Characterization of ddx4 and dnd Homologs in Snakeskin Gourami (Trichopodus pectoralis) and Their Expression Levels during Larval Development and in Gonads of Males and Females
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Fish Sampling
2.2. Fish Sampling and Total RNA Extraction
2.3. Cloning of the Full-Length ddx4 and dnd1 cDNAs
2.4. Tissue Expression Analysis of ddx4 and dnd1
2.5. Histological Study and In Situ Hybridization
2.6. Fish Breeding, Larva Sampling, and Fish Sampling
2.7. Quantitative Expression Analysis of ddx4 and dnd1 mRNAs during Larval Development and in Adult Male and Female Fish
2.7.1. Total RNA Extraction and cDNA Synthesis
2.7.2. Quantitative RT-PCR Analysis
3. Results
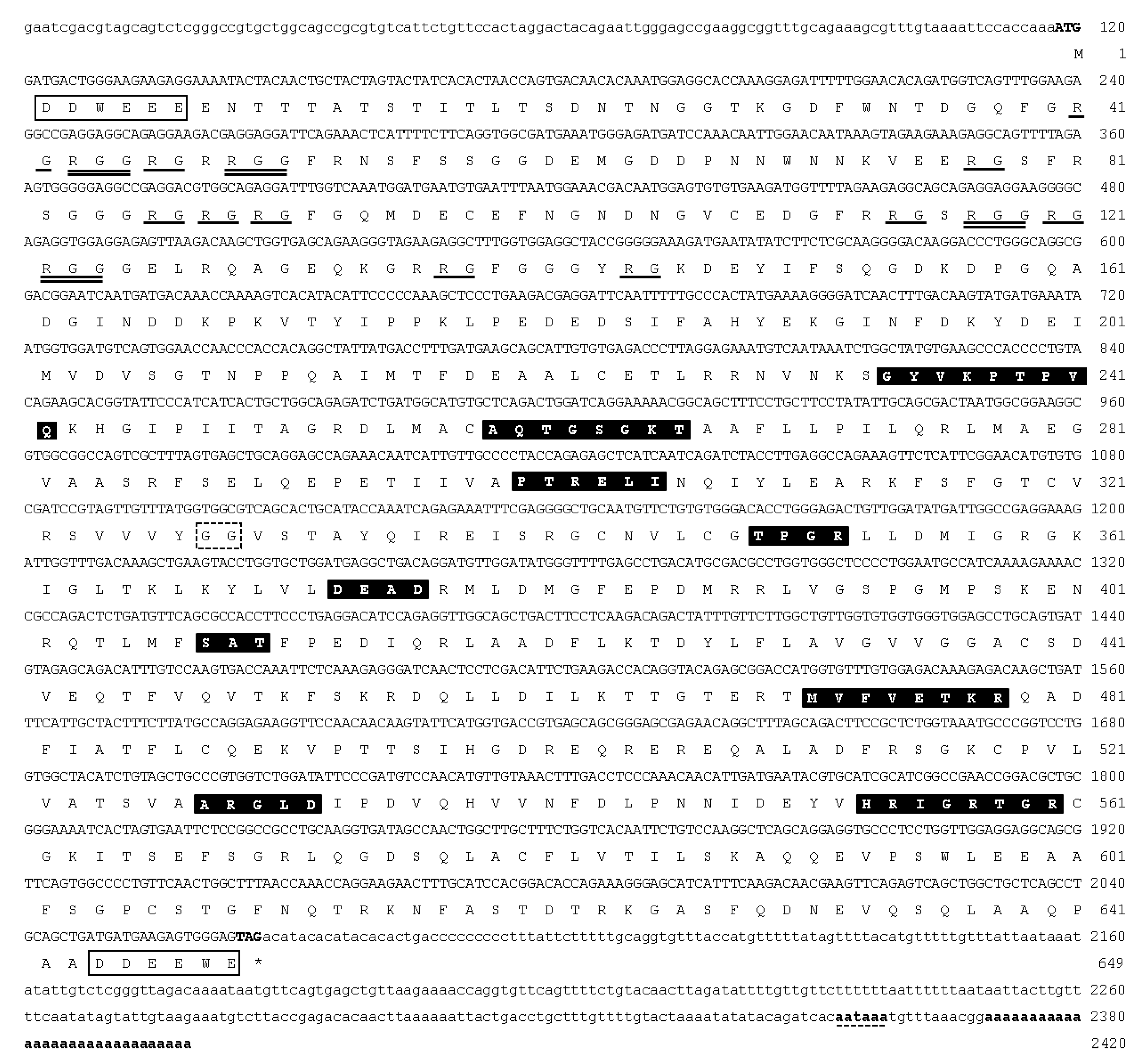
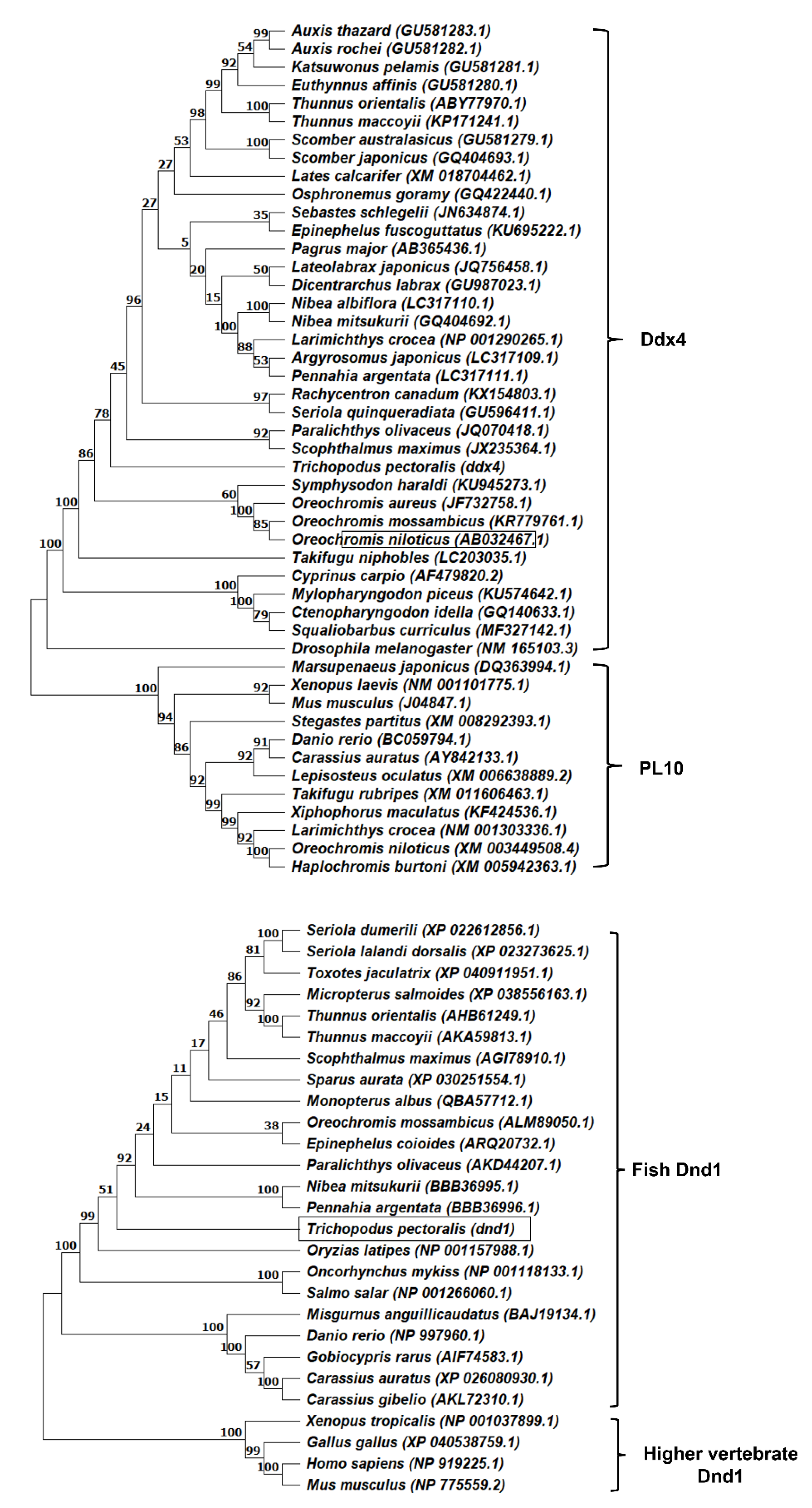
3.1. Molecular Cloning and Characterization of ddx4 and dnd1 in Snakeskin Gourami
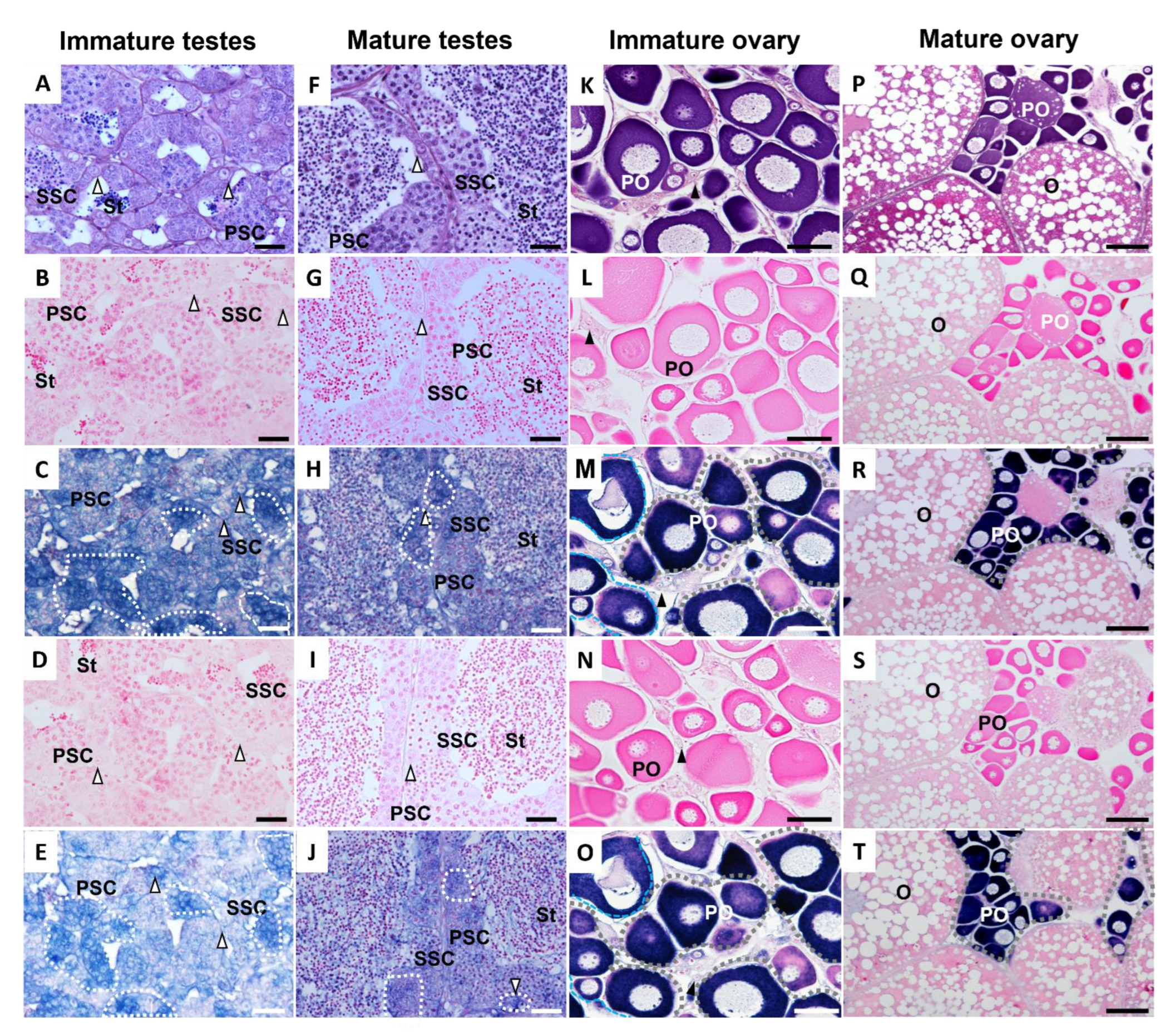
3.2. Tissue Distribution of ddx4 and dnd1 and In Situ Hybridization
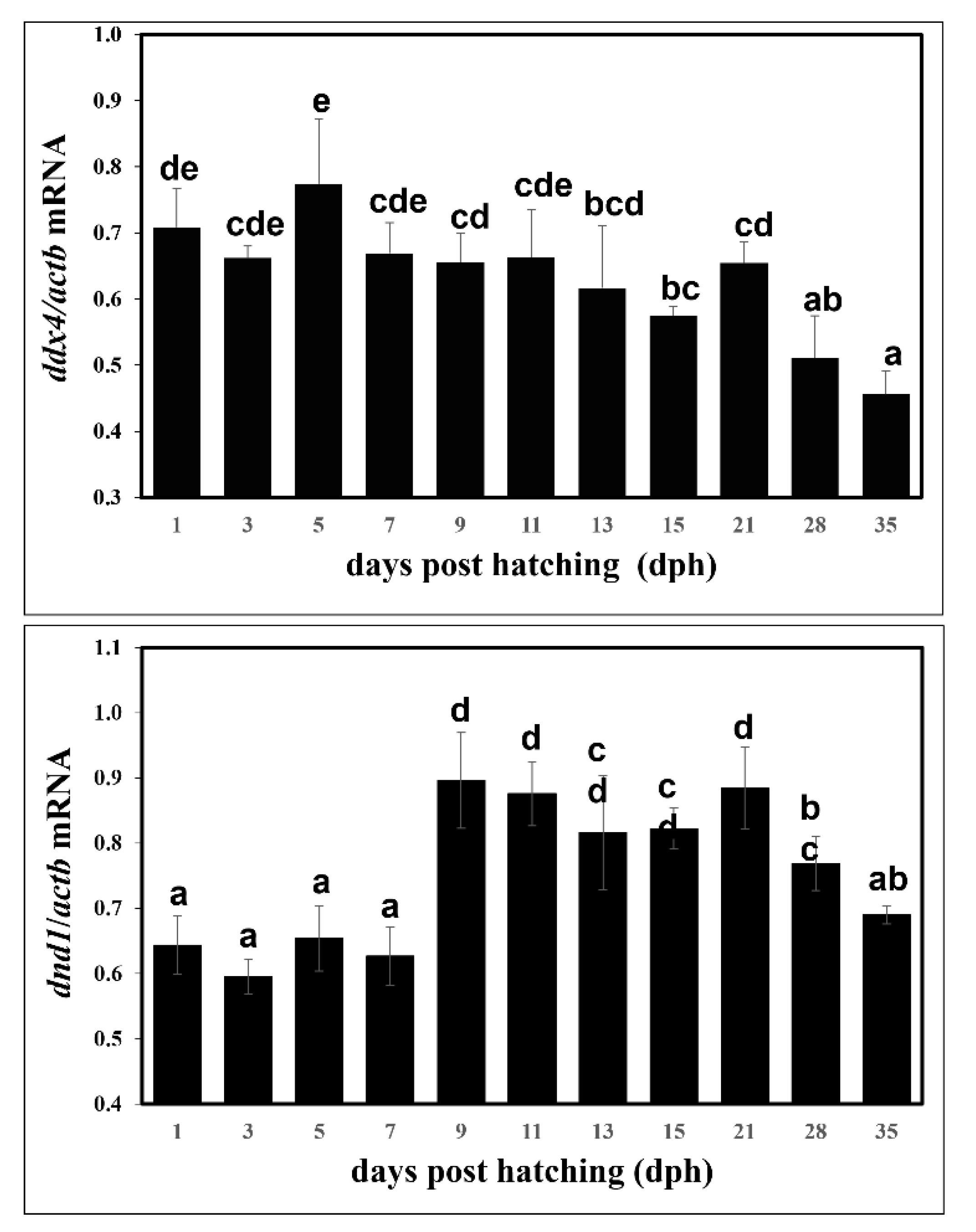
3.3. Expression Levels of ddx4 and dnd1 during Early Development
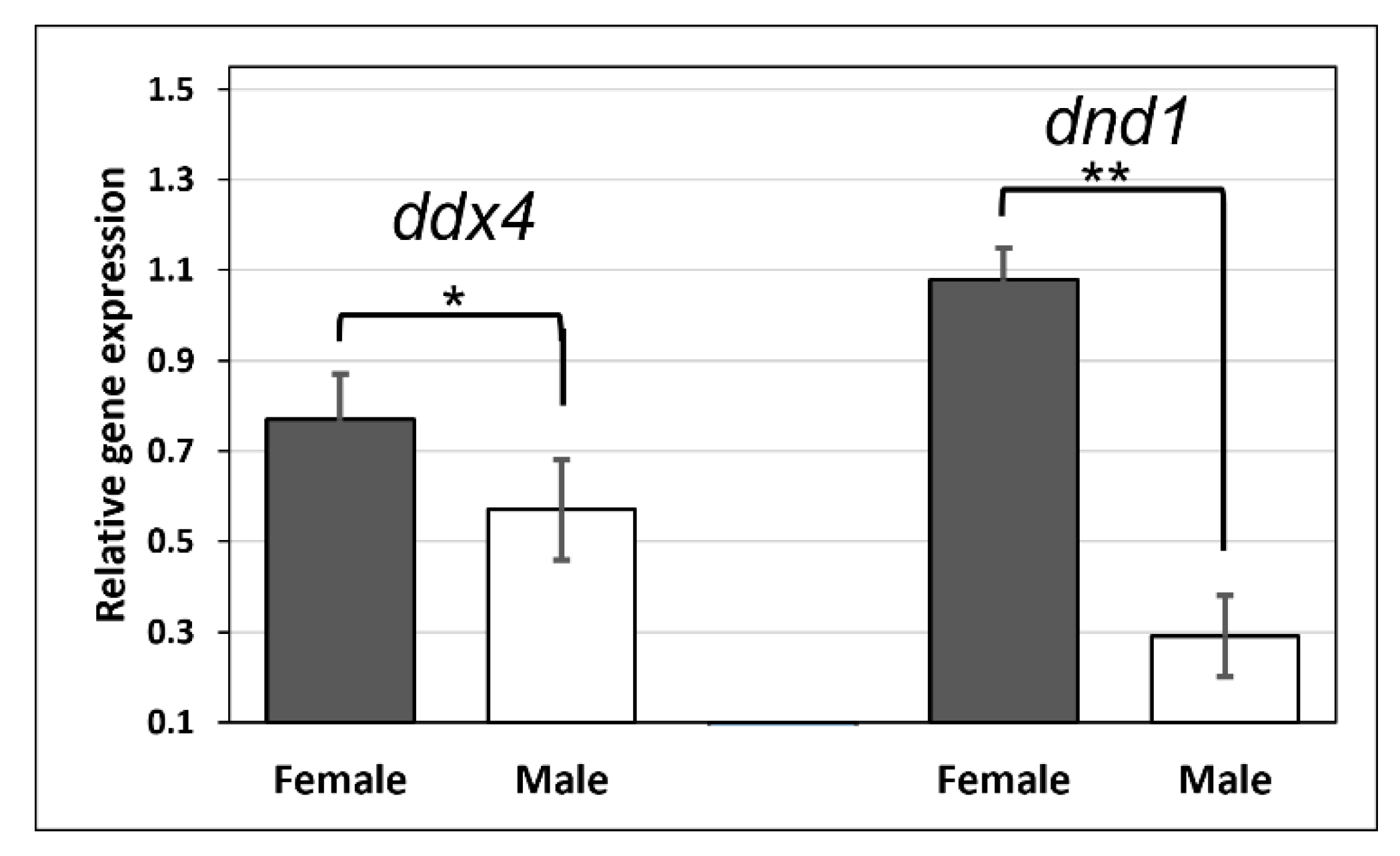
3.4. Sexually Dimorphic Expression of ddx4 and dnd1 in Males and Females
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beardmorea, J.A.; Mairal, G.C.; Lewis, R.I. Monosex male production in finfish as exemplified by tilapia: Applications, problems, and prospects. Aquaculture 2001, 197, 283–301. [Google Scholar] [CrossRef]
- Piferrer, F. Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 2001, 197, 229–281. [Google Scholar] [CrossRef]
- Li, X.Y.; Mei, J.; Ge, C.T.; Liu, X.L.; Gui, J.F. Sex determination mechanisms and sex control approaches in aquaculture animals. Sci. China Life Sci. 2022, 65, 1091–1122. [Google Scholar] [CrossRef]
- Zhai, G.; Shu, T.; Chen, K.; Lou, Q.; Jia, J.; Huang, J.; Shi, C.; Jin, X.; He, J.; Jiang, D.; et al. Successful production of an all-female common carp (Cyprinus carpio L.) population using cyp17a1-deficient neomale carp. Engineering 2022, 8, 181–189. [Google Scholar] [CrossRef]
- Castrillon, D.H.; Quade, B.J.; Wang, T.; Quigley, C.; Crum, C.P. The human VASA gene is specifically expressed in the germ cell lineage. Proc. Nat. Acad. Sci. USA 2000, 97, 9585–9590. [Google Scholar] [CrossRef]
