The Modulatory Effects of Lacticaseibacillus paracasei Strain NSMJ56 on Gut Immunity and Microbiome in Early-Age Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Feed Additive Prepartaion
2.2. Animals and Experimental Design and Sample Collection
2.3. Antioxidant Activity Analysis
2.4. Gut Tissue Histology Analysis
2.5. Gut Lamina Propria Cell Isolation
2.6. Flow Cytometry Analysis
2.7. RNA Extraction and qRT-PCR Analysis
2.8. Volatile Fatty Acid Analysis
2.9. 16S rRNA Sequencing Analysis
2.10. Linear Discriminant Analysis Effect Size (LEfSe) Analysis
2.11. Statistical Analysis
3. Results
3.1. Effect of Supplementation of NSMJ56 on Growth Performance of Early-Age Broiler Chickens
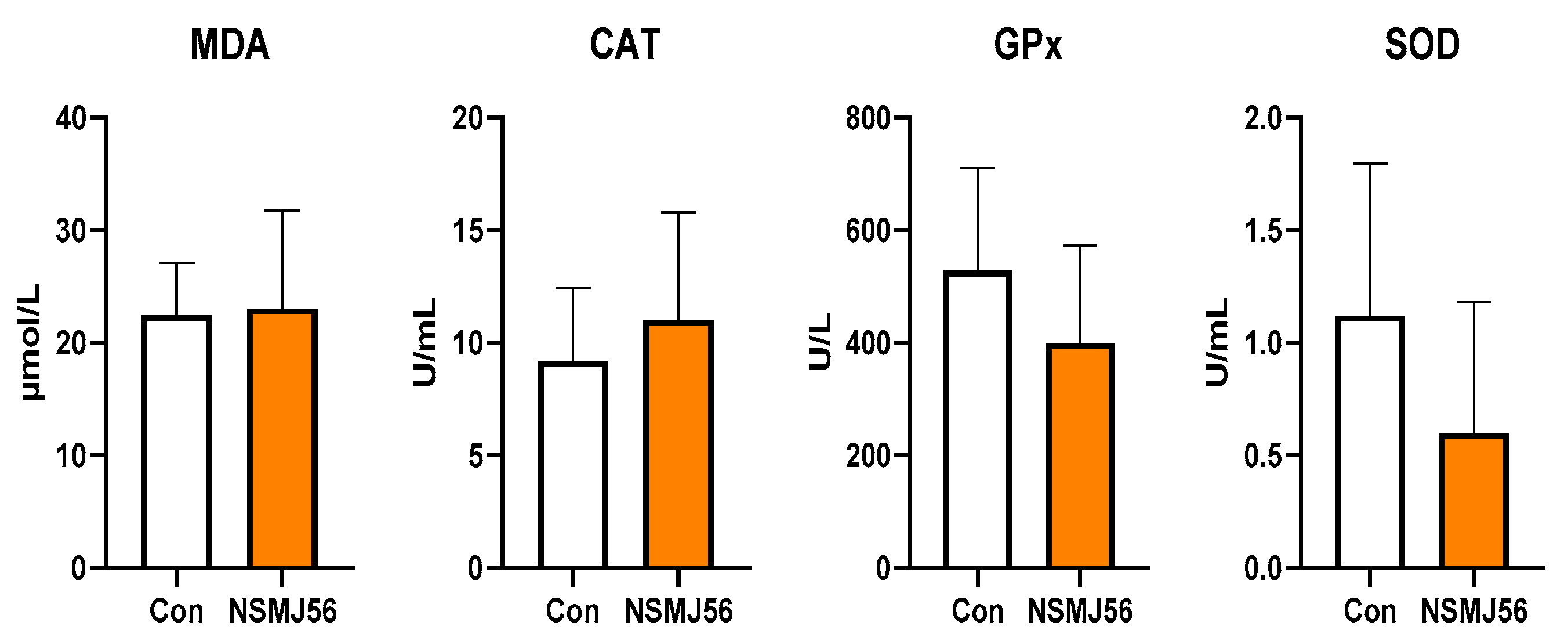
3.2. Effect of Supplementation of NSMJ56 on Antioxidant Activity Parameters in Sera
3.3. Changes of Small Intestine Morphology of Early-Age Broiler Chickens by NSMJ56
