Body Weight Development in Adult Dogs Fed a High Level Resistant Starch Diet
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Management, and Housing
2.2. Experimental Design
2.2.1. Experiment 1
2.2.2. Experiment 2
2.3. Preparation of Experimental Diets
2.4. Sampling and Analysis
2.5. Statistical Analysis
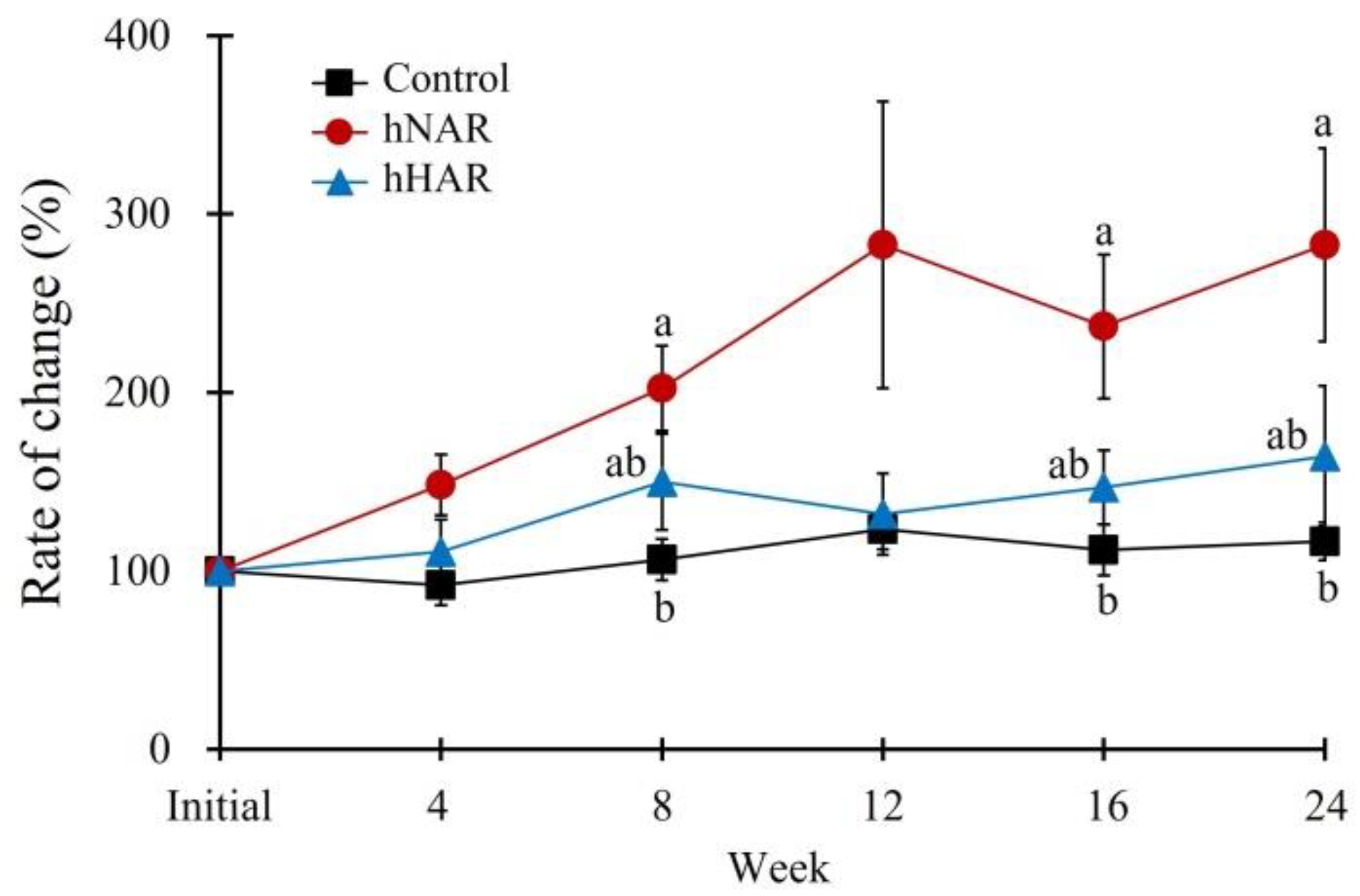
3. Results
3.1. Experiment 1
3.1.1. Food Intake and Body Parameters
3.1.2. Hematological and Biochemical Parameters
3.2. Experiment 2
3.2.1. Feed Intake and Body Parameters
3.2.2. Hematological and Biochemical Parameters
4. Discussion
4.1. Body Parameters and Feeding
4.2. Safety and Blood Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandler, M.; Cunningham, S.; Lund, E.M.; Khanna, C.; Naramore, R.; Patel, A.; Day, M.J. Obesity and associated comorbidities in people and companion animals: A one health perspective. J. Comp. Pathol. 2017, 156, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Belotta, A.F.; Teixeira, C.R.; Padovani, C.R.; Rahal, S.C.; Mayer, M.N.; Mamprim, M.J. Sonographic evaluation of liver hemodynamic indices in overweight and obese dogs. J. Vet. Intern. Med. 2018, 32, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Gossellin, J.; Wren, J.A.; Sunderland, S.J. Canine obesity: An overview. J. Vet. Pharmacol. Ther. 2007, 30 (Suppl. S1), 1–10. [Google Scholar] [CrossRef] [PubMed]
- German, A.J. Obesity prevention and weight management after loss. Vet. Clin. N. Am. 2016, 46, 913–929. [Google Scholar] [CrossRef]
- Burkholder, W.J.; Toll, P.W. Obesity. In Small Animal Clinical Nutrition, 4th ed.; Hand, M.S., Thatcher, C.D., Reimillard, R.L., Roudebush, P., Morris, M.L., Novotny, B.J., Eds.; Mark Morris Institute: Topeka, KS, USA, 2000; pp. 401–430. [Google Scholar]
- German, A.J. The growing problem of obesity in dogs and cats. J. Nutr. 2006, 136, 1940S–1946S. [Google Scholar] [CrossRef] [PubMed]
- Kipperman, B.S.; German, A.J. The responsibility of veterinarians to address companion animal obesity. Animals 2018, 8, 143. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch–A review. Comp. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Biliaderis, C.G. The structure and interactions of starch with food constituents. Can. J. Physiol. Pharmacol. 1991, 69, 60–78. [Google Scholar] [CrossRef]
- Berry, C.S. Resistant starch formation and measurement of starch that survives exhaustive digestion with amylolytic enzymes during the determination of dietary fiber. J. Cereal Sci. 1986, 4, 301–314. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, 33S–50S. [Google Scholar]
- Miller, B.; Pang, J.E.; Bramall, L. Rice: A high or low glycemic index food? Am. J. Clin. Nutr. 1992, 56, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Leeman, A.M.; Karlsson, M.E.; Eliasson, A.C.; Bjor, M.E. Resistant starch formation in temperature treated potato starches varying in amylose/amylopectin ratio. Carbohydr. Polym. 2006, 65, 306–313. [Google Scholar] [CrossRef]