- Raz, E. Primordial germ-cell development: The zebrafish perspective. Nat. Rev. Genet. 2003, 4, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Duangkaew, R.; Jangprai, A.; Ichida, K.; Yoshizaki, G.; Boonanuntanasarn, S. Characterization and expression of a vasa homolog in the gonads and primordial germ cells of the striped catfish (Pangasianodon hypophthalmus). Theriogenology 2019, 131, 61–71. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, X.; Du, S.; Wang, Y.; Zhao, H.; Du, T.; Yu, J.; Wu, L.; Song, Z.; Liu, Q.; et al. Germline Specific Expression of a vasa Homologue Gene in the Viviparous Fish Black Rockfish (Sebastes schlegelii) and Functional Analysis of the vasa 3′ Untranslated Region. Front. Cell Dev. Biol. 2020, 8, 575788. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Xiao, Y.; Yang, Y.; Wang, Y.; Song, Z.; You, F.; An, H.; Xiao, Z.; Xu, S.; et al. The dnd RNA Identifies Germ Cell Origin and Migration in Olive Flounder (Paralichthys olivaceus). Biomed Res. Int. 2015, 2015, 428591. [Google Scholar]
- Gribouval, L.; Sourdaine, P.; Lareyre, J.J.; Bellaiche, J.; Gac, F.L.; Mazan, S.; Guiardiere, C.; Auvray, P.; Gautier, A. The nanos1 gene was duplicated in early Vertebrates and the two paralogs show different gonadal expression profiles in a shark. Sci. Rep. 2018, 8, 6942. [Google Scholar] [CrossRef]
- Sun, Z.H.; Zhou, L.; Lia, Z.; Liu, X.C.; Li, S.S.; Wang, Y.; Gui, J.F. Sexual dimorphic expression of dnd in germ cells during sex reversal and its requirement for primordial germ cell survival in protogynous hermaphroditic grouper. Comp. Biochem. Physiol. B. 2017, 208–209, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Zhou, L.; Wang, Y.; Xia, W.; Gui, J.F. Differential expression and dynamic changes of SOX3 during gametogenesis and sex reversal in protogynous hermaphroditic fish. J. Exp. Zool. A Ecol. Genet. Physiol. 2007, 307, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Z.; Liu, W.; Li, Z.; Wang, Y.; Zhou, L.; Yi, M.S.; Gui, J.F. Molecular characterization and expression pattern of a germ cell marker gene dnd in gibel carp (Carassius gibelio). Gene 2016, 591, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.S.; Toyooka, Y.; Akasu, R.; Katoh-Fukui, Y.; Nakahara, Y.; Suzuki, R.; Yokoyama, M.; Noce, T. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 2000, 14, 841–853. [Google Scholar] [CrossRef]
- Weidinger, G.; Stebler, J.; Slanchev, K.; Dumstrei, K.; Wise, C.; Lovell-Badge, R.; Thisse, C.; Thisse, B.; Raz, E. Dead end, a Novel Vertebrate Germ Plasm Component, Is Required for Zebrafish Primordial Germ Cell Migration and Survival. Curr. Biol. 2003, 13, 1429–1434. [Google Scholar] [CrossRef]