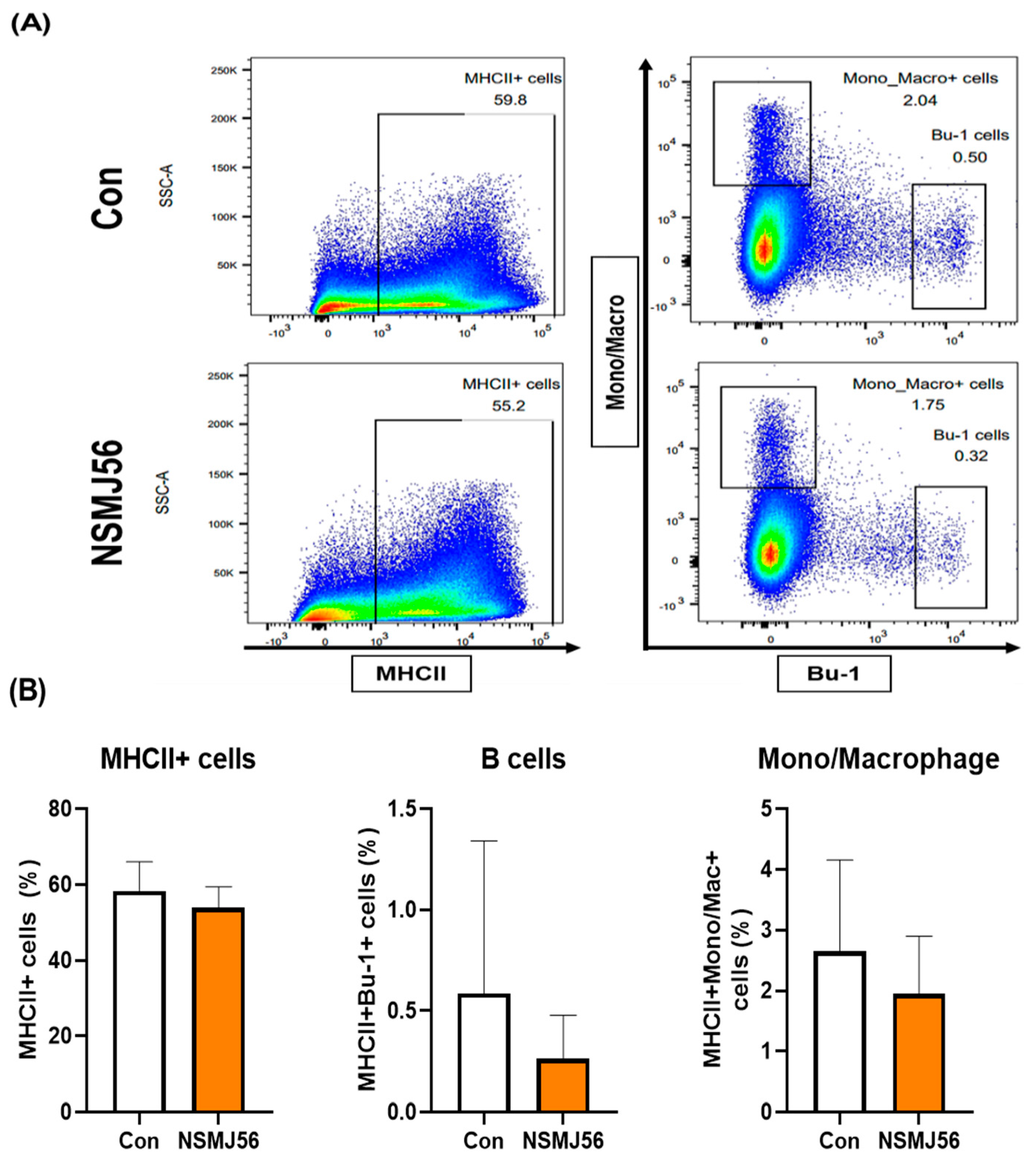
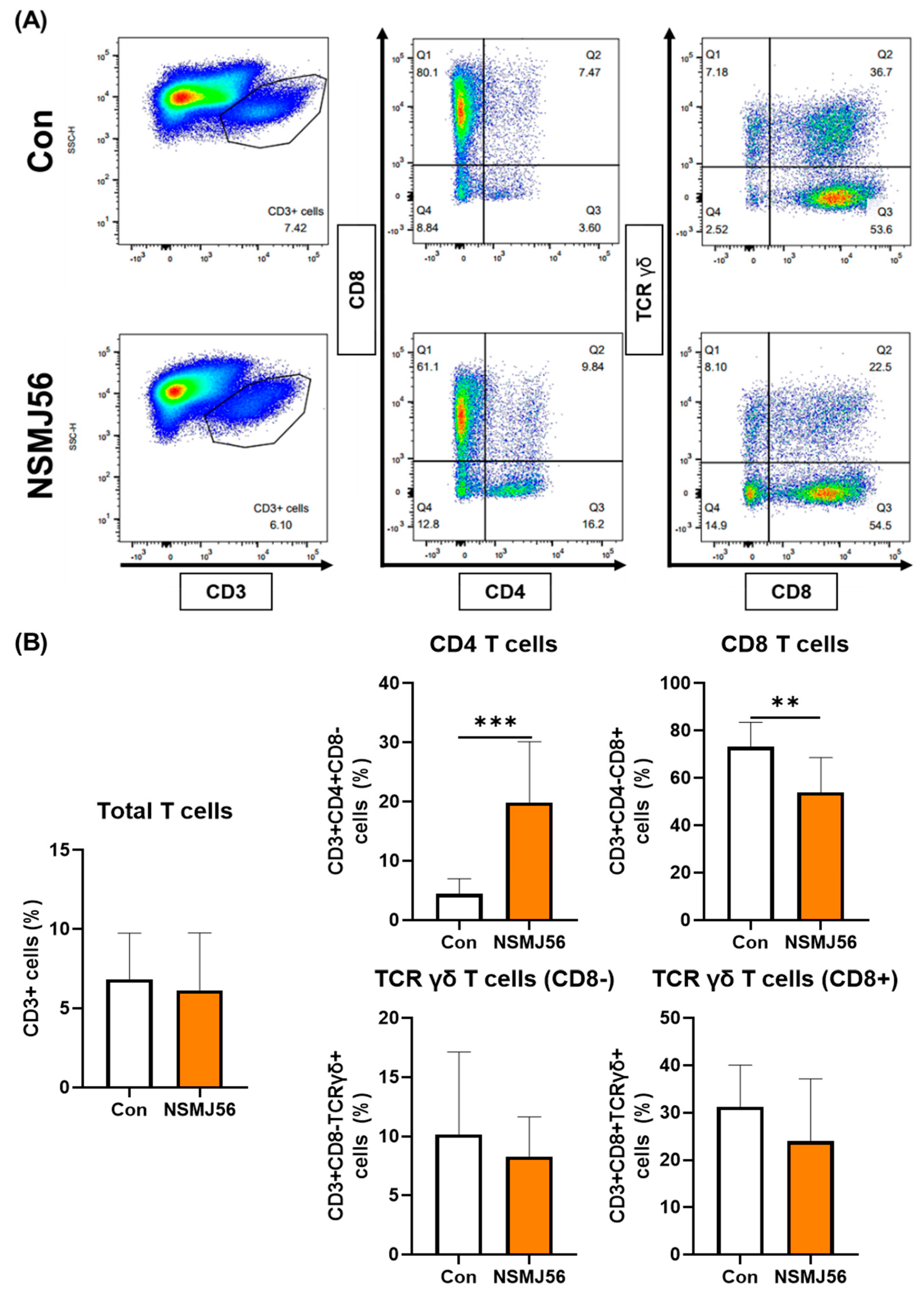
3.4. Changes in Immune Cells in the Small Intestinal Lamina Propria of Early-Age Broiler Chickens by NSMJ56
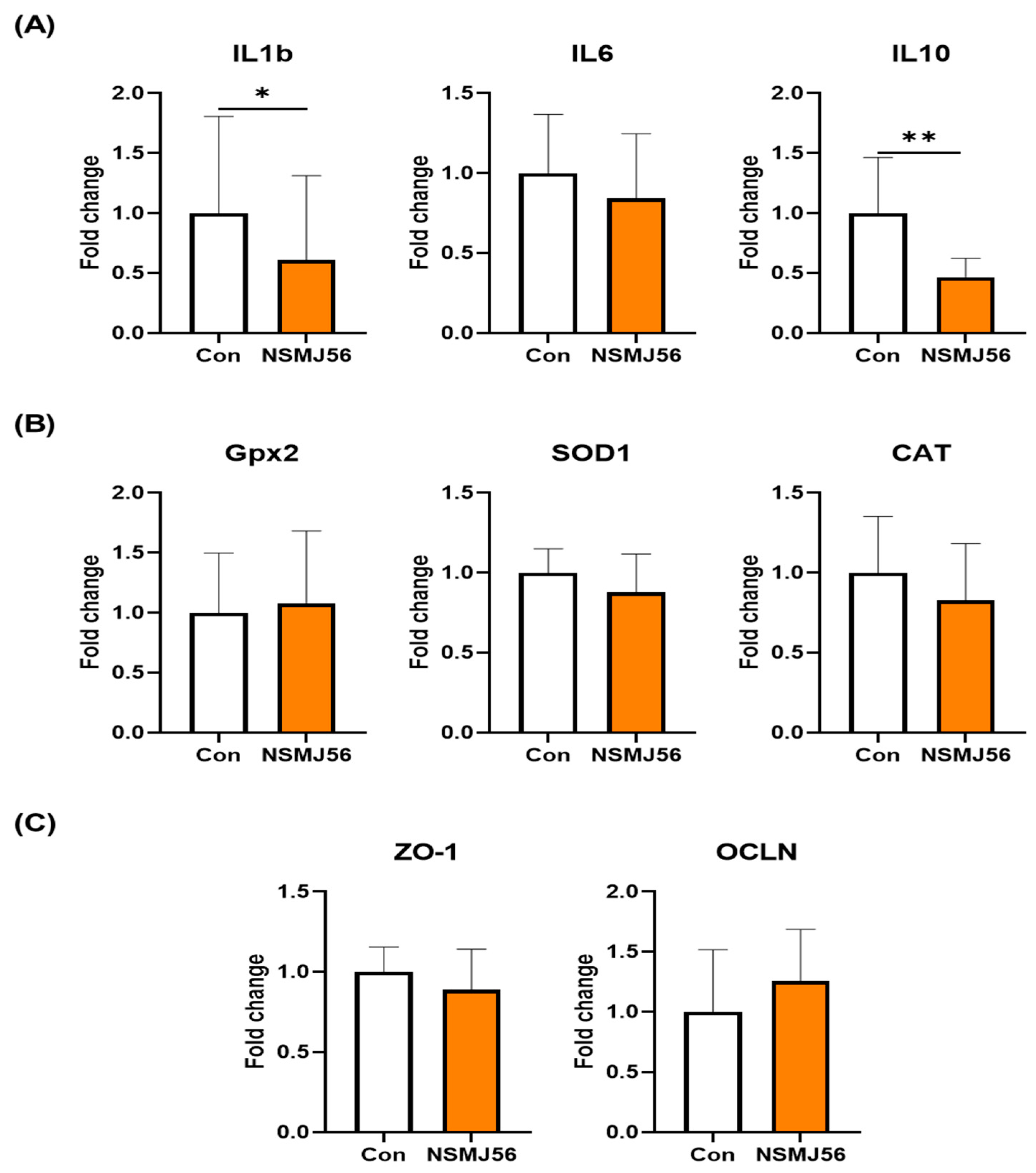
3.5. Changes of Gut Immunity and Gut Health-Related Gene Expression in the Early-Age Broiler Chickens Small Intestine Tissue by NSMJ56
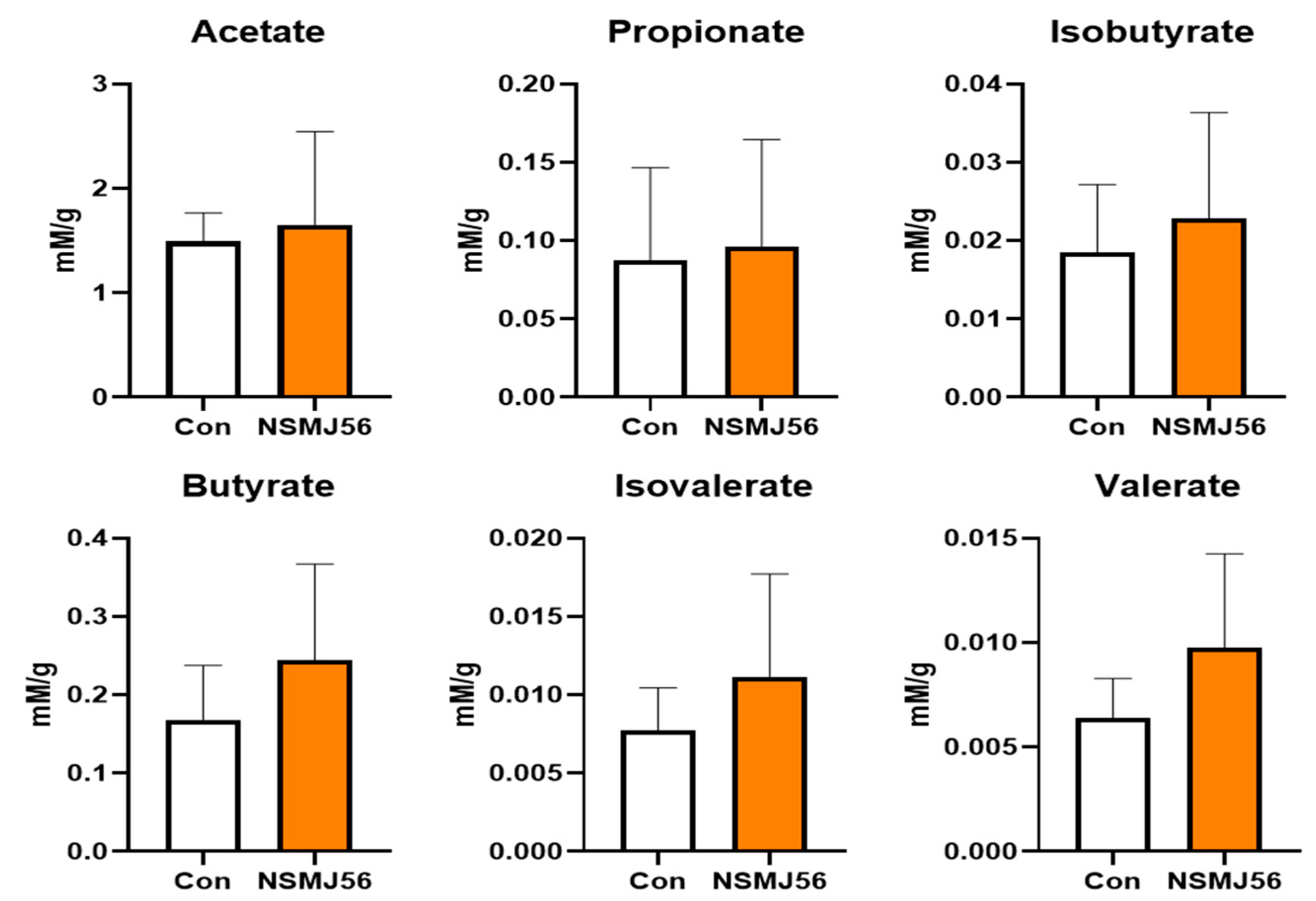
3.6. Alteration in Cecal Content VFA of Early-Age Broiler Chickens by NSMJ56
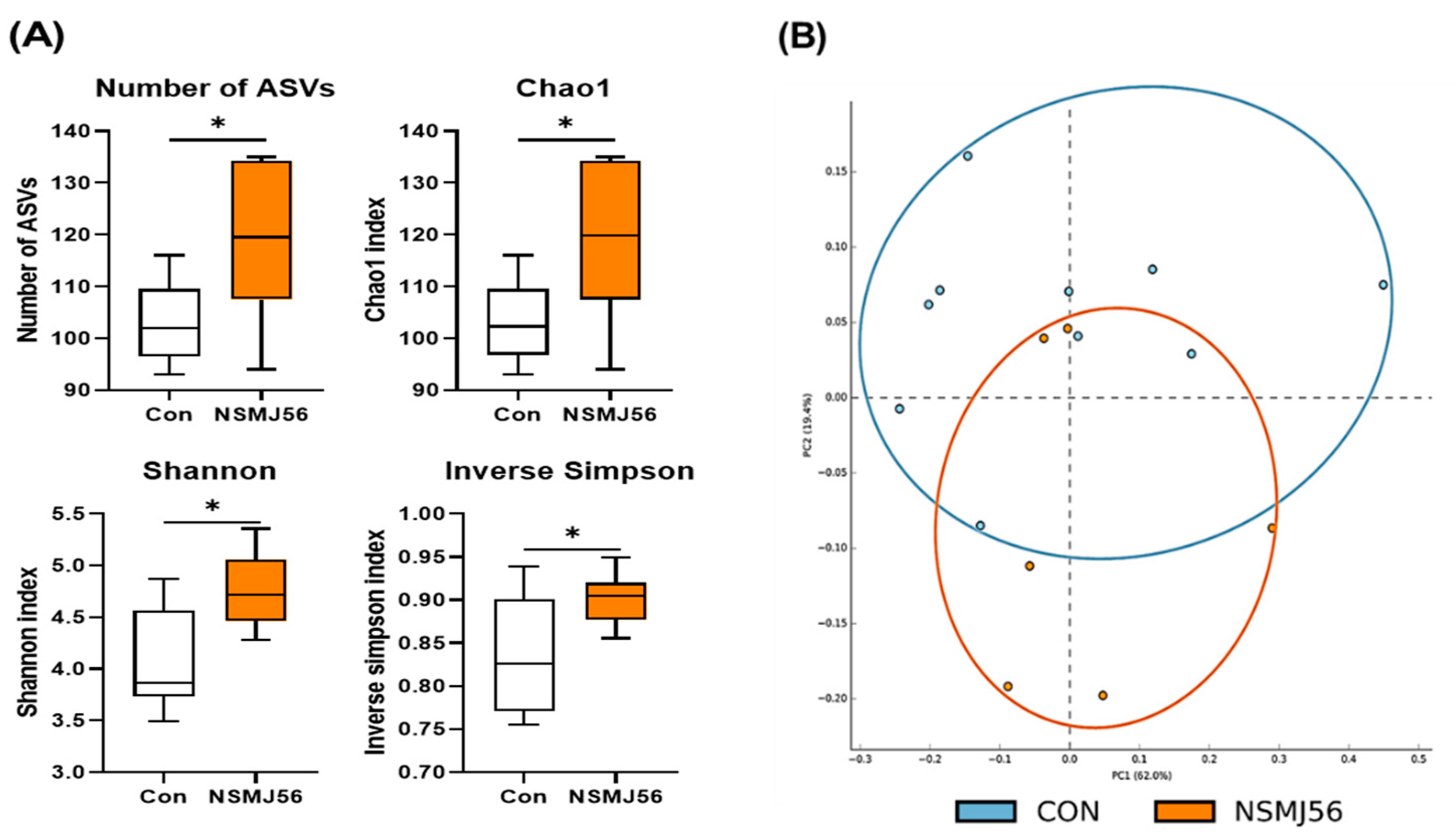
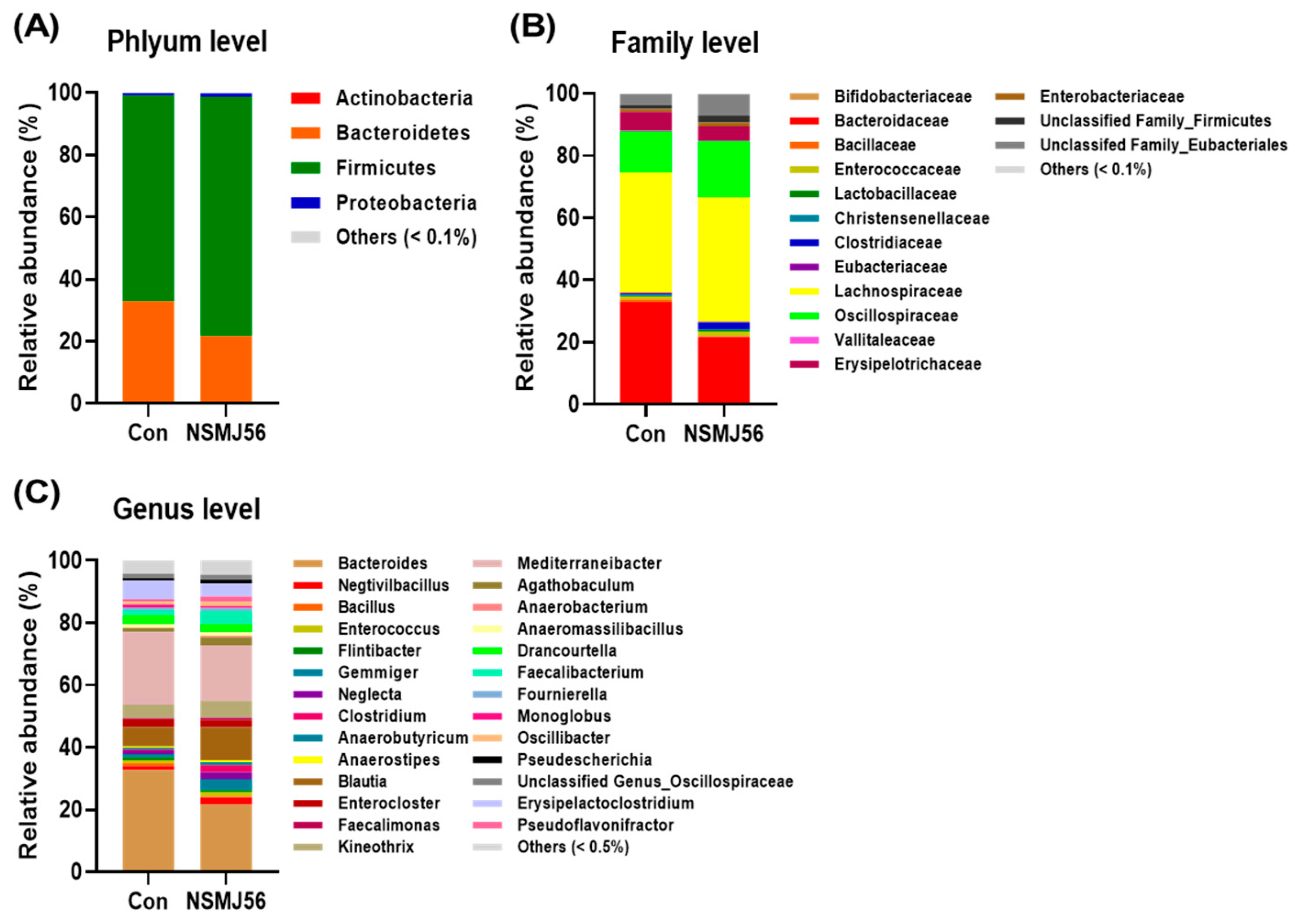
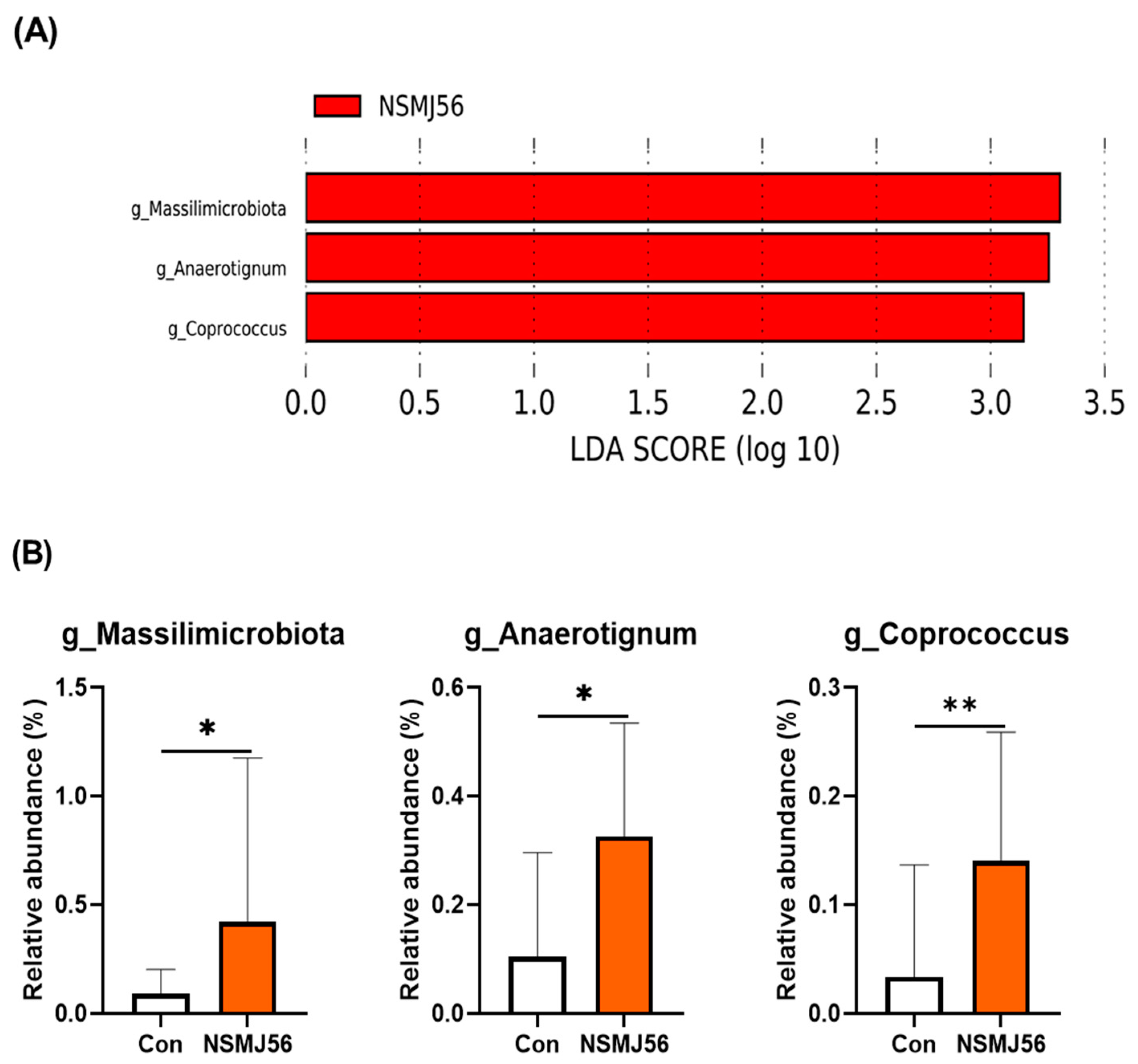
3.7. Effects of NSMJ56 Feeding on the Gut Microbiome of Early-Age Broiler Chickens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nawab, A.; Liu, W.; Li, G.; Ibtisham, F.; FOX, D.P.; Zhao, Y.; Wo, J.; Xiao, M.; Nawab, Y.; An, L. The potential role of probiotics (nutraceuticals) in gut