- Lehman, U.; Robin, F. Slow digestible starch—Its structure and health implications: A review. Trends Food Sci. Technol. 2007, 18, 346–355. [Google Scholar] [CrossRef]
- Zhu, L.J.; Liua, Q.Q.; Wilson, J.D.; Gu, M.H.; Shi, Y.C. Digestibility and physicochemical properties of rice (Oryza sativa L.) flours and starches differing in amylose content. Carbohydr. Polym. 2011, 86, 1751–1759. [Google Scholar] [CrossRef]
- Asp, N.G. Nutritional classification of food carbohydrates. J. Clin. Nutr. 1994, 59, S679–S681. [Google Scholar] [CrossRef]
- Haralampu, S.G. Resistant starch—A review of the physical properties and biological impact of RS3. Carbohydr. Polym. 2000, 41, 285–292. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Tasman-Jones, C.; Englystm, H.; Harris, P.J. Comparative effects of three resistant starch preparations on transit time and short-chain fatty acid production in rats. Nutr. Cancer 2000, 36, 230–237. [Google Scholar] [CrossRef]
- Morita, T.; Kasaoka, S.; Hase, K.; Kiriyama, S. Psyllium shifts the fermentation site of high-amylose cornstarch toward the distal colon and increases fecal butyrate concentration in rats. J. Nutr. 1999, 129, 2081–2087. [Google Scholar] [CrossRef]
- Raben, A.; Tagliabue, A.; Christensen, N.J.; Madsn, J.; Holst, J.J.; Astrup, A. Resistant starch: The effect on postprandial glycemia, hormonal response and satiety. Am. J. Clin. Nutr. 1994, 60, 544–551. [Google Scholar] [CrossRef]
- Reader, D.; Johnson, M.L.; Hollander, P.; Franz, M. Response of resistant starch in a food bar vs. two commercially available bars in persons with type II diabetes mellitus. Diabetes 1997, 46, 254A. [Google Scholar]
- Han, K.H.; Fukushima, M.; Kato, T.; Kojima, M.; Ohba, K.; Shimada, K.; Sekikawa, M.; Nakano, M. Enzyme-resistant fractions of beans lowered serum cholesterol and increased sterol excretions and hepatic mRNA levels in rats. Lipids 2003, 38, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Flores, H.E.; Chang, Y.K.; Martinez-Bustos, F.; Sgarbieri, V. Effect of high fiber products on blood lipids and lipoproteins in hamsters. Nutr. Res. 2004, 24, 85–93. [Google Scholar] [CrossRef]
- Higgins, J.A.; Dana, H.R.; Donahoo, W.T.; Brown, I.L.; Bell, M.L.; Bessesen, D.H. Resistant starch consumption promotes lipid oxidation. Nutr. Met. 2004, 1, 1–8. [Google Scholar] [CrossRef]
- Jacobasach, G.; Schmiedl, D.; Kruschewski, M.; Schmehl, K. Dietary resistant starch and chronic inflammatory bowel diseases. Int. J. Colorectal. Dis. 1999, 14, 201–211. [Google Scholar] [CrossRef]
- Lopez, H.W.; Levrat-Verny, M.-A.; Coudray, C.; Besson, C.; Krespine, V.; Messager, A.; Demigné, C.; Rémésy, C. Class 2 resistant starches lower plasma and liver lipids and improve mineral retention in rats. J. Nutr. 2001, 131, 1283–1289. [Google Scholar] [CrossRef]
- Brown, I.L.; McNaught, K.J.; Ganly, R.N.; Conway, P.L.; Evans, A.J.; Topping, D.L.; Wang, X. Probiotic Compositions. International Patent WO 96(08261), A1, November 1996. [Google Scholar]