- Hartung, O.; Forbes, M.M.; Marlow, F.L. Zebrafish vasa is required for germ-cell differentiation and maintenance. Mol. Reprod. Dev. 2014, 81, 946–961. [Google Scholar] [CrossRef]
- Ye, M.; Chen, Y. Zebrafish as an emerging model to study gonad development. Comput. Struct. Biotechnol. J. 2020, 18, 2373–2380. [Google Scholar] [CrossRef]
- Raghuveer, K.; Senthilkumaran, B. Cloning and differential expression pattern of vasa in the developing and recrudescing gonads of catfish, Clarias gariepinus. Comp. Biochem. Physiol. A 2010, 157, 79–85. [Google Scholar] [CrossRef]
- Lin, F.; Zhao, C.Y.; Xu, S.H.; Ma, D.Y.; Xiao, Z.Z.; Xiao, Y.S.; Xu, C.A.; Liu, Q.H.; Li, J. Germline-specific and sexually dimorphic expression of a dead end gene homologue in turbot (Scophthalmus maximus). Theriogenology 2013, 80, 665–672. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Jiang, J.; Gao, J.; Wang, J.; Zhou, X.; Zhang, Q. Cloning, expression promoter analysis of vasa gene in Japanese flounder (Paralichthys olivaceus). Comp. Biochem. Physiol. B 2014, 167, 41–50. [Google Scholar] [CrossRef]
- Yoon, J.H.; Cho, Y.S.; Lee, H.B.; Park, J.Y.; Lim, H.K. Dead-End (dnd) Gene Cloning and Gonad-Specific Expression Pattern in Starry Flounder (Platichthys stellatus). Animals 2021, 11, 2256. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Diehl-Jones, W.; Lasko, P. Localization of vasa protein to the Drosophila pole plasm is independent of its RNA-binding and helicase activities. Development 1994, 120, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Luking, A.; Stahl, U.; Schmidt, U. The protein family of RNA helicases. Crit. Rev. Biochem. Mol. Biol. 1998, 33, 259–296. [Google Scholar] [CrossRef]
- Rocak, S.; Linder, P. DEAD-box proteins: The driving forces behind RNA metabolism. Nat. Rev. Mol. Cell Biol. 2004, 5, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Parsyan, A.; Svitkin, Y.; Shahbazian, D.; Gkogkas, C.; Lasko, P.; Merrick, W.C.; Sonenberg, N. mRNA helicases: The tacticians of translational control. Nat. Rev. Mol. Cell Biol. 2011, 12, 235–245. [Google Scholar] [CrossRef]
- Raz, E. The function and regulation of vasa-like genes in germ-cell development. Genome Biol. 2000, 1, reviews1017.1. [Google Scholar] [CrossRef]
- Tzung, K.W.; Goto, R.; Saju, J.M.; Sreenivasan, R.; Saito, T.; Arai, K.; Yamaha, E.; Hossain, M.S.; Calvert, M.E.K.; Orban, L. Early depletion of primordial germ cells in zebrafish promotes testis formation. Stem Cell Rep. 2015, 4, 61–73. [Google Scholar] [CrossRef]
