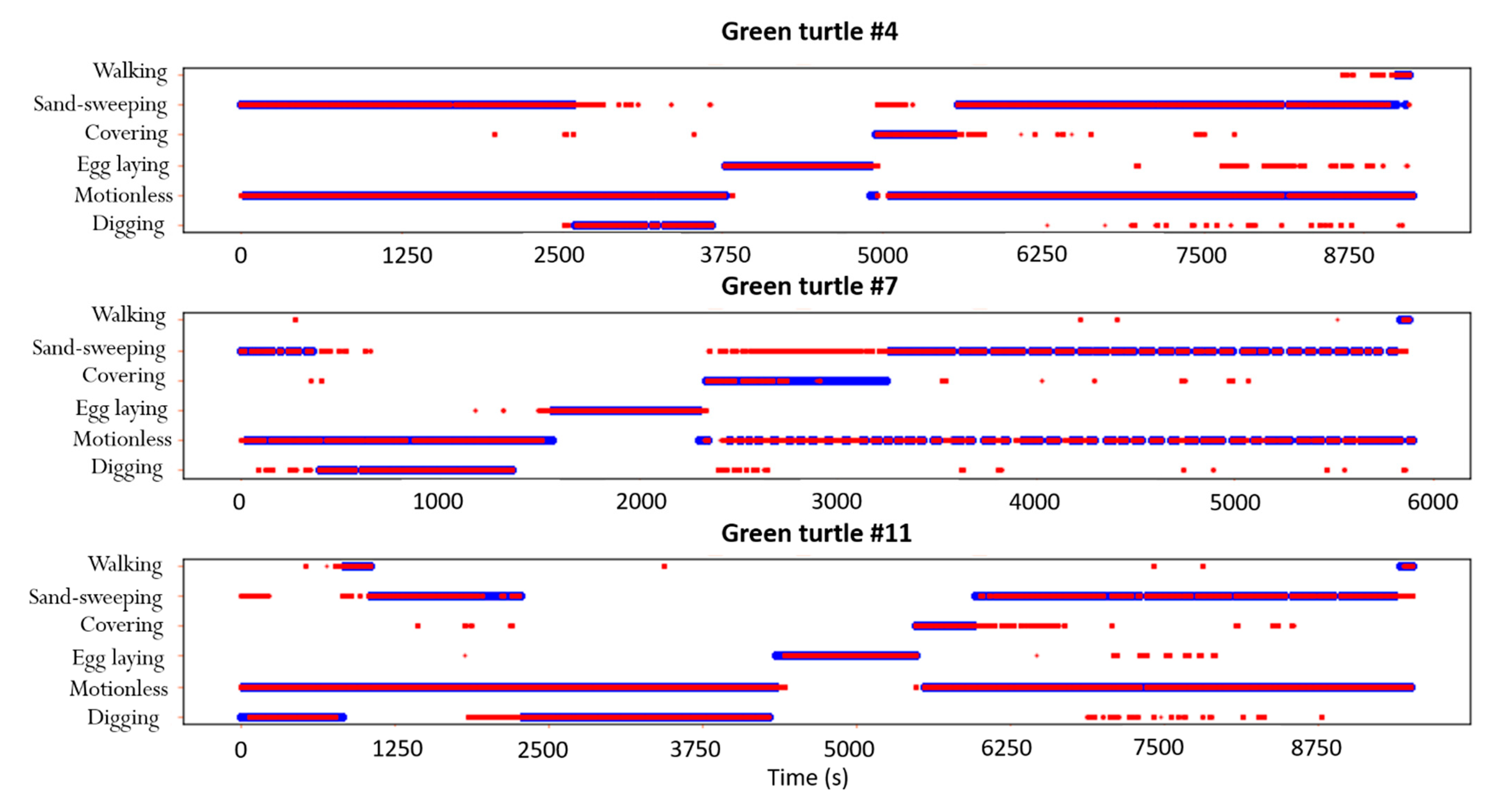
3. Results
4. Discussion
4.1. Automatic Identification of Nesting Behaviors
4.2. Automatic Identification of Number of Eggs Laid
4.3. Perspective of Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, J.D.; Limpus, C.J.; Godfrey, M.H. Nest site selection, oviposition, eggs, development, hatching and emergence of loggerhead turtles. In Loggerhead Sea Turtles; Bolten, A., Witherington, B.E., Eds.; Smithsonian Books Press: Washington, DC, USA, 2003; pp. 125–143. [Google Scholar]
- Broderick, A.C.; Glen, F.; Godley, B.J.; Hays, G.C. Variation in reproductive output of marine turtles. J. Exp. Mar. Biol. Ecol. 2003, 288, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Congdon, J.D.; Gibbons, J.W. Morphological constraint on egg size: A challenge to optimal egg size theory? Proc. Natl. Acad. Sci. USA 1987, 84, 4145–4147. [Google Scholar] [CrossRef] [Green Version]
- Wallace, B.P.; Sotherland, P.R.; Tomillo, P.S.; Reina, R.D.; Spotila, J.R.; Paladino, F.V. Maternal investment in reproduction and its consequences in leatherback turtles. Oecologia 2007, 152, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Broderick, A.C.; Glen, F.; Godley, B.J.; Hays, G.C. Estimating the number of green and loggerhead turtles nesting annually in the Mediterranean. Oryx 2002, 36, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Chevallier, D.; Girondot, M.; Berzins, R.; Chevalier, J.; de Thoisy, B.; Fretey, J.; Kelle, L.; Lebreton, J. Survival and breeding interval of an endangered marine vertebrate, the leatherback turtle Dermochelys coriacea, in French Guiana. Endanger. Species Res. 2020, 41, 153–165. [Google Scholar] [CrossRef]
- Johnson, S.A.; Ehrhart, L.M. Reproductive ecology of the florida green turtle: Clutch frequency. J. Herpetol. 1996, 30, 407–410. [Google Scholar] [CrossRef] [Green Version]
- Weber, N.; Weber, S.B.; Godley, B.J.; Ellick, J.; Witt, M.; Broderick, A.C. Telemetry as a tool for improving estimates of marine turtle abundance. Biol. Conserv. 2013, 167, 90–96. [Google Scholar] [CrossRef]
- Briane, J.P.; Rivalan, P.; Girondot, M. The inverse problem applied to the observed clutch frequency of leatherback turtles from Yalimapo Beach, French Guiana. Chelonian Conserv. Biol. 2007, 6, 63–69. [Google Scholar] [CrossRef]
- Hancock, J.; Vieira, S.; Lima, H.; Schmitt, V.; Pereira, J.; Rebelo, R.; Girondot, M. Overcoming field monitoring restraints in estimating marine turtle internesting period by modelling individual nesting behaviour using capture-mark-recapture data. Ecol. Modell. 2019, 402, 76–84. [Google Scholar] [CrossRef]
- Reina, R.D.; Mayor, P.A.; Spotila, J.R.; Piedra, R.; Paladino, F.V. Nesting ecology of the leatherback turtle, Dermochelys coriacea, at Parque Nacional Marino Las Baulas, Costa Rica: 1988–1989 to 1999–2000. Copeia 2002, 2002, 653–664. [Google Scholar] [CrossRef]
- Blanco, G.S.; Morreale, S.J.; Vélez, E.; Piedra, R.; Montes, W.M.; Paladino, F.V.; Spotila, J.R. Reproductive output and ultrasonography of an endangered population of East Pacific green turtles. J. Wildl. Manag. 2012, 76, 841–846. [Google Scholar] [CrossRef]