- Kim, Y.J.; Kim, J.G.; Lee, W.K.; So, K.M.; Kim, J.K. Trial data of the anti-obesity potential of a high resistant starch diet for canines using Dodam rice and the identification of discriminating markers in feces for metabolic profiling. Metabolomics 2019, 15, 21. [Google Scholar] [CrossRef]
- Ribeiro, D.M.; Peixoto, M.C.; Putarov, T.C.; Monti, M.; Pacheco, P.D.G.; Loureiro, B.A.; Pereira, G.T.; Carciofi, A.C. The effects of age and dietary resistant starch on digestibility, fermentation end products in faeces and postprandial glucose and insulin responses of dogs. Arch. Anim. Nutr. 2019, 73, 485–504. [Google Scholar] [CrossRef]
- Beloshapka, A.N.; Cross, T.W.L.; Swanson, K.S. Graded dietary resistant starch concentrations on apparent total tract macronutrient digestibility and fecal fermentative end products and microbial populations of healthy adult dogs. J. Anim. Sci. 2021, 99, skaa409. [Google Scholar] [CrossRef]
- Bradshaw, J.W.S. The evolutionary basis for the feeding behaviour of domestic dogs (Canis familiaris) and cats (Felis catus). J. Nutr. 2006, 136, 1927S–1931S. [Google Scholar] [CrossRef]
- Cabrita, A.R.; Guilherme-Fernandes, J.; Valente, I.M.; Almeida, A.; Lima, S.A.; Fonseca, A.J.; Maia, M.R. Nutritional Composition and Untargeted Metabolomics Reveal the Potential of Tetradesmus obliquus, Chlorella vulgaris and Nannochloropsis oceanica as Valuable Nutrient Sources for Dogs. Animals 2022, 12, 2643. [Google Scholar] [CrossRef]
- Beloshapka, A.N.; Buff, P.R.; Fahey, G.C.; Swanson, K.S. Compositional analysis of whole grains, processed grains, grain co-products, and other carbohydrate sources with applicability to pet animal nutrition. Foods 2016, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Beaton, L. Grain-free petfood: A top trend in the U.S. pet market. Petfood Ind. 2014, 56, 68–70. [Google Scholar]
- Cho, J.H.; Song, Y.C.; Lee, J.H.; Lee, J.Y.; Son, Y.B.; Oh, S.H.; Han, S.I.; Kim, C.S.; Chung, K.H.; Park, D.S.; et al. ‘Dodam (Milyang261)’, functional rice as a resistant starch with a high amylose content. Kor. Soc. Breed. Sci. 2019, 51, 515–522. [Google Scholar] [CrossRef]
- Association of American Feed Control Officials (AAFCO). Model Bill and Regulation; Official Publication: Champaign, IL, USA, 2016. [Google Scholar]
- Seo, K.; Cho, H.W.; Chun, J.; Jeon, J.; Kim, C.; Kim, M.; Park, K.; Kim, K. Evaluation of fermented oat and black soldier fly larva as food ingredients in senior dog diets. Animals 2021, 11, 3509. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- de Deckere, E.A.; Kloots, W.J.; Van Amelsvoort, J.M. Resistant starch decreases serum total cholesterol and triacylglycerol concentrations in rats. J. Nutr. 1993, 123, 2142–2151. [Google Scholar] [CrossRef]
- Kishida, T.; Nogami, H.; Himeno, S.; Ebihara, K. Heat moisture treatment of high amylose cornstarch increases its resistant starch content but not its physiologic effects in rats. J. Nutr. 2001, 131, 2716–2721. [Google Scholar] [CrossRef]