- Kedde, M.; Strasser, M.J.; Boldajipour, B.; Vrielink, J.A.F.O.; Slanchev, K.; Sage, C.; Nagel, R.; Voorhoeve, P.M.; Duijse, J.; Ørom, U.A.; et al. RNA-Binding Protein Dnd1 Inhibits MicroRNA Access to Target mRNA. Cell 2007, 131, 1273–1286. [Google Scholar] [CrossRef]
- Liu, L.; Hong, N.; Xu, H.; Li, M.; Yan, Y.; Purwanti, Y.; Yi, M.; Li, Z.; Wang, L.; Hong, Y. Medaka dead end encodes a cytoplasmic protein and identifies embryonic and adult germ cells. Gene Expr. Patterns 2009, 9, 541–548. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kobayashi, H.K.; Nagahama, Y. Differential expression of vasa homologue gene in the germ cells during oogenesis and spermatogenesis in a teleost fish, tilapia, Oreochromis niloticus. Mech. Dev. 2000, 99, 139–142. [Google Scholar] [CrossRef]
- Boonanuntanasarn, S.; Bunlipatanon, P.; Ichida, K.; Yoohat, K.; Mengyu, O.; Detsathit, S.; Yazawa, R.; Yoshizaki, G. Characterization of a vasa homolog in the brown-marbled grouper (Epinephelus fuscoguttatus) and its expression in gonad and germ cells during larval development. Fish Physiol. Biochem. 2016, 42, 1621–1636. [Google Scholar] [CrossRef]
- Horvay, K.; Claußen, M.; Katzer, M.; Landgrebe, J.; Pieler, T. Xenopus Dead end mRNA is a localized maternal determinant that serves a conserved function in germ cell development. Dev. Biol. 2006, 291, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, C.; Aggarwal, S.; Zhu, R. The mouse dead-end gene isoform α is necessary for germ cell and embryonic viability. Biochem. Biophys. Res. Commun. 2007, 355, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, R.; Takeuchi, Y.; Higuchi, K.; Yatabe, T.; Kabeya, N.; Yoshizaki, G. Chub mackerel gonads support colonization, survival, and proliferation of intraperitoneally transplanted xenogenic germ cells. Biol. Reprod. 2010, 82, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; Wen, H.; Ni, M.; Qian, K.; Zhang, P.; Chai, S. The Characteristics of vasa Gene from Japanese Sea Bass (Lateolabrax japonicas) and Its Response to the External Hormones. J. Ocean Univ. China. 2015, 14, 717–723. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy; WF Freeman & Co.: San Francisco, CA, USA, 1973. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Jangprai, A.; Boonanuntanasarn, S.; Yoshizaki, G. Characterization of melanocortin 4 receptor in Snakeskin Gourami and its expression in relation to daily feed intake and short-term fasting. Gen. Comp. Endocrinol. 2011, 173, 27–37. [Google Scholar] [CrossRef]
