1. Introduction
Aquaculture production has expanded quickly throughout the past decade. Nile tilapia is the most important species of freshwater fish in tropical and subtropical regions [
1]. It is an omnivorous fish that can tolerate various environmental conditions. It is an important economic species, because it accepts both natural and synthetic feeds [
2]. Aquatic culture is growing worldwide, raising the need for feed components to encourage production [
3]. Half of the aquaculture production costs have been reported to be feed costs [
4]. Since protein is the most expensive feed component [
5], the nutritional value of fish feed depends mainly on the protein quality of the ingredients used in feed formulation [
6]. The market price of fish meal (FM) has risen recently due to the increased need for FM in aquaculture feed manufacturing and the depletion of marine fishery resources [
7]. Therefore, assessing low-FM feed preparations for the target cultured species is essential.
The use of dietary plant protein supplements is usually restricted because of essential amino acid deficiency and the presence of anti-nutrient factors [
8]. Animal protein components are characterized by a high content of digestible protein with a good amino acid profile, digestible dry matter, and digestible energy [
9]. Earthly animal by-product meals such as blood meals and blood products are considered the safest and essentially most unexploited available source of animal protein in the aquatic feed industry.
Spray-dried blood and spray-dried plasma proteins are feed ingredients of high value for farm animals [
10]. Bovine hemoglobin powder (BH) is produced by splitting hemoglobin from hygienically gathered and isolated eligible healthy bovine blood. Spray drying (low-temperature processing method) resulted in slight biological degradation of amino acids in the high protein-containing BH (92% of dry matter) [
11] and high lysine and leucine contents. However, it still shows a low isoleucine content [
12]. Dried bovine hemoglobin powder improved fish growth, health, antioxidant capacity, blood hematology, and increased resistance to
Aeromonas veronii [
13]. Due to the aforementioned characteristics, SDBH could be a potential protein ingredient in fish diets. Therefore, this study evaluated the SDBH as a possible protein ingredient in Nile tilapia diets and assessed the effects of this inclusion on the growth, growth-related genes, digestive enzyme activity, blood biochemical parameters, intestinal histomorphology, immune status, and immune-related genes in Nile tilapia,
O. niloticus. 4. Discussion
The present study assessed the possible use of spray-dried bovine hemoglobin as a novel protein source in Nile tilapia diets. The study evaluated the effects of SDBH inclusion on growth, growth-related genes, digestive enzyme activity, blood biochemical parameters, intestinal histomorphology, immune status, and immune-related genes in Nile tilapia,
O. niloticus. The results of the present study reported increased FBW, TWG, SGR, and PER by increasing the level of SDBH inclusion. Dietary SDBH7.5 and SDBH10 decreased daily feed intake relative to body weight and FCR. The SDBH10 group showed the highest performance compared to other groups. Yao et al. [
26] showed that the optimum dietary inclusion with hemoglobin powder protein was 17.3% in hybrid grouper (
Epinephelus fuscoguttatus × Epinephelus lanceolatus) based on the maximum response weight gain. They also reported that the highest inclusion levels (43.38 and 52.05%) showed a reduction in the growth performance, feed intake, feed utilization, and feeding-related gene expression of the fish due to insufficient essential amino acids (threonine and arginine) and poor palatability and gut growth. Ding et al. [
27] showed a reduced growth rate of largemouth bass (
Micropterus salmoides) with dietary inclusion of chicken hemoglobin powder up to 7.66%, which may be due to the effect of chicken hemoglobin powder inclusion on the feed intake and the protein and amino acids’ digestibility. Chookird et al. [
28] reported decreased shrimp growth with increasing the level of hemoglobin powder dietary inclusion (4.26–34.17%). De Araújo et al. [
29] demonstrated the effects of dietary spray-dried plasma (0, 16.6, 33.2, 49.7, and 66.3 g kg−1) on the growth and intestinal health of Nile tilapia. They reported improved fish growth, hematological profile, and intestinal health, and the optimal level of inclusion was 51.83 g kg
−1. Gisbert et al. [
10] indicated that dietary spray-dried plasma protein from porcine (3 and 6%) increased the growth of gilthead sea bream (
Sparus aurata) fingerlings, where the body weight was increased by 10.5% in fish fed 3% spray-dried plasma protein compared to the control group. Twahirwa et al. [
30] reported decreased intestinal trypsin activity and lower amylase in black carp,
Mylopharyngodon piceus, with increasing dietary blood meal levels (40 and 100%).