- Kabir, M.; Rizkalla, S.W.; Quignard-Boulangé, A.; Guerre-Millo, M.; Boillot, J.; Ardouin, B.; Luo, J.; Slama, G. A high glycemic index starch diet affects lipid storage–related enzymes in normal and to a lesser extent in diabetic rats. J. Nutr. 1998, 128, 1878–1883. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Polakof, S.; Díaz-Rubio, M.E.; Dardevet, D.; Martin, J.-F.; Pujos-Guillot, E.; Scalbert, A.; Sebedio, J.-L.; Mazur, A.; Comte, B. Resistant starch intake partly restores metabolic and inflammatory alterations in the liver of high-fat-diet-fed rats. J. Nutr. Biochem. 2013, 24, 1920–1930. [Google Scholar] [CrossRef]
- Xu, J.; Ma, Z.; Li, X.; Liu, L.; Hu, X. A more pronounced effect of type III resistant starch vs. type II resistant starch on ameliorating hyperlipidemia in high fat diet-fed mice is associated with its supramolecular structural characteristics. Food Funct. 2020, 11, 1982–1995. [Google Scholar] [CrossRef]
- Si, X.; Strappe, P.; Blanchard, C.; Zhou, Z. Enhanced anti-obesity effects of complex of resistant starch and chitosan in high fat diet fed rats. Carbohyd. Polym. 2017, 157, 834–841. [Google Scholar] [CrossRef]
- Lerer-Metzger, M.; Rizkallal, S.W.; Luo, J.; Champ, M.; Kabir, M.; Bruzzo, F.; Bornet, F.; Slama, G. Effects of long-term low-glycaemic index starchy food on plasma glucose and lipid concentrations and adipose tissue cellularity in normal and diabetic rats. Brit. J. Nutr. 1996, 75, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Dulman, O.M.; Anton, A.; Solcan, G. Variations in standard blood count and biochemical parameters in dogs with atopic dermatitis. Bull. UASVM Vet. Med. 2015, 72, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Mahassni, S.H.; Sebaa, R.B. Obesity and the immune system in Saudi Arabian adolescent females. Int. J. Biochem. Biotech. Sci. 2012, 1, 439–450. [Google Scholar]
- Radakovich, L.B.; Truelove, M.P.; Pannone, S.C.; Olver, C.S.; Santangelo, K.S. Clinically healthy overweight and obese dogs differ from lean controls in select CBC and serum biochemistry values. Vet. Clin. Pathol. 2017, 46, 221–226. [Google Scholar] [CrossRef]
- Chmelar, J.; Chung, K.J.; Chavakis, T. The role of innate immune cells in obese adipose tissue inflammation and development of insulin resistance. Thromb. Haemost. 2013, 109, 399–406. [Google Scholar] [PubMed]
- Chen, Y.F.; Wu, Z.M.; Xie, C.; Bai, S.; Zhao, L.D. Expression level of IL-6 secreted by bone marrow stromal cells in mice with aplastic anemia. Int. Sch. Res. Not. 2013, 2013, 986219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mori, N.; Lee, P.; Kondo, K.; Kido, T.; Saito, T.; Arai, T. Potential use of cholesterol lipoprotein profile to confirm obesity status in dogs. Vet. Res. Commun. 2011, 35, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Tribuddharatana, T.; Kongpiromchean, Y.; Sribhen, K.; Sribhen, C. Biochemical alterations and their relationships with the metabolic syndrome components in canine obesity. Agric. Nat. Resour. 2011, 45, 622–628. [Google Scholar]