- Esteban, N.; Mortimer, J.A.; Hays, G.C. How numbers of nesting sea turtles can be overestimated by nearly a factor of two. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, A.D. Nest site fidelity and clutch frequency of loggerhead turtles are better elucidated by satellite telemetry than by nocturnal tagging efforts: Implications for stock estimation. J. Exp. Mar. Biol. Ecol. 2010, 383, 48–55. [Google Scholar] [CrossRef]
- Chambault, P.; De Thoisy, B.; Kelle, L.; Berzins, R.; Bonola, M.; Delvaux, H.; Le Maho, Y.; Chevallier, D. Inter-nesting behavioural adjustments of green turtles to an estuarine habitat in French Guiana. Mar. Ecol. Prog. Ser. 2016, 555, 235–248. [Google Scholar] [CrossRef]
- Brown, D.D.; Kays, R.; Wikelski, M.; Wilson, R.; Klimley, A.P. Observing the unwatchable through acceleration logging of animal behavior. Anim. Biotelem. 2013, 1, 20. [Google Scholar] [CrossRef] [Green Version]
- Fossette, S.; Gleiss, A.C.; Myers, A.E.; Garner, S.; Liebsch, N.; Whitney, N.M.; Hays, G.C.; Wilson, R.P.; Lutcavage, M.E. Behaviour and buoyancy regulation in the deepest-diving reptile: The leatherback turtle. J. Exp. Biol. 2010, 213, 4074–4083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hays, G.C.; Marshall, G.J.; Seminoff, J.A. Flipper beat frequency and amplitude changes in diving green turtles, Chelonia mydas. Mar. Biol. 2007, 150, 1003–1009. [Google Scholar] [CrossRef]
- Okuyama, J.; Nakajima, K.; Noda, T.; Kimura, S.; Kamihata, H.; Kobayashi, M.; Arai, N.; Kagawa, S.; Kawabata, Y.; Yamada, H.; et al. Ethogram of immature green turtles: Behavioral strategies for somatic growth in large marine herbivores. PLoS ONE 2013, 8, e65783. [Google Scholar] [CrossRef]
- Yasuda, T.; Arai, N. Changes in flipper beat frequency, body angle and swimming speed of female green turtles Chelonia mydas. Mar. Ecol. Prog. Ser. 2009, 386, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Jeantet, L.; Dell’Amico, F.; Forin-Wiart, M.-A.; Coutant, M.; Bonola, M.; Etienne, D.; Gresser, J.; Regis, S.; Lecerf, N.; Lefebvre, F.; et al. Combined use of two supervised learning algorithms to model sea turtle behaviours from tri-axial acceleration data. J. Exp. Biol. 2018, 221, jeb177378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeantet, L.; Planas-Bielsa, V.; Benhamou, S.; Geiger, S.; Martin, J.; Siegwalt, F.; Lelong, P.; Gresser, J.; Etienne, D.; Hiélard, G.; et al. Behavioural inference from signal processing using animal-borne multi-sensor loggers: A novel solution to extend the knowledge of sea turtle ecology. R. Soc. Open Sci. 2020, 7, 200139. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Noda, T.; Yasuda, T.; Okuyama, J.; Arai, N.; Kobayashi, M. Decision tree classification of behaviors in the nesting process of green turtles (Chelonia mydas) from tri-axial acceleration data. J. Ethol. 2013, 31, 315–322. [Google Scholar] [CrossRef]
- Jeantet, L.; Vigon, V.; Geiger, S.; Chevallier, D. Fully convolutional neural network: A solution to infer animal behaviours from multi-sensor data. Ecol. Modell. 2021, 450, 109555. [Google Scholar] [CrossRef]