The somatic growth, feed consumption, and energy-storing in vertebrates are highly maintained evolutionary modifications controlled by peripheral signals, such as
IGF-1, leptin, and several hypothalamic neuropeptides, which regulate the appetite [
31,
32]. The growth hormone (GH) and IGF-1 axis regulate the most significant peripheral endocrine factors related to growth and nutritional status in fish [
33,
34]. Pituitary GH promotes the synthesis and release of IGF-1 from the liver, which affects skeletal muscle and the gonads to motivate somatic growth [
35,
36]. Hence, the GH–IGF-1 system is crucial for growth regulation, and thus IGF-1 concentration increases or decreases in anabolic or catabolic conditions, respectively [
34,
37].
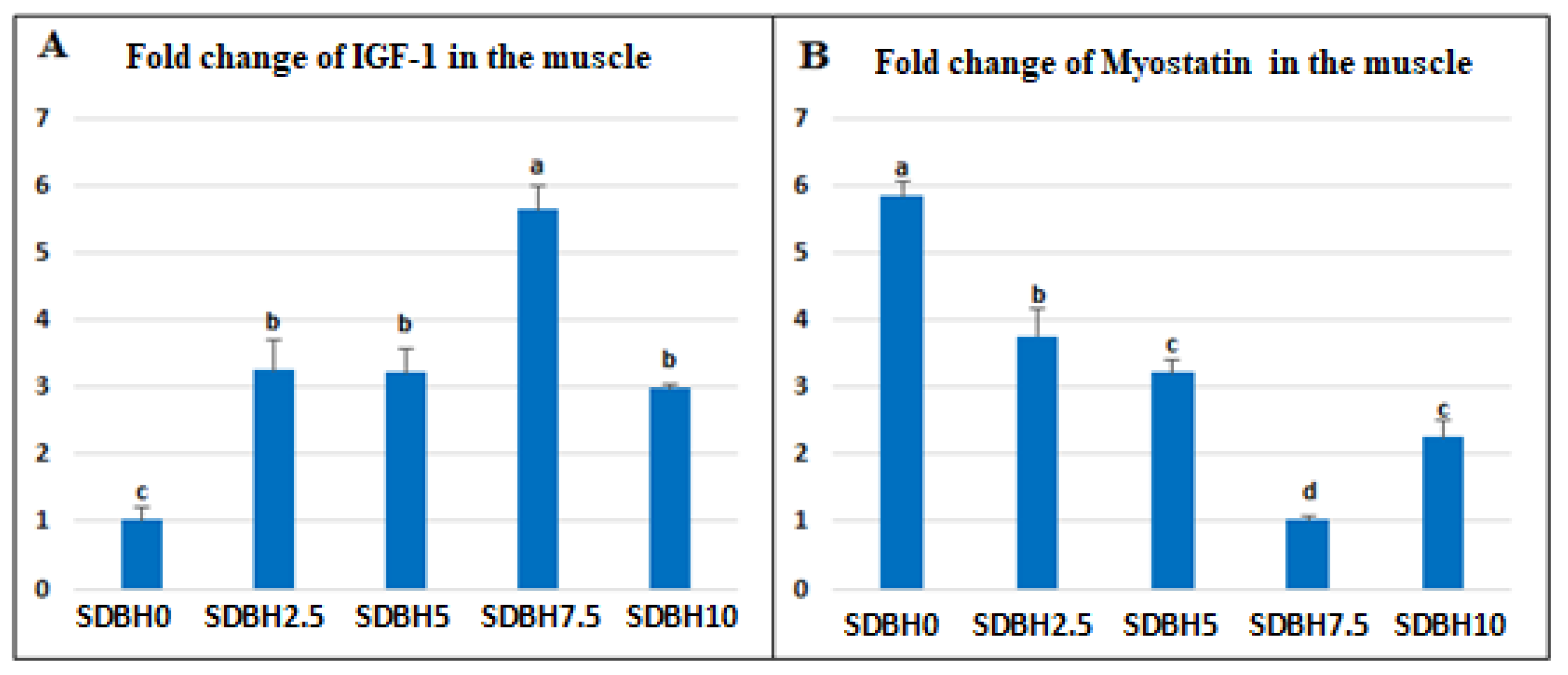
Myostatin (MSTN), or growth and differentiation factor 8 (GDF-8), a member of the Transforming Growth Factor-β (TGF-β) superfamily, was first detected in mice by McPherron et al. [
38] and controlled the growth of skeletal muscle in various mammalian species adversely. MSTN plays an inhibitory role in developing and managing skeletal muscle size. Consequently, mutations that deactivate or decrease the protein activity markedly increase muscle size [
38,
39]. Myostatin inhibits myoblast differentiation and proliferation [
40,
41], which was achieved by downregulation of the myogenic gene expression [
40,
42]. The MSTN gene has been identified in various vertebrate species, including numerous fish species [
43,
44,
45].