- Piantedosi, D.; Di Loria, A.; Guccione, J.; De Rosa, A.; Fabbri, S.; Cortese, L.; Carta, S.; Ciaramella, P. Serum biochemistry profile, inflammatory cytokines, adipokines and cardiovascular findings in obese dogs. Vet. J. 2016, 216, 72–78. [Google Scholar] [CrossRef]
- Meena, Y.K.; Gupta, S.; Pannu, S.P.; Tiwari, R. Therapeutic response of dietary interventions to obesity in dogs. Pharma Innov. 2021, 10, 2864–2872. [Google Scholar]
- Nandini, M.K.; Kamran, C.A.; Yathiraj, S.; Upendra, H.A.; Kumar, V.G.; Rao, S. Hematological, biochemical and lipid profile changes associated with obesity in dogs. Indian Vet. J. 2012, 89, 79–81. [Google Scholar]
- Preet, G.S.; Turkar, S.; Uppal, S.K.; Randhawa, C.S.; Chhabra, S. Risk factors and metabolic alterations in healthy obese companion dogs in India. Pharma Innov. 2019, 8, 677–682. [Google Scholar]
- Ueda, S.; Takenaka, O.; Omoto, K. Glutamic-pyruvic transaminase (GPT) in non-human primates II. Implication of the interspecific variation in enzyme activity. J. Human Evol. 1982, 11, 109–115. [Google Scholar] [CrossRef]
- Nakata, Y.; Iwai, M.; Kimura, S.; Shimazu, T. Prolonged decrease in hepatic connexin32 in chronic liver injury induced by carbon tetrachloride in rats. J. Hepatol. 1996, 25, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W. Association between elevated serum hepatic enzyme activity and total body fat in obese humans. Ann. Clin. Lab. Sci. 2003, 33, 257–264. [Google Scholar] [PubMed]
- Westerbacka, J.; Tiikkainen, M.; Tamminen, M.; Vehkavaara, S.; Fredriksson, J. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: Implications for sex differences in markers of cardiovascular risk. Diabetologia 2004, 47, 1360–1369. [Google Scholar] [CrossRef]
- Schindhelm, R.K.; Diamant, M.; Dekker, J.M.; Tushuizen, M.E.; Teerlink, T.; Heine, R.J. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes/Metab. Res. Rev. 2006, 22, 437–443. [Google Scholar] [CrossRef]
- Wang, A.; Liu, M.; Shang, W.; Liu, J.; Dai, Z.; Strappe, P.; Zhou, Z. Attenuation of metabolic syndrome in the ob/ob mouse model by resistant starch intervention is dose dependent. Food Funct. 2019, 10, 7940–7951. [Google Scholar] [CrossRef]
- Martinez, O.D.M.; Theodoro, J.M.V.; Grancieri, M.; Toledo, R.C.L.; Queiroz, V.A.V.; de Barros, F.A.R.; Martino, H.S.D. Dry heated whole sorghum flour (BRS 305) with high tannin and resistant starch improves glucose metabolism, modulates adiposity, and reduces liver steatosis and lipogenesis in Wistar rats fed with a high-fat high-fructose diet. J. Cereal Sci. 2021, 99, 103201. [Google Scholar] [CrossRef]
| Items | Ingredients | Basal Diet | High-Fat Diet | ||
|---|---|---|---|---|---|
| NAR 1 | HAR 2 | Control 3 | hNAR 4 | hHAR 5 | |
| Ingredients, % | |||||
| Chicken breast powder | - | - | 12 | 17 | 17 |
| Egg yolk powder | - | - | 12 | 12 | 12 |