- Linder, P.; Lasko, P.F.; Ashburner, M.; Leroy, P.; Nielsen, P.J.; Nishi, K.; Schnier, J.; Slonimski, P. Birth of the D-E-A-D box. Nature 1989, 337, 121–122. [Google Scholar] [CrossRef]
- Schmid, S.R.; Linder, P. D-E-A-D protein family of putative RNA helicases. Mol. Micro. 1992, 6, 283–292. [Google Scholar] [CrossRef]
- Pause, A.; Methot, N.; Sonenberg, N. The HRIGRXXR region of the DEAD box RNA helicase eukaryotic translation initiation factor 4A is required for RNA binding and ATP hydrolysis. Mol. Cell. Biol. 1993, 13, 6789–6798. [Google Scholar] [PubMed]
- Tanner, N.K.; Cordin, O.; Banroques, J.; Doere, M.; Linder, P. The Q motif: A newly identified motif in DEAD box helicases may regulate ATP binding and hydrolysis. Mol. Cell. 2003, 11, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Cordin, O.; Banroques, J.; Tanner, N.K.; Linder, P. The DEAD-box protein family of RNA helicases. Gene 2006, 367, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Kiledjian, M.; Dreyfuss, G. Primary structure and binding activity of the hnRNP U protein: Binding RNA through RGG box. EMBO J. 1992, 11, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Slanchev, K.; Steblera, J.; Goudarzic, M.; Cojocarud, V.; Weidingere, G.; Raz, E. Control of dead end localization and activity-implications for the function of the protein in antagonizing miRNA function. Mech. Dev. 2009, 126, 270–277. [Google Scholar] [CrossRef]
- Taguchi, A.; Watanabe, K.; ORII, H. Intracellular localizations of the dead end protein in Xenopus primordial germ cells. Int. J. Dev. Biol. 2014, 58, 793–798. [Google Scholar] [CrossRef]
- Škugor, A.; Slanchev, K.; Torgersen, J.S.; Tveiten, H.; Andersen, Ø. Conserved mechanisms for germ cell-specific localization of nanos3 transcripts in teleost species with aquaculture significance. Mar. Biotechnol. 2014, 16, 256–264. [Google Scholar] [CrossRef]
- Wargelius, A.; Leininger, S.; Skaftnesmo, K.O.; Kleppe, L.; Andersson, E.; Taranger, G.L.; Schulz, R.W.; Edvardsen, R.B. Dnd knockout ablates germ cells and demonstrates germ cell independent sex differentiation in Atlantic salmon. Sci. Rep. 2016, 6, 21284. [Google Scholar] [CrossRef]
- Yoshizaki, G.; Sakatani, S.; Tominaga, H.; Takeuchi, T. Cloning and characterization of a vasa-like gene in rainbow trout and its expression in the germ cell lineage. Mol. Reprod. Dev. 2000, 55, 364–371. [Google Scholar] [CrossRef]