- Bonola, M.; Girondot, M.; Robin, J.P.; Martin, J.; Siegwalt, F.; Jeantet, L.; Lelong, P.; Grand, C.; Chambault, P.; Etienne, D.; et al. Fine scale geographic residence and annual primary production drive body condition of wild immature green turtles (Chelonia mydas) in Martinique Island (Lesser Antilles). Biol. Open 2019, 8, bio048058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustard, R.; Greenham, P. Nesting behavior of the green sea turtle on a great barrier reef island. Herpetologica 1969, 25, 93–102. [Google Scholar]
- Hailman, J.P.; Elowson, M.A. Ethogram of the nesting female loggerhead (Carette caretta). Herpetologica 1992, 48, 1–30. [Google Scholar]
- Lindborg, R.; Neidhardt, E.; Smith, J.R.; Schwartz, B.; Hernandez, V.; Savage, A.; Witherington, B. An Ethogram Describing the nesting behavior of green sea turtles (Chelonia mydas). Herpetologica 2019, 75, 114. [Google Scholar] [CrossRef]
- Péron, C.; Chevallier, D.; Galpin, M.; Chatelet, A.; Anthony, E.J.; Le Maho, Y.; Gardel, A. Beach morphological changes in response to marine turtles nesting: A preliminary study of Awala-Yalimapo beach, French Guiana (South America). J. Coast. Res. 2013, 65, 99–104. [Google Scholar] [CrossRef]
- Geiger, S. Package “rblt”. Available online: https://cran.microsoft.com/web/packages/rblt/rblt.pdf (accessed on 9 February 2022).
- Milletari, F.; Navab, N.; Ahmadi, S.A. V-Net: Fully convolutional neural networks for volumetric medical image segmentation. In Proceedings of the 2016 Fourth International Conference on 3D Vision (3DV), Stanford, CA, USA, 25–28 October 2016; pp. 565–571. [Google Scholar] [CrossRef] [Green Version]
- Bom, R.A.; Bouten, W.; Piersma, T.; Oosterbeek, K.; van Gils, J.A. Optimizing acceleration-based ethograms: The use of variable-time versus fixed-time segmentation. Mov. Ecol. 2014, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Ladds, M.A.; Salton, M.; Hocking, D.P.; McIntosh, R.R.; Thompson, A.P.; Slip, D.J.; Harcourt, R.G. Using accelerometers to develop time-energy budgets of wild fur seals from captive surrogates. PeerJ 2018, 6, e5814. [Google Scholar] [CrossRef]
- Wang, G. Machine learning for inferring animal behavior from location and movement data. Ecol. Inform. 2019, 49, 69–76. [Google Scholar] [CrossRef]
- Wallace, B.P.; Kilham, S.S.; Paladino, F.V.; Spotila, J.R. Energy budget calculations indicate resource limitation in Eastern Pacific leatherback turtles. Mar. Ecol. Prog. Ser. 2006, 318, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Heerah, K.; Cox, S.L.; Blevin, P.; Guinet, C.; Charrassin, J.-B. Validation of dive foraging indices using archived and transmitted acceleration data: The case of the Weddell seal. Front. Ecol. Env. 2019, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Harcourt, R.; Sequeira, A.M.M.; Zhang, X.; Roquet, F.; Komatsu, K.; Heupel, M.; McMahon, C.; Whoriskey, F.; Meekan, M.; Carroll, G.; et al. Animal-borne telemetry: An integral component of the ocean observing toolkit. Front. Mar. Sci. 2019, 6, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Cox, S.L.; Orgeret, F.; Gesta, M.; Rodde, C.; Heizer, I.; Weimerskirch, H.; Guinet, C. Processing