| Anchovy powder | - | - | 0.5 | 0.5 | 0.5 |
| Rice flour (Normal amylose type) | - | - | 12 | 12 | - |
| Rice flour (High amylose type) | - | - | - | - | 12 |
| Sweet potato powder | - | - | 10 | - | - |
| Lard | - | - | - | 8 | 8 |
| Cooked rice (Normal amylose type) | - | - | 20 | 18.7 | 18.7 |
| Egg-shell powder | - | - | 1 | 1 | 1 |
| Potassium Gluconate | - | - | 1 | 1.2 | 1.2 |
| Vitamin-Mineral premix 6 | - | - | 0.5 | 0.6 | 0.6 |
| Water | - | - | 31 | 29 | 29 |
| Chemical compositions, % (DM basis, analyzed) | |||||
| Dry matter (DM) | 86.9 | 86.7 | 53.0 | 56.2 | 55.6 |
| Crude protein (CP) | 6.67 | 7.57 | 32.6 | 36.8 | 37.1 |
| Ether extract (EE) | 0.43 | 1.46 | 16.4 | 27.9 | 29.5 |
| Crude fiber (CF) | 0.03 | 0.18 | 1.0 | 0.5 | 0.7 |
| Crude ash (CA) | 0.55 | 0.68 | 3.8 | 3.6 | 3.7 |
| Nitrogen-free extract (NFE) | 92.1 | 89.7 | 51.5 | 34.0 | 34.4 |
| Calcium (Ca) | 0.08 | 0.09 | 0.8 | 0.7 | 0.8 |
| Phosphorus (P) | 0.15 | 0.21 | 0.5 | 0.5 | 0.5 |
| Resistant starch (RS) | 2.68 | 15.57 | 1.4 | 1.3 | 7.5 |
| ME, kcal/kg (calculated) | - | - | 4155 | 4748 | 4816 |
| Items | NAR 1 | HAR 2 |
|---|---|---|
| Ingredients, % | ||
| Chicken breast powder | 12.7 | 12.7 |
| Egg yolk powder | 12 | 12 |
| Rice flour (Normal amylose type) | 28.76 | - |
| Rice flour (High amylose type) | - | 28.76 |
| Lard | 2.9 | 2.9 |
| Green laver powder | 1 | 1 |
| Calcium carbonate | 1.04 | 1.04 |
| Potassium Citrate | 0.6 | 0.6 |
| Monocalcium phosphate | 0.4 | 0.4 |
| Vitamin-Mineral premix 3 | 0.4 | 0.4 |
| Salt (NaCl) | 0.2 | 0.2 |
| Water | 40 | 40 |
| Chemical compositions, % (DM basis, analyzed) | ||
| Dry matter (DM) | 55.7 | 52.8 |
| Crude protein (CP) | 33.9 | 33.1 |
| Ether extract (EE) | 16.9 | 17.4 |
| Crude fiber (CF) | 0.2 | 0.7 |
| Crude ash (CA) | 4.9 | 4.6 |
| Nitrogen-free extract (NFE) | 44.2 | 44.1 |
| Ca | 0.9 | 0.9 |
| P | 0.6 | 0.6 |
| ME, kcal/kg (DM basis, calculated) | 4165 | 4184 |
| Items | Normal Diet | High-Fat Diet | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control 1 | hNAR 2 | hHAR 3 | ||||||||
| ADFI (g/d) | 151 | ± | 11 | 146 | ± | 10 | 141 | ± | 13 | 0.850 |
| MER (kcal/d) | 330 | ± | 22 | 332 | ± | 21 | 319 | ± | 26 | 0.946 |
| MEI (kcal/d) | 332 | ± | 23 | 389 | ± | 28 | 378 | ± | 33 | 0.342 |
| Calculated MEI/kg mBW | 103 | ± | 5 | 119 | ± | 5 | 121 | ± | 17 | 0.304 |
| Body weight (kg) | ||||||||||
| Initial | 4.8 | ± | 0.6 | 4.8 | ± | 0.5 | 4.6 | ± | 0.6 | 0.946 |
| Final | 4.7 | ± | 0.6 | 5.5 | ± | 0.6 | 5.3 | ± | 0.6 | 0.618 |
| p-value 4 | 0.913 | 0.416 | 0.382 | |||||||
| BWG (kg) | −0.1 | ± | 0.1 b | 0.9 | ± | 0.3 a | 0.7 | ± | 0.1 a | 0.006 |
| Rate of gain (%) | −1.8 | ± | 2.9 b | 21 | ± | 9 a | 17 | ± | 3 a | 0.009 |
| Body condition score | ||||||||||
| Initial | 5.0 | ± | 0.3 | 4.8 | ± | 0.3 | 4.8 | ± | 0.3 | 0.794 |
| Final | 5.0 | ± | 0.3 | 5.3 | ± | 0.3 | 5.1 | ± | 0.3 | 0.853 |
| p-vlaue 4 | 0.989 | 0.278 | 0.349 | |||||||