Consequently, the increased fish growth in SDBH-included groups in the current study may be attributed to the increased growth hormone level and decreased leptin hormone level that was noted by increasing the SDBH inclusion level, in addition to increasing the digestive enzymes’ activity (amylase and protease), which results in increased nutrient digestibility. Leptin is produced in the white adipose tissue and regulates energy homeostasis, spending, feed intake, reproduction, and obesity [
46,
47]. In fish, leptin is produced and distributed in the liver, with the highest leptin expression noted by [
48,
49,
50,
51]. It has been shown that leptin secretion in fish may be altered due to diet composition [
52]. In the study of Liang et al. [
53], grass carp (
Ctenopharyngodon idellus) fed a plant-protein-based diet showed reduced leptin levels compared to a fishmeal-based diet. Moreover, leptin synthesis is regulated by pituitary growth hormone; increasing the level of GH leads to reduced leptin secretion in fish [
54]. In the current study, although leptin secretion decreased with increasing the level of SDBH inclusion, the daily feed intake relative to the BW was decreased in the SDBH7.5 and SDBH10 groups. We can explain this by the effect of dietary SDBH in improving the feed conversion rate, nutrient utilization, and uptake rather than increasing the feed intake. This has the advantage of fish eating less feed and converting it to more weight gain.
The increased growth in SDBH-included groups also may be due to the up-regulation of
IGF-1 and down-regulation of
myostatin. The results showed that the expression of IGF-1 was upregulated in the SDBH-included groups in a linear trend up to the inclusion level of 7.5%, then down-regulated but still higher than the non-included group. The IGF-1 pathway can regulate skeletal muscle protein synthesis via rapamycin (TOR) activation and inhibit skeletal muscle protein breakdown through the phosphorylation of Forkhead box O (FoxO) transcription factors [
55,
56]. So the highest inclusion levels (10%) did not stimulate more expression of IGF-1 in the muscle. This influence is correlated with the level of protein absorption in the intestine that AA and peptide transporters regulate. The current study found that the expression of these transporters was increased up to the inclusion level of 7.5% and then reduced at the highest level (10%). So the level of 10% SDBH in the diets might result in extra protein in the intestine during digestion without absorption. This indicated that IGF-1 expression might be modified by muscle AA [
57].
The serum glucose level was decreased in the SDBH7.5 and SDBH10 groups. The hypoglycemic effect of SDBH is due to the reduced daily feed intake relative to BW in these groups and the up-regulation of mRNA expression of
insulin growth factor-1 (
IGF-1); its hypoglycemic effect is reported in fish [
58].