- Shinomiya, A.; Tanaka, M.; Kobayashi, T.; Nagahama, Y.; Hamaguchi, S. The vasa-like gene, olvas, identifies the migration path of primordial germ cells during embryonic body formation stage in the medaka, Oryzias latipes. Dev. Growth Differ. 2000, 42, 317–326. [Google Scholar] [CrossRef]
- Xu, H.; Gui, J.; Hong, Y. Differential expression of vasa RNA and protein during spermatogenesis and oogenesis in the gibel carp (Carassius auratus gibelio), a bisexually and gynogenetically reproducing vertebrate. Dev. Dyn. 2005, 233, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Lv, D.; Song, P.; Peng, M.; Chen, Y.; Guo, M.; Yang, Q.; Hu, Y. Cloning and characterization of a rice field eel vasa-like gene cDNA and its expression in gonads during natural sex transformation. Biochem. Genet. 2007, 45, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, K.; Takeuchi, Y.; Miwa, M.; Higuchi, K.; Morita, T.; Mitsuboshi, T.; Miyaki, K.; Kadomura, K.; Yoshizaki, G. cDNA cloning and expression analysis of a vasa-like gene in Pacific Bluefin tuna Thunnus orientalis. Fish Sci. 2009, 75, 71–79. [Google Scholar] [CrossRef]
- Li, C.J.; Liu, L.; Chen, X.H.; Zhang, T.; Gan, F.; Cheng, B.L. Identification of a vasa homologue gene in grass carp and its expression pattern in tissues and during embryogenesis. Comp. Biochem. Physiol. B 2010, 157, 159–166. [Google Scholar] [CrossRef]
- Blazquez, M.; Gonzalez, A.; Mylonas, C.C.; Piferrer, F. Cloning and sequence analysis of a vasa homolog in the European sea bass (Dicentrarchus labrax): Tissue distribution and mRNA expression levels during early development and sex differentiation. Gen. Comp. Endocrinol. 2011, 170, 322–333. [Google Scholar] [CrossRef]
- Cao, M.; Yang, Y.; Xu, H.; Duan, J.; Cheng, N.; Wang, J.; Hu, W.; Zhao, H. Germ cell specific expression of Vasa in rare minnow, Gobiocypris rarus. Comp. Biochem. Physiol. Part A 2012, 162, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Presslauer, C.; Nagasawa, K.; Fernandes, J.M.O.; Babiak, I. Expression of vasa and nanos3 during primordial germ cell formation and migration in Atlantic cod (Gadus morhua L.). Theriogenology 2012, 78, 1262–1277. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Wen, H.; He, F.; Li, J.; Liu, M.; Ma, R.; Zhang, Y.; Hu, J.; Qi, B. Cloning and Expression Analysis of vasa During the Reproductive Cycle of Korean Rockfish, Sebastes schlegeli. J. Ocean Univ. China 2013, 12, 115–124. [Google Scholar] [CrossRef]