| Items | Normal Diet | High-Fat Diet | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control 1 | hNAR 2 | hHAR 3 | ||||||||
| Leukocytes | ||||||||||
| WBC (×106/mL) (Ref. range: 5.05–16.76) | 7.1 | ± | 1.2 | 8 | ± | 0.9 | 8.8 | ± | 1.3 | 0.590 |
| NEU (×106/mL) (Ref. range: 2.95–11.64) | 4.8 | ± | 0.9 | 5.5 | ± | 0.6 | 5.8 | ± | 0.9 | 0.718 |
| LYM (×106/mL) (Ref. range: 1.05–5.10) | 1.6 | ± | 0.1 | 1.8 | ± | 0.3 | 2.1 | ± | 0.3 | 0.394 |
| MONO (×106/mL) (Ref. range: 0.16–1.12) | 0.4 | ± | 0.1 | 0.4 | ± | 0.1 | 0.5 | ± | 0.1 | 0.815 |
| EOS (×106/mL) (Ref. range: 0.06–1.23) | 0.2 | ± | 0.1 | 0.3 | ± | 0.1 | 0.5 | ± | 0.1 | 0.221 |
| BASO (×106/mL) (Ref. range: 0–0.1) | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.269 |
| Erythrocytes | ||||||||||
| RBC (×109/mL) (Ref. range: 5.65–8.87) | 6.8 | ± | 0.2 | 6.8 | ± | 0.1 | 6.9 | ± | 0.2 | 0.958 |
| HGB (g/dL) (Ref. range: 13.1–20.5) | 15 | ± | 0.5 | 16 | ± | 0.3 | 15 | ± | 0.4 | 0.697 |
| HCT (%) (Ref. range: 37.3–61.7) | 43 | ± | 1.6 | 46 | ± | 1.1 | 45 | ± | 1.1 | 0.447 |
| MCH (pg) (Ref. range: 21.2–25.9) | 22 | ± | 0.3 | 23 | ± | 0.3 | 23 | ± | 0.4 | 0.170 |
| Thrombocytes | ||||||||||
| PLT (K/µL) (Ref. range: 148–484) | 284 | ± | 39 | 330 | ± | 63 | 266 | ± | 37 | 0.624 |
| PCT (%) (Ref. range: 0.14–0.46) | 0.4 | ± | 0.0 | 0.4 | ± | 0.1 | 0.4 | ± | 0.0 | 0.476 |
| Items | Normal Diet | High-Fat Diet | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control 1 | hNAR 2 | hHAR 3 | ||||||||
| GLU (mg/dL) (Ref. range: 70–138) | 89 | ± | 3.4 | 98 | ± | 8.0 | 97 | ± | 4.7 | 0.480 |
| CREA (mg/dL) (Ref. range: 0.5–1.6) | 0.6 | ± | 0.1 | 0.6 | ± | 0.03 | 0.6 | ± | 0.1 | 0.676 |
| BUN (mg/dL) (Ref. range: 6.0–31) | 16 | ± | 1.3 | 13 | ± | 0.6 | 15 | ± | 0.9 | 0.136 |
| PHOS (mg/dL) (Ref. range: 2.5–6.0) | 4.3 | ± | 0.2 | 4.3 | ± | 0.1 | 4.4 | ± | 0.3 | 0.873 |
| T-Pro (g/dL) (Ref. range: 5.0–7.4) | 6.6 | ± | 0.1 | 6.6 | ± | 0.2 | 6.7 | ± | 0.1 | 0.679 |
| ALB (g/dL) (Ref. range: 2.7–4.4) | 3.2 | ± | 0.1 | 3.2 | ± | 0.2 | 3.2 | ± | 0.1 | 0.965 |
| ALT (U/L) (Ref. range: 12–118) | 71 | ± | 11 | 143 | ± | 26 | 101 | ± | 24 | 0.090 |
| T-BIL (mg/dL) (Ref. range: 0.1–0.3) | 0.2 | ± | 0.02 | 0.2 | ± | 0.04 | 0.3 | ± | 0.03 | 0.247 |
| T-CHO (mg/dL) (Ref. range: 29–291) | 155 | ± | 19 b | 192 | ± | 9 ab | 231 | ± | 20 a | 0.015 |
| Items | NAR 1 | HAR 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| ADFI (g/d) | 146 | ± | 28 | 150 | ± | 22 | 0.909 |
| MER (kcal/d) | 305 | ± | 53 | 302 | ± | 44 | 0.972 |
| MEI (kcal/d) | 339 | ± | 71 | 332 | ± | 66 | 0.936 |
| Calculated MEI/kg mBW | 113 | ± | 8 | 112 | ± | 7 | 0.908 |
| Body weight (kg) | |||||||
| Initial | 4.3 | ± | 1.1 | 4.3 | ± | 0.9 | 0.972 |
| Final | 4.2 | ± | 1.1 | 4.0 | ± | 0.8 | 0.872 |
| p-vlaue 3 | 0.952 | 0.821 | |||||
| BWG (kg) | −0.1 | ± | 0.00 | −0.3 | ± | 0.1 | 0.032 |
| Rate of gain (%) | −3.0 | ± | 1.0 | −6.7 | ± | 1.5 | 0.094 |
| Body condition score | |||||||
| Initial | 4.5 | ± | 0.3 | 4.5 | ± | 0.5 | 0.998 |