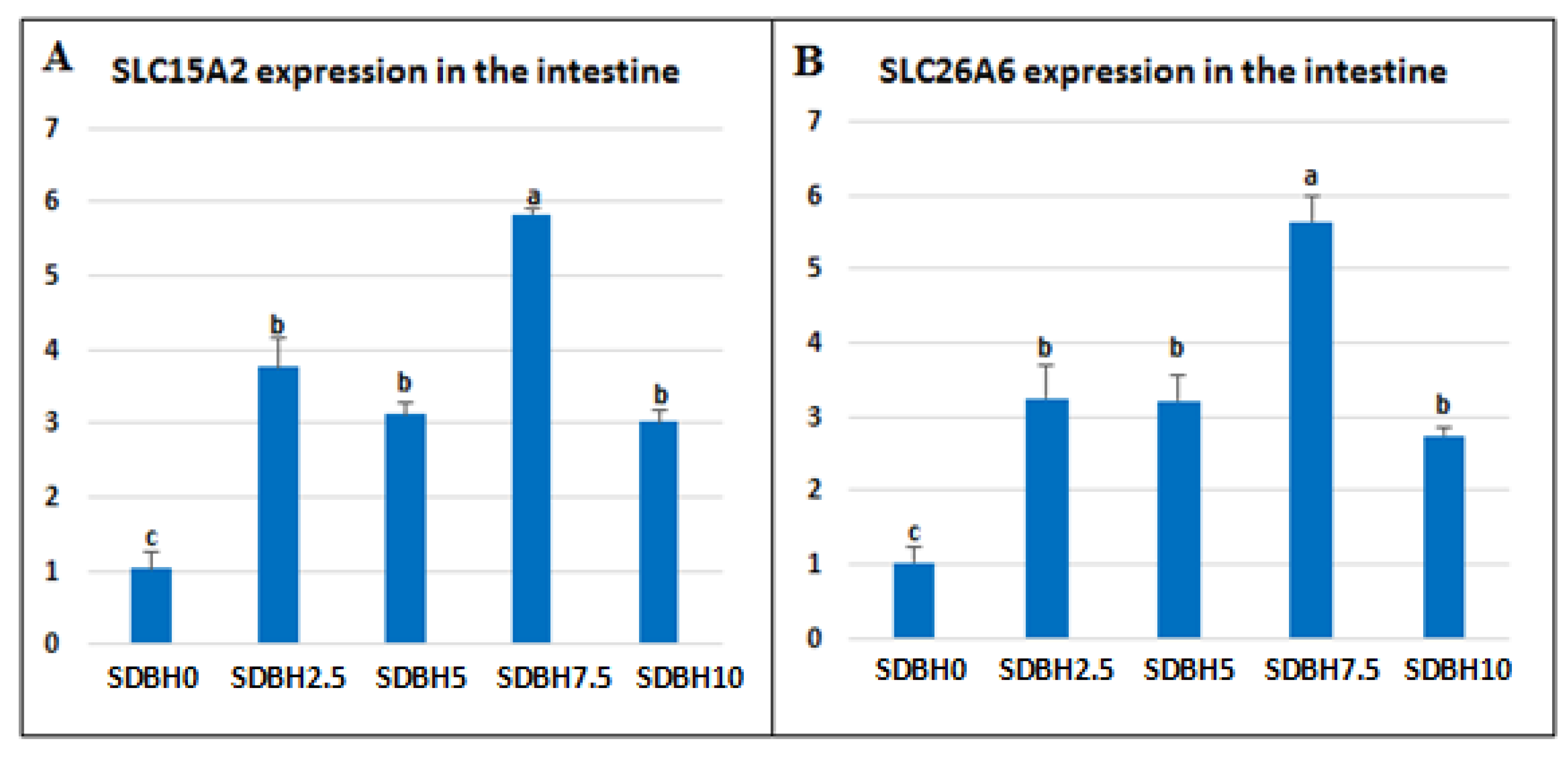
During the digestive process, dietary proteins are broken down to free amino acids and di- or tripeptides in the intestinal lumen; then, they are absorbed through AA and peptide transporters, such as peptide transporter 1 (PepT1, SLC15A1), proton-coupled oligopeptide transporter (SLC15A2, peptide transporter 2, PepT2), and SLC26A6 (PAT-1, putative anion transporter-1) [
59,
60]. Peptide transporter 1 (PepT1) could transport all potential 400 dipeptides and 8000 tripeptides [
61,
62]. The entire intestine may be implicated in peptide absorption because PepT1 has been well-expressed in the fish intestine from proximal to distal parts [
63,
64,
65]. The present results showed up-regulation in the intestinal mRNA expression of SLC15A2 and SLC26A6 in SDBH-included groups. The highest expression was reported in the SDBH7.5, then began to downregulate in the highest inclusion level (10%) but was still higher than the non-included group. This means that the highest protein absorption was in the inclusion levels of 2.5–7.5% SDBH, then decreased at the highest level (10%). Inconsistent with our results, the mRNA expression of PepT1 in the proximal and distal intestine was down-regulated in high levels of fish protein hydrolysate in turbot and yellow catfish (
Pelteobagrus fulvidraco) [
66,
67,
68]. Because PepT1 can be controlled by its transmissible substrates [
60], the present results indicated that the highest level of SDBH in the diets (10%) might result in an extra of di- or tripeptides in the intestine during digestion.