- Pacchiarini, T.; Cross, I.; Leite, R.B.; Gavaia, P.; Ortiz-Delgado, J.B.; Pousão-Ferreira, P.; Rebordinos, L.; Sarasquete, C.; Cabrita, E. Solea senegalensis vasa transcripts: Molecular characterisation, tissue distribution and developmental expression profiles. Reprod. Fertil. Dev. 2013, 25, 646–660. [Google Scholar] [CrossRef]
- Úbeda-Manzanaro, M.; Rebordinos, L.; Sarasquete, C. Cloning and characterization of Vasa gene expression pattern in adults of the Lusitanian toadfish Halobatrachus didactylus. Aquat. Biol. 2014, 21, 37–46. [Google Scholar] [CrossRef][Green Version]
- Ichida, K.; Jangprai, A.; Khaosa-art, P.; Yoshizaki, G.; Boonanuntanasarn, S. Characterization of a vasa homolog in Mekong giant catfish (Pangasianodon gigas): Potential use as a germ cell marker. Anim. Reprod. Sci. 2021, 234, 106869. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Feng, G.; Chang, P.; Zhang, X.; Zhou, Q.; Zhong, X.; Qi, C.; Xie, S.; Zhao, H. Germ cell-specific expression of dead end (dnd) in rare minnow (Gobiocypris rarus). Fish. Physiol. Biochem. 2015, 41, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.C.N.; Streit, D.P., Jr.; Octavera, A.; Miwa, M.; Kabeya, N.; Yoshizaki, G. The germ cell marker dead end reveals alternatively spliced transcripts with dissimilar expression. Sci. Rep. 2019, 9, 2407. [Google Scholar] [CrossRef] [PubMed]
- Kleppe, L.; Edvardsen, R.B.; Furmanek, T.; Andersson, E.; Skaftnesmo, K.O.; Segafredo, F.T.; Wargelius, A. Transcriptomic analysis of dead end knockout testis reveals germ cell and gonadal somatic factors in Atlantic salmon. BMC Genom. 2020, 21, 99. [Google Scholar] [CrossRef]
- Nagasawa, K.; Fernandes, J.M.O.; Yoshizaki, G.; Miwa, M.; Babiak, I. Identification and Migration of Primordial Germ Cells in Atlantic Salmon, Salmo salar: Characterization of Vasa, Dead End, and Lymphocyte Antigen 75 Genes. Mol. Reprod. Dev. 2013, 80, 118–131. [Google Scholar] [CrossRef]
- Hay, B.; Jan, L.Y.; Jan, Y.N. A protein component of Drosophila polar granules is encoded by vasa and has extensive sequence similarity to ATP-dependent helicases. Cell 1988, 55, 577–587. [Google Scholar] [CrossRef]
- Li, M.; Hong, N.; Xu, H.; Yi, M.; Li, C.; Gui, J.; Hong, Y. Medaka vasa is required for migration but not survival of primordial germ cells. Mech. Dev. 2009, 126, 366–381. [Google Scholar] [CrossRef]




| Primer Name | 5′ to 3′ Nucleotide Sequences | Purposes |