of acceleration and dive data on-board satellite relay tags to investigate diving and foraging behaviour in free-ranging marine predators. Methods Ecol. Evol. 2017, 9, 64–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korpela, J.; Suzuki, H.; Matsumoto, S.; Mizutani, Y.; Samejima, M.; Maekawa, T.; Nakai, J.; Yoda, K. Machine learning enables improved runtime and precision for bio-loggers on seabirds. Commun. Biol. 2020, 3, 633. [Google Scholar] [CrossRef] [PubMed]


| Individual | CCL | CCW | First Recorded Behavior | Nb of Laid Eggs | Comments |
|---|---|---|---|---|---|
| #1 | 126 | 122 | Egg laying | - | |
| #2 | 111 | 103 | Digging | - | |
| #3 | 122 | 109 | Sand-sweeping | - | |
| #4 | 112 | 96 | Sand-sweeping | - | |
| #5 | 115 | 110 | Digging | 106 | |
| #6 | 114 | 113 | Digging | 111 | |
| #7 | 102 | 94 | Digging | 93 | |
| #8 | 112 | 94 | Sand-sweeping | 117 | |
| #9 | 108 | 98 | Digging | 103 | |
| #10 | 128 | 110 | Digging | 173 | |
| #11 | 119 | 104 | Sand-sweeping | 93 | |
| #12 | 105 | 96 | Sand-sweeping | - | Did not lay eggs |
| #13 | 117 | 104 | Digging | - | |
| #14 | 118 | 106 | Sand-sweeping | 97 |
| Recall | Precision | |
|---|---|---|
| Digging | 0.87 | 0.79 |
| Motionless | 0.92 | 0.90 |
| Egg laying | 0.97 | 0.79 |
| Filling and packing | 0.49 | 0.72 |
| Sand-sweeping | 0.73 | 0.84 |
| Walking | 0.61 | 0.70 |
| Accuracy | 0.95 |
| Individual | Nb of Observed Eggs | Nb of Estimated Eggs | Difference | Relative Error |
|---|---|---|---|---|
| #5 | 106 | 101 | −5 | 0.05 |
| #6 | 111 | 109 | −2 | 0.02 |
| #7 | 93 | 93 | 0 | 0.00 |
| #8 | 117 | 118 | 1 | 0.01 |
| #9 | 103 | 117 | 14 | 0.14 |
| #10 | 173 | 150 | −23 | 0.13 |
| #11 | 93 | 88 | −5 | 0.05 |
| #14 | 97 | 112 | 15 | 0.15 |
| MEAN | −1 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeantet, L.; Hadetskyi, V.; Vigon, V.; Korysko, F.; Paranthoen, N.; Chevallier, D. Estimation of the Maternal Investment of Sea Turtles by Automatic Identification of Nesting Behavior and Number of Eggs Laid from a Tri-Axial Accelerometer. Animals 2022, 12, 520. https://doi.org/10.3390/ani12040520
Jeantet L, Hadetskyi V, Vigon V, Korysko F, Paranthoen N, Chevallier D. Estimation of the Maternal Investment of Sea Turtles by Automatic Identification of Nesting Behavior and Number of Eggs Laid from a Tri-Axial Accelerometer. Animals. 2022; 12(4):520. https://doi.org/10.3390/ani12040520
Chicago/Turabian StyleJeantet, Lorène, Vadym Hadetskyi, Vincent Vigon, François Korysko, Nicolas Paranthoen, and Damien Chevallier. 2022. "Estimation of the Maternal Investment of Sea Turtles by Automatic Identification of Nesting Behavior and Number of Eggs Laid from a Tri-Axial Accelerometer" Animals 12, no. 4: 520. https://doi.org/10.3390/ani12040520
APA StyleJeantet, L., Hadetskyi, V., Vigon, V., Korysko, F., Paranthoen, N., & Chevallier, D. (2022). Estimation of the Maternal Investment of Sea Turtles by Automatic Identification of Nesting Behavior and Number of Eggs Laid from a Tri-Axial Accelerometer. Animals, 12(4), 520. https://doi.org/10.3390/ani12040520