health of domestic animals; an alternative to antibiotic growth promoters. J. Hell. Vet. Med. Soc. 2018, 69, 1169–1188. [Google Scholar] [CrossRef]
- Lan, Y.; Verstegen, M.W.A.; Tamminga, S.; Williams, B.A. The role of the commensal gut microbial community in broiler chickens. Worlds Poult. Sci. J. 2005, 61, 95–104. [Google Scholar] [CrossRef]
- Lee, K.; Lillehoj, H.S.; Siragusa, G.R. Direct-fed microbials and their impact on the intestinal microflora and immune system of chickens. J. Poult. Sci. 2010, 47, 106–114. [Google Scholar] [CrossRef]
- Rubio, L.A. Possibilities of early life programming in broiler chickens via intestinal microbiota modulation. Poult. Sci. 2019, 98, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Dai, R.; Yang, L.; He, C.; Xu, K.; Liu, S.; Zhao, W.; Xiao, L.; Luo, L.; Zhang, Y.; et al. Inheritance and establishment of gut microbiota in chickens. Front. Microbiol. 2017, 8, 1967. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Tahoun, A.; Rizk, A.M.; Suzuki, T.; Elmonir, W.; Nassef, E.; Shukry, M.; Germoush, M.O.; Farrag, F.; Bin-Jumah, M.; et al. Evaluation of Bifidobacteria and Lactobacillus probiotics as alternative therapy for Salmonella typhimurium infection in broiler chickens. Animals 2020, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, R.; Jia, H.; Zhu, Z.; Li, H.; Ma, Y. Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens. Open Life Sci. 2021, 16, 311–322. [Google Scholar] [CrossRef]
- Černý, J.; Stříž, I. Adaptive innate immunity or innate adaptive immunity? Clin. Sci. 2019, 133, 1549–1565. [Google Scholar] [CrossRef]
- Abaidullah, M.; Peng, S.; Kamran, M.; Song, X.; Yin, Z. Current findings on gut microbiota mediated immune modulation against viral diseases in chicken. Viruses 2019, 11, 681. [Google Scholar] [CrossRef]