| Final | 4.3 | ± | 0.3 | 4.0 | ± | 0.4 | 0.620 |
| p-vlaue 3 | 0.537 | 0.468 | |||||
| Items | NAR 1 | HAR 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Leukocytes | |||||||
| WBC (×106/mL) (Ref. range: 5.05–16.76) | 8.2 | ± | 0.8 | 6.6 | ± | 0.7 | 0.206 |
| NEU (×106/mL) (Ref. range: 2.95–11.64) | 4.6 | ± | 0.5 | 3.8 | ± | 0.5 | 0.309 |
| LYM (×106/mL) (Ref. range: 1.05–5.10) | 2.7 | ± | 0.6 | 2.2 | ± | 0.3 | 0.393 |
| MONO (×106/mL) (Ref. range: 0.16–1.12) | 0.4 | ± | 0.1 | 0.3 | ± | 0.1 | 0.639 |
| EOS (×106/mL) (Ref. range: 0.06–1.23) | 0.5 | ± | 0.1 | 0.3 | ± | 0.1 | 0.256 |
| BASO (×106/mL) (Ref. range: 0–0.1) | 0.01 | ± | 0.01 | 0.00 | ± | 0.00 | 0.356 |
| Erythrocytes | |||||||
| RBC (×109/mL) (Ref. range: 5.65–8.87) | 6.7 | ± | 0.2 | 6.9 | ± | 0.3 | 0.602 |
| HGB (g/dL) (Ref. range: 13.1–20.5) | 16 | ± | 0.4 | 17 | ± | 0.7 | 0.680 |
| HCT (%) (Ref. range: 37.3–61.7) | 46 | ± | 0.7 | 49 | ± | 2.2 | 0.316 |
| MCH (pg) (Ref. range: 21.2–25.9) | 24 | ± | 0.6 | 24 | ± | 0.4 | 0.735 |
| Thrombocytes | |||||||
| PLT (K/µL) (Ref. range: 148–484) | 483 | ± | 85 | 403 | ± | 62 | 0.478 |
| PCT (%) (Ref. range: 0.14–0.46) | 0.4 | ± | 0.1 | 0.4 | ± | 0.1 | 0.575 |
| Items | NAR 1 | HAR 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| GLU (mg/dL) (Ref. range: 70–138) | 107 | ± | 4 | 101 | ± | 2 | 0.196 |
| CREA (mg/dL) (Ref. range: 0.5–1.6) | 0.8 | ± | 0.1 | 0.9 | ± | 0.04 | 0.122 |
| BUN (mg/dL) (Ref. range: 6.0–31) | 18 | ± | 1 | 17 | ± | 1 | 0.568 |
| PHOS (mg/dL) (Ref. range: 2.5–6.0) | 3.4 | ± | 0.1 | 3.6 | ± | 0.4 | 0.618 |
| T-Pro (g/dL) (Ref. range: 5.0–7.4) | 6.5 | ± | 0.2 | 6.7 | ± | 0.4 | 0.697 |
| ALB (g/dL) (Ref. range: 2.7–4.4) | 2.7 | ± | 0.1 | 2.8 | ± | 0.1 | 0.303 |
| ALT (U/L) (Ref. range: 12–118) | 46 | ± | 8 | 39 | ± | 8 | 0.545 |
| T-BIL (mg/dL) (Ref. range: 0.1–0.3) | 0.03 | ± | 0.01 | 0.05 | ± | 0.01 | 0.134 |
| T-CHO (mg/dL) (Ref. range: 29–291) | 220 | ± | 32 | 168 | ± | 19 | 0.208 |
| TG (mg/dL) (Ref. range: 23–102) | 44 | ± | 1 | 27 | ± | 8 | 0.273 |
| NEFA, mEq/L (Ref. range: 0.13–1.25) | 0.8 | ± | 0.1 | 0.6 | ± | 0.1 | 0.328 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, K.; Cho, H.-W.; Chun, J.L.; So, K.M.; Kim, K.H. Body Weight Development in Adult Dogs Fed a High Level Resistant Starch Diet. Animals 2022, 12, 3440. https://doi.org/10.3390/ani12233440
Seo K, Cho H-W, Chun JL, So KM, Kim KH. Body Weight Development in Adult Dogs Fed a High Level Resistant Starch Diet. Animals. 2022; 12(23):3440. https://doi.org/10.3390/ani12233440
Chicago/Turabian StyleSeo, Kangmin, Hyun-Woo Cho, Ju Lan Chun, Kyoung Min So, and Ki Hyun Kim. 2022. "Body Weight Development in Adult Dogs Fed a High Level Resistant Starch Diet" Animals 12, no. 23: 3440. https://doi.org/10.3390/ani12233440
APA StyleSeo, K., Cho, H.-W., Chun, J. L., So, K. M., & Kim, K. H. (2022). Body Weight Development in Adult Dogs Fed a High Level Resistant Starch Diet. Animals, 12(23), 3440. https://doi.org/10.3390/ani12233440