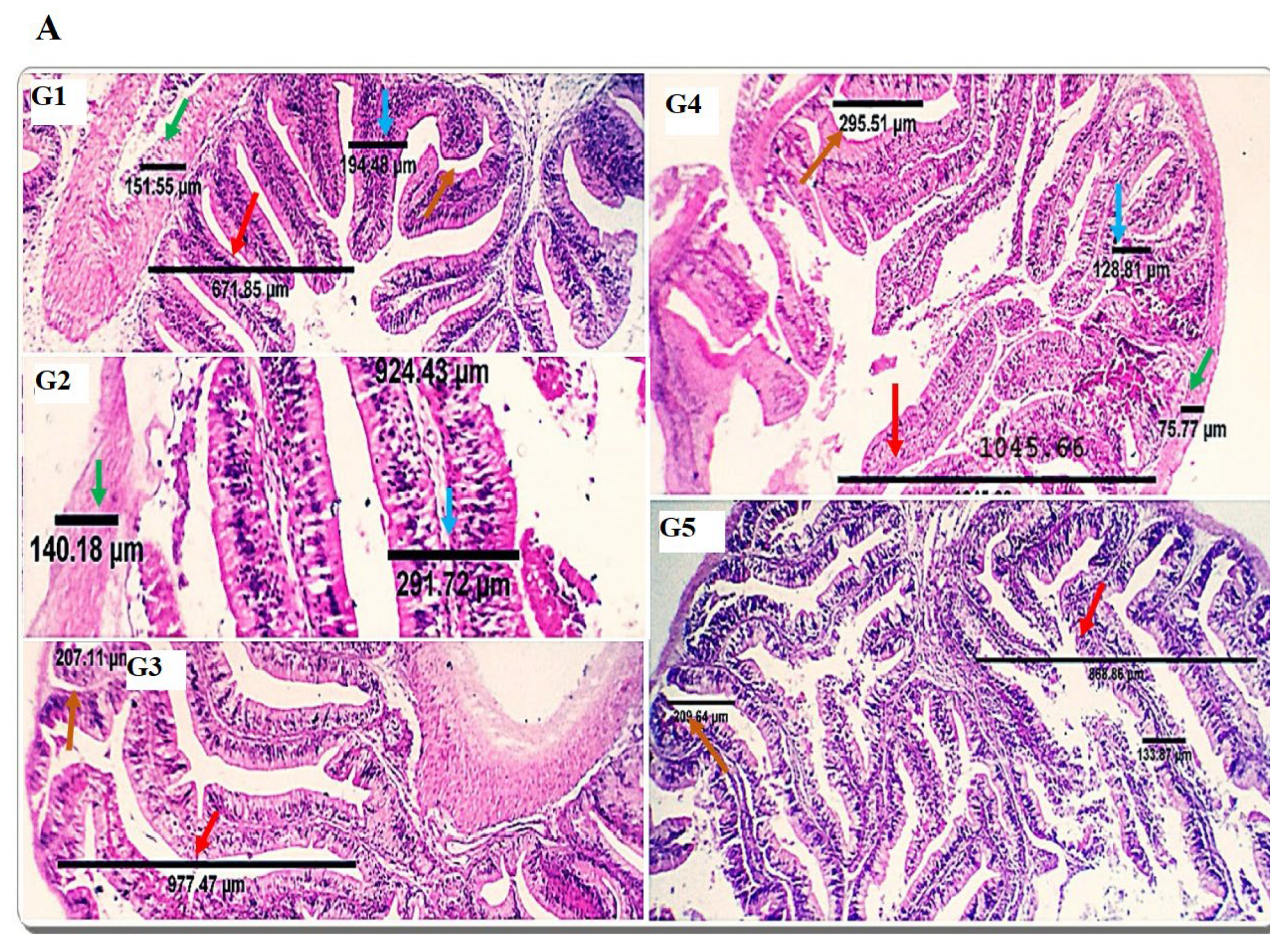
The gut is the primary feed digestion and nutrient uptake site, and optimal nutrient utilization relies on its functional efficacy [
69]. Because dietary protein affects the structure of intestinal microvilli [
70], research studies have investigated the effects of different dietary protein sources on the histomorphology of the gut. In the current study, dietary inclusion with SDBH showed a significant increase in the intestinal VH, VW, VH: CD, and MCT, with the highest values in the SDBH7.5 group and then decreased in the SDBH10 group. These results confirm the results of the intestinal mRNA expression of SLC15A2 and SLC26A6. This implies that the gut of Nile tilapia may badly affect the uptake and absorption of free AA and peptides from the high level of SDBH through the regulation of AA and peptide transporters. It has been reported that high inclusion levels of hemoglobin powder protein (43.38 and 52.05%) in hybrid grouper decreased the enterocyte and microvillus height of the foregut and midgut and may damage gut health [
26]. Amer et al. [
71] reported improved gut development by dietary inclusion with whey protein concentrate of up to 5% in Nile tilapia diets. Gisbert et al. [