- Erik, K.; Knudsen, B. Development of antibiotic resistance and options to replace antimicrobials in animal diets. Proc. Nutr. Soc. 2001, 60, 291–299. [Google Scholar] [CrossRef]
- Castanon, J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Vahjen, W.; Dadi, T.; Saliu, E.-M.; Boroojeni, F.G.; Zentek, J. Synergistic effects of probiotics and phytobiotics on the intestinal microbiota in young broiler chicken. Microorganisms 2019, 7, 684. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ma, C.; Sun, Z.; Wang, L.; Huang, S.; Su, X.; Xu, J.; Zhang, H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome 2017, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.P.; Wu, A.M.; Ding, X.M.; Lei, Y.; Bai, J.; Zhang, K.; Chio, J.S. Effects of probiotic-supplemented diets on growth performance and intestinal immune characteristics of broiler chickens. Poult. Sci. 2013, 92, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.; Hughes, R.J.; Hao Van, T.T.; Moore, R.J.; Stanley, D. At-hatch administration of probiotic to chickens can introduce beneficial changes in gut microbiota. PLoS ONE 2018, 13, e0194825. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Han, S.-S.; Kim, Z.-H.; Kim, M.H.; Kang, H.K.; Jin, H.M.; Lee, M.H. In-vitro characterization of growth inhibition against the gut pathogen of potentially probiotic lactic acid bacteria strains isolated from fermented products. Microorganisms 2021, 9, 2141. [Google Scholar] [CrossRef]
- Kim, B.G.; Lindemann, M.D. A spreadsheet method for experimental animal allotment. J. Anim. Sci. 2007, 85, 112. [Google Scholar]
- Aviagen, W. Ross 308: Broiler Nutrition Specifications. Available online: https://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerNutritionSpecifications2022-EN.pdf (accessed on 21 October 2022).