|---|---|---|
| Tpe-dndF1 | AAYGGNCARMGNAARTAYGG | Cloning, RT-PCR |
| Tpe-dndR1 | TGNCCNSWRAARTTCATCAT | Cloning |
| Tpe-dndF2 | CTGATCCCCCTATTTAGCTCTGTG | RACE PCR |
| Tpe-dndR2 | CACAGAGCTAAATAGGGGGATCAG | RACE PCR |
| Tpe-dndF3 | GCTCTGGGAGTTTAGACTGATGATG | In situ hybridization |
| Tpe-dndR3 | CATGAAGCCGTCATGTAGGGTGTG | In situ hybridization |
| Tpe-dndF4 | GCTGTGAGGTCTTCATCAGCCAGA | qRT-PCR |
| Tpe-dndR4 | TCCAGCATGTAACCGTGAAGCAAG | qRT-PCR, RT-PCR |
| Tpe-ddx4F1 | GAYGABATMHTKGTVGAYGTBAGYGG | Cloning |
| Tpe-ddx4F2 | AAGCCBACYCCDGTVCAGAARYAYGG | Cloning, in situ hybridization, RT-PCR |
| Tpe-ddx4R1 | CCABKWVGGMACYTCCTGYTGRGG | Cloning |
| Tpe-ddx4R2 | TTHCCRCAKCGDCCDGTKCKBCCRA | Cloning, in situ hybridization |
| Tpe-ddx4R3 | AGTCGCTGCAATATAGGAAGCAGG | RACE PCR, RT-PCR |
| Tpe-ddx4R4 | CAGGTGTCCCACACAGAACATTGC | RACE PCR |
| Tpe-ddx4F3 | AGGTTGGCAGCTGACTTCCTCAAG | RACE PCR |
| β-actinF | ACTACC TCA AGATCCTG | RT-PCR |
| β-actinR | TTGCTGATCCACATCTGCTG | RT-PCR |
| Tpe-ddx4F4 | AGTCGCTTTAGTGAGCTGCAGGAG | qRT-PCR |
| Tpe-ddx4R5 | CTGATTTGGTATGCAGTGCTGACG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakharuthai, C.; Sreebun, S.; Kabpha, A.; Phuong, T.V.; Boonanuntanasarn, S. Characterization of ddx4 and dnd Homologs in Snakeskin Gourami (Trichopodus pectoralis) and Their Expression Levels during Larval Development and in Gonads of Males and Females. Animals 2022, 12, 3415. https://doi.org/10.3390/ani12233415
Nakharuthai C, Sreebun S, Kabpha A, Phuong TV, Boonanuntanasarn S. Characterization of ddx4 and dnd Homologs in Snakeskin Gourami (Trichopodus pectoralis) and Their Expression Levels during Larval Development and in Gonads of Males and Females. Animals. 2022; 12(23):3415. https://doi.org/10.3390/ani12233415
Chicago/Turabian StyleNakharuthai, Chatsirin, Somkiat Sreebun, Apinat Kabpha, Tran Vinh Phuong, and Surintorn Boonanuntanasarn. 2022. "Characterization of ddx4 and dnd Homologs in Snakeskin Gourami (Trichopodus pectoralis) and Their Expression Levels during Larval Development and in Gonads of Males and Females" Animals 12, no. 23: 3415. https://doi.org/10.3390/ani12233415
APA StyleNakharuthai, C., Sreebun, S., Kabpha, A., Phuong, T. V., & Boonanuntanasarn, S. (2022). Characterization of ddx4 and dnd Homologs in Snakeskin Gourami (Trichopodus pectoralis) and Their Expression Levels during Larval Development and in Gonads of Males and Females. Animals, 12(23), 3415. https://doi.org/10.3390/ani12233415