10] reported no significant effect of spray-dried plasma protein (SDPP) on the intestinal villi width and length, with an increased number of goblet cells in the gilthead sea bream fed SDPP by a level of 6%, followed by 3%.
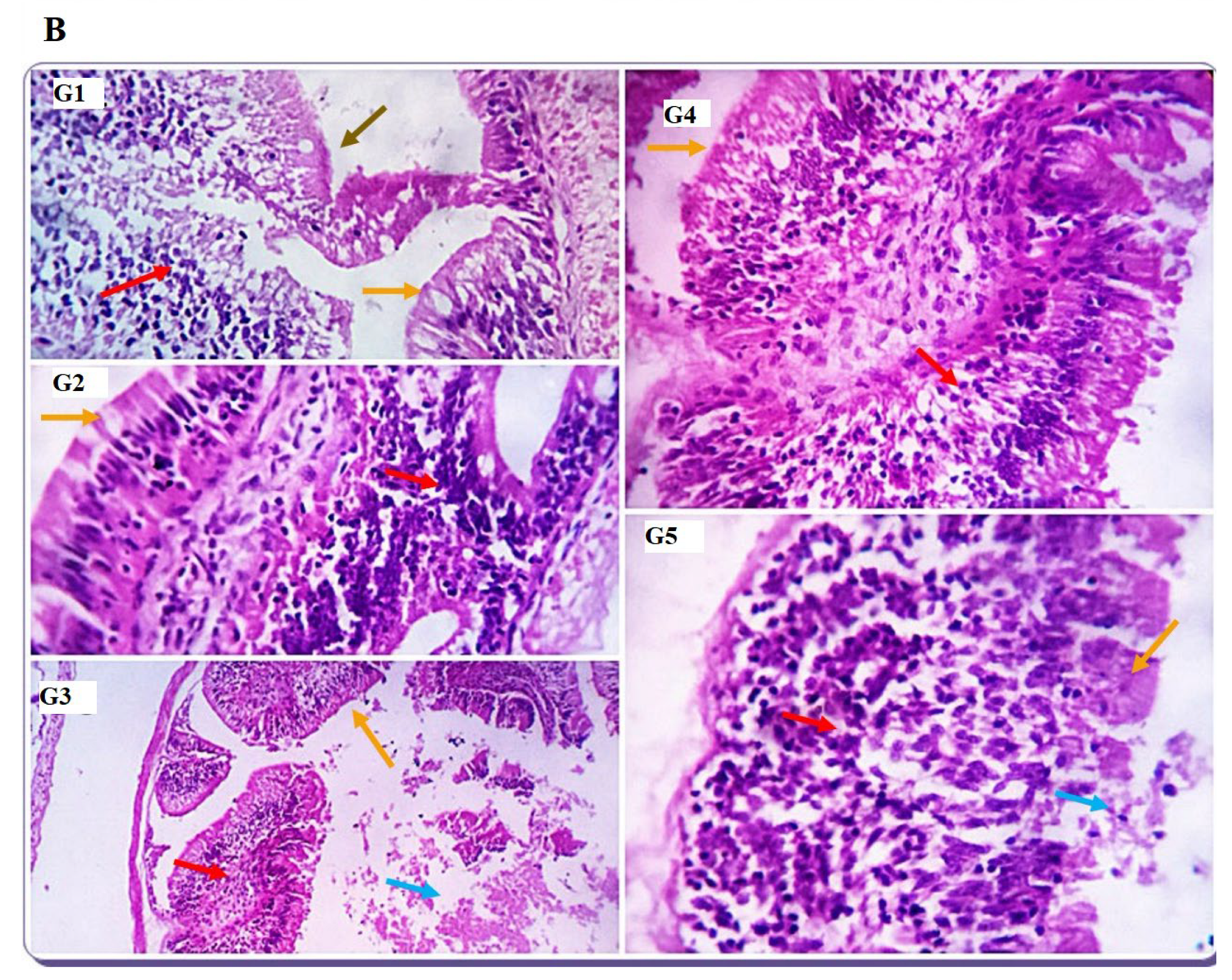
The first line of defense against infection is innate immunity, considered primitive and, thus, the general form of host defense [
72]. Interleukin (IL-10) is an immune-regulating cytokine that has many functions. It was primarily recognized as a cytokine synthesis inhibitory factor [
73].
IL-10 is considered an anti-inflammatory cytokine and shows a vital function in controlling inflammation [
74]. Transforming growth factor-beta (
TGF-β) is a pleiotropic cytokine released during an immune response with significant immunomodulatory functions in the regulation, differentiation, proliferation, migration, survival, activation, and deactivation of macrophages and other immune cells [
75]. TGF-β is mainly produced by macrophages, platelets, and fibroblasts [
76].
Toll-like receptors (
TLRs) are a significant component of innate immunity. TLR2 is concerned with recognizing specific microbial structures such as zymosan, lipoteichoic acid (LTA), and peptidoglycan (PGN). Its binding leads to primary myeloid differentiation response gene 88 (MyD88), a dependent signaling pathway to induction of numerous cytokines [
77]. The current study showed increased lysozyme activity, NO, and C3 serum levels by SDBH inclusion, with the highest values recorded in the SDBH5 group. The phagocytic % and the phagocytic index were increased by increasing the SDBH inclusion level. TGF-β, TLR2, and IL-10 were up-regulated in all SDBH-included groups compared to the SDBH0 group, with the highest expression observed in the SDBH5 group. These results demonstrated the immunomodulating effect of SDBH in Nile tilapia. The improved immune responses may be attributed to the high immunoglobulin content in SDBH [
78]. Gisbert et al. [
10] indicated higher nonspecific serum immune responses in gilthead sea bream fingerlings fed spray-dried plasma protein than the control group. Twahirwa et al. [
30] detected reduced lysozyme activity in black carp (
Mylopharyngodon piceus) fed bloodmeal-included diets (20–100%) compared with the control group, while the complement C3 and C4 was not different from the control group.
Concerning the economic efficiency of the diets, the feed costs and feed cost/kg gain were decreased in the highest SDBH inclusion levels (SDBH7.5 and SDBH10 diets), which was a consequence of the improved growth performance and the lower price of SDBH in comparison with fishmeal.