- Bucková, B.; Revajová, V. Immunophenotyping of intraepithelial (IEL) and lamina propria lymphocytes (LPL) in the chicken intestine by flow cytometry. Folia Vet. 2014, 58, 75–77. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J.; Kogut, M.H. Gut immunity: Its development and reasons and opportunities for modulation in monogastric production animals. Anim. Health Res. Rev. 2018, 19, 46–52. [Google Scholar] [CrossRef]
- Bar-Shira, E.; Friedman, A. Development and adaptations of innate immunity in the gastrointestinal tract of the newly hatched chick. Dev. Comp. Immunol. 2006, 30, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Van der Wielen, P.W.J.J.; Keuzenkamp, D.A.; Lipman, L.J.A.; Van Knapen, F.; Biesterveld, S. Spatial and temporal variation of the intestinal bacterial community in commercially raised broiler chickens during growth. Microb. Ecol. 2002, 44, 286–293. [Google Scholar] [CrossRef]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, M.S.; Mohanta, R.K.; Patra, A.K. Probiotics in livestock and poultry nutrition and health. In Advances in Probiotics for Sustainable Food and Medicine; Springer: Berlin/Heidelberg, Germany, 2021; pp. 149–179. [Google Scholar]
- Ognik, K.; Cholewińska, E.; Krauze, M.; Abramowicz, K.; Matusevicius, P. The effect of a probiotic preparation containing Enterococcus faecium DSM 7134 for chickens on growth performance, immune status, and the histology and microbiological profile of the jejunum. Anim. Prod. Sci. 2017, 59, 101–108. [Google Scholar] [CrossRef]
- Forte, C.; Manuali, E.; Abbate, Y.; Papa, P.; Vieceli, L.; Tentellini, M.; Trabalza-Marinucci, M.; Moscati, L. Dietary Lactobacillus acidophilus positively influences growth performance, gut morphology, and gut microbiology in rurally reared chickens. Poult. Sci. 2018, 97, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Brisbin, J.T.; Gong, J.; Sharif, S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim. Health Res. Rev. 2008, 9, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Shibata, E.; Nishimura, H.; Igarashi, Y.; Isobe, N.; Yoshimura, Y. Effects of probiotics on the localization of T cell subsets in the intestine of broiler chicks. J. Poult. Sci. 2013, 50, 275–281. [Google Scholar] [CrossRef]
- Asgari, F.; Madjd, Z.; Falak, R.; Bahar, M.A.; Nasrabadi, M.H.; Raiani, M.; Shekarabi, M. Probiotic feeding affects T cell populations in blood and lymphoid organs in chickens. Benef. Microbes 2016, 7, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Fong, F.L.Y.; Shah, N.P.; Kirjavainen, P.; El-Nezami, H. Mechanism of action of probiotic bacteria on intestinal and systemic immunities and antigen-presenting cells. Int. Rev. Immunol. 2016, 35, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Swaggerty, C.L. Effects of prebiotics and probiotics on the host immune response. In Direct-Fed Microbials and Prebiotics for Animals; Springer: Berlin/Heidelberg, Germany, 2012; pp. 61–72. [Google Scholar]
- Zaghari, M.; Sarani, P.; Hajati, H. Comparison of two probiotic preparations on growth performance, intestinal microbiota, nutrient digestibility and cytokine gene expression in broiler chickens. J. Appl. Anim. Res. 2020, 48, 166–175. [Google Scholar] [CrossRef]
- Kaiser, P.; Rothwell, L.; Goodchild, M.; Bumstead, N. The chicken proinflammatory cytokines interleukin-1β and interleukin-6: Differences in gene structure and genetic location compared with their mammalian orthologues. Anim. Genet. 2004, 35, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Dawei, Z.; Wenjun, Y.; Changhai, Q.; Jiang, Y. Probiotic Cocktails Alleviate Heat-induced Intestinal Barrier Dysfunction and Oxidative Stress in Broiler Chickens. Anim. Husb. Feed. Sci. 2019, 11, 107–114. [Google Scholar]
- Broom, L.J.; Kogut, M.H. The role of the gut microbiome in shaping the immune system of chickens. Vet. Immunol. Immunopathol. 2018, 204, 44–51. [Google Scholar] [CrossRef]
- Józefiak, D.; Rutkowski, A.; Martin, S. Carbohydrate fermentation in the avian ceca: A review. Anim. Feed Sci. Technol. 2004, 113, 1–15. [Google Scholar] [CrossRef]
- Gong, J.; Si, W.; Forster, R.J.; Huang, R.; Yu, H.; Yin, Y.; Yang, C.; Han, Y. 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: From crops to ceca. FEMS Microbiol. Ecol. 2007, 59, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Danzeisen, J.L.; Kim, H.B.; Isaacson, R.E.; Tu, Z.J.; Johnson, T.J. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS ONE 2011, 6, e27949. [Google Scholar] [CrossRef]
- Yin, Y.; Lei, F.; Zhu, L.; Li, S.; Wu, Z.; Zhang, R.; Gao, G.F.; Zhu, B.; Wang, X. Exposure of different bacterial inocula to newborn chicken affects gut microbiota development and ileum gene expression. ISME J. 2010, 4, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Barko, P.C.; McMichael, M.A.; Swanson, K.S.; Williams, D.A. The gastrointestinal microbiome: A review. J. Vet. Intern. Med. 2018, 32, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Ocejo, M.; Oporto, B.; Hurtado, A. 16S rRNA amplicon sequencing characterization of caecal microbiome composition of broilers and free-range slow-growing chickens throughout their productive lifespan. Sci. Rep. 2019, 9, 2506. [Google Scholar] [CrossRef]
- Torok, V.A.; Ophel-Keller, K.; Loo, M.; Hughes, R.J. Application of methods for identifying broiler chicken gut bacterial species linked with increased energy metabolism. Appl. Environ. Microbiol. 2008, 74, 783–791. [Google Scholar] [CrossRef]
- Zenner, C.; Hitch, T.C.A.; Riedel, T.; Wortmann, E.; Tiede, S.; Buhl, E.M.; Abt, B.; Neuhaus, K.; Velge, P.; Overmann, J.; et al. Early-life immune system maturation in chickens using a synthetic community of cultured gut bacteria. mSystems 2021, 6, e01300-20. [Google Scholar] [CrossRef]
- Liu, L.; Lin, L.; Zheng, L.; Tang, H.; Fan, X.; Xue, N.; Li, M.; Liu, M.; Li, X. Cecal microbiome profile altered by Salmonella enterica, serovar Enteritidis inoculation in chicken. Gut Pathog. 2018, 10, 34. [Google Scholar] [CrossRef]
- Ueki, A.; Goto, K.; Ohtaki, Y.; Kaku, N.; Ueki, K. Description of Anaerotignum aminivorans gen. nov., sp. nov., a strictly anaerobic, amino-acid-decomposing bacterium isolated from a methanogenic reactor, and reclassification of Clostridium propionicum, Clostridium neopropionicum and Clostridium lactatifermentans as species of the genus Anaerotignum. Int. J. Syst. Evol. Microbiol. 2017, 67, 4146–4153. [Google Scholar]
| Items | Experimental Diets (1) | |
|---|---|---|
| Con | NSMJ56 | |
| Ingredient composition, g/kg | ||
| Corn | 535.44 | 535.44 |
| Soybean meal | 388.00 | 388.00 |
| Cornstarch | 5.00 | 4.00 |
| L. paracasei (NSMJ56) | - | 1.00 |
| Soybean oil | 20.00 | 20.00 |
| L-Arg | 0.93 | 0.93 |
| L-His | 0.29 | 0.29 |
| L-Ile | 1.00 | 1.00 |
| L-Lys-HCL | 3.49 | 3.49 |
| L-Met | 2.35 | 2.35 |
| L-Cys | 1.43 | 1.43 |
| L-Thr | 1.43 | 1.43 |
| L-Val | 2.01 | 2.01 |
| Limestone | 10.58 | 10.58 |
| Dicalcium phosphate | 19.05 | 19.05 |
| Salt | 4.00 | 4.00 |
| Vitamin premix (2) | 2.00 | 2.00 |
| Mineral premix (3) | 2.00 | 2.00 |
| Choline chloride | 1.00 | 1.00 |
| Calculated chemical compositions | ||
| Nitrogen-corrected metabolizable energy, kcal/kg | 2976 | 2972 |
| Crude protein, % | 23.00 | 23.00 |
| Calcium, % | 0.96 | 0.96 |
| Non-phytate phosphorus, % | 0.48 | 0.48 |
| Calculated amino acids compositions, g/kg | ||
| SID Arg | 13.12 | 13.12 |
| SID His | 4.74 | 4.74 |
| SID Ile | 8.71 | 8.71 |
| SID Leu | 15.25 | 15.25 |
| SID Lys | 12.80 | 12.80 |
| SID Met | 4.75 | 4.75 |
| SID Cys | 3.96 | 3.96 |
| SID Phe | 9.17 | 9.17 |
| SID Thr | 8.13 | 8.13 |
| SID Trp | 2.28 | 2.28 |
| SID Val | 10.12 | 10.12 |
| Gene | Forward | Reverse | Product Size (bp) (1) |
|---|---|---|---|
| IL1b | GCTCTACATGTCGTGTGTGATGAG | TGTCGATGTCCCGCATGA | 80 |
| IL6 | CTCCTCGCCAATCTGAAGTC | GGATTGTGCCCGAACTAAAA | 164 |
| IL10 | CATGCTGCTGGGCCTGAA | CGTCTCCTTGATCTGCTTGATG | 94 |
| Gpx2 | ACGGCACCAACGAGGAGAT | TTCAGGTAGGCGAAGACGG | 133 |
| SOD1 | AGGGGGTCATCCACTTCC | CCCATTTGTGTTGTCTCCAA | 122 |
| CAT | TTACGGAGGTAGAACAGATGG | TGTCAGGATACGCAAAGAGA | 105 |
| ZO-1 | GCCAACTGATGCTGAACCAA | GGGAGAGACAGGACAGGACT | 141 |
| OCLN | GATGGACAGCATCAACGACC | CTTGCTTTGGTAGTCTGGGC | 142 |
| GAPDH | GTCCTCTCTGGCAAAGTCCAAG | TCACAAGTTTCCCGTTCTCAGC | 139 |
| Item | Experimental Diets (2) | ||
|---|---|---|---|
| Con | NSMJ56 | p-Value (3) | |
| Body weight gain, g/bird | 156.20 ± 8.56 | 152.50 ± 16.02 | 0.6324 |
| Feed intake, g/bird | 175.00 ± 9.69 | 169.00 ± 11.32 | 0.3466 |
| Gain to feed ratio, g/g | 0.89 ± 0.03 | 0.90 ± 0.06 | 0.7519 |
| Item | Experimental Diets (2) | ||
|---|---|---|---|
| Con | NSMJ56 | p-Value (3) | |
| Villus height, µm | 469.56 ± 33.19 | 459.86 ± 96.84 | 0.1797 |
| Crypt depth, µm | 107.13 ± 22.66 | 96.29 ± 7.44 | 0.2915 |
| Villus height:crypt depth | 4.53 ± 0.89 | 4.74 ± 0.61 | 0.6442 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, S.S.; Yoon, J.H.; Jung, J.Y.; Joo, S.Y.; An, S.H.; Ban, B.C.; Kong, C.; Kim, M. The Modulatory Effects of Lacticaseibacillus paracasei Strain NSMJ56 on Gut Immunity and Microbiome in Early-Age Broiler Chickens. Animals 2022, 12, 3413. https://doi.org/10.3390/ani12233413
Joo SS, Yoon JH, Jung JY, Joo SY, An SH, Ban BC, Kong C, Kim M. The Modulatory Effects of Lacticaseibacillus paracasei Strain NSMJ56 on Gut Immunity and Microbiome in Early-Age Broiler Chickens. Animals. 2022; 12(23):3413. https://doi.org/10.3390/ani12233413
Chicago/Turabian StyleJoo, Sang Seok, June Hyeok Yoon, Ji Young Jung, Sung Yong Joo, Su Hyun An, Byeong Cheol Ban, Changsu Kong, and Myunghoo Kim. 2022. "The Modulatory Effects of Lacticaseibacillus paracasei Strain NSMJ56 on Gut Immunity and Microbiome in Early-Age Broiler Chickens" Animals 12, no. 23: 3413. https://doi.org/10.3390/ani12233413
APA StyleJoo, S. S., Yoon, J. H., Jung, J. Y., Joo, S. Y., An, S. H., Ban, B. C., Kong, C., & Kim, M. (2022). The Modulatory Effects of Lacticaseibacillus paracasei Strain NSMJ56 on Gut Immunity and Microbiome in Early-Age Broiler Chickens. Animals, 12(23), 3413. https://doi.org/10.3390/ani12233413





