Progress of Studies on Plant-Derived Polysaccharides Affecting Intestinal Barrier Function in Poultry
Abstract
Simple Summary
Abstract
1. Introduction
2. Source and Structure of Plant-Derived Polysaccharides
3. Effects of Plant-Derived Polysaccharides on Intestinal Barrier Function in Poultry
3.1. Plant-Derived Polysaccharides Improve Intestinal Microbial Barrier in Poultry
3.2. Plant-Derived Polysaccharides Improve Intestinal Chemical Barrier in Poultry
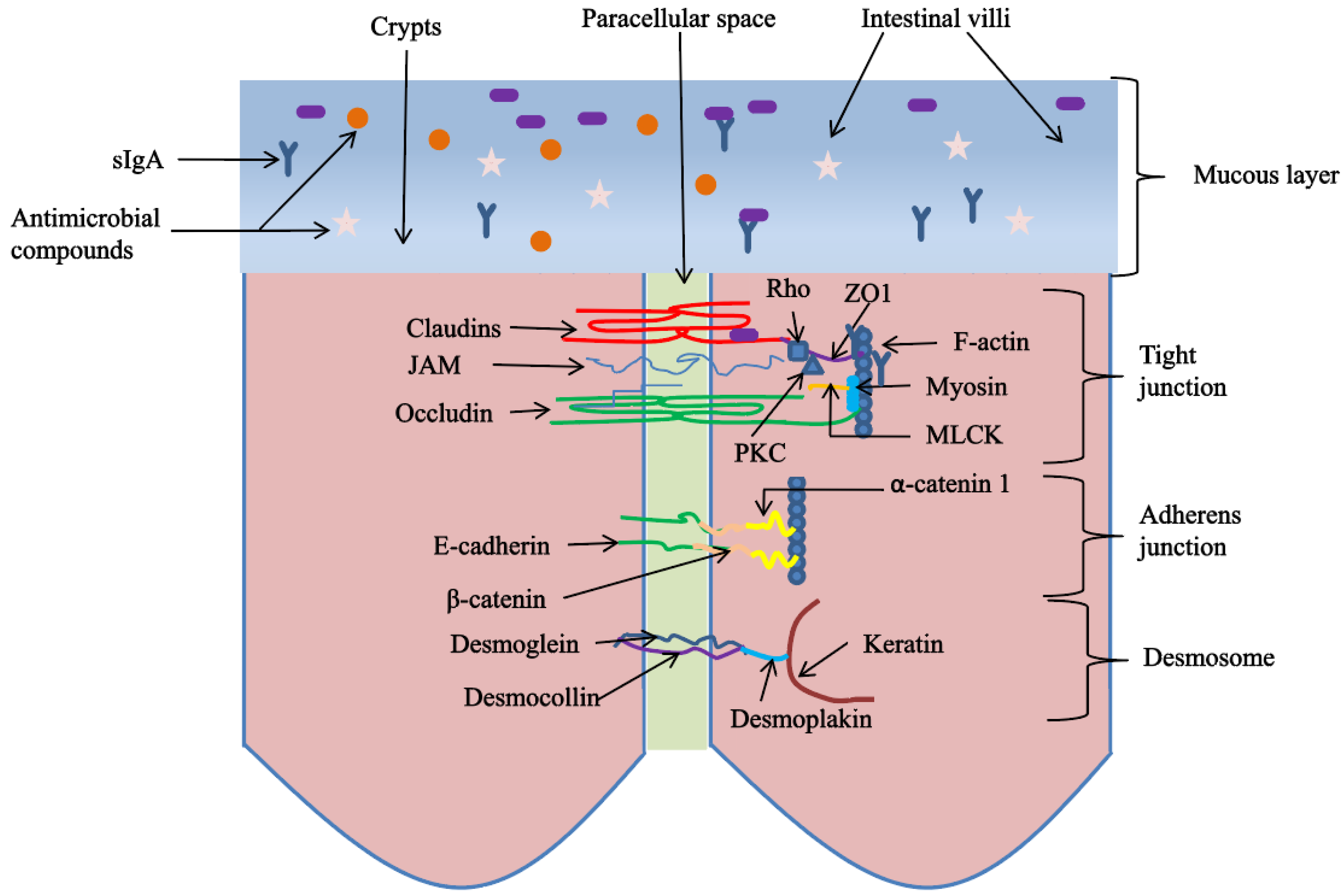
3.3. Plant-Derived Polysaccharides Improve Intestinal Physical Barrier in Poultry
3.4. Plant-Derived Polysaccharides Improve the Intestinal Immune Barrier in Poultry
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, D.; Yang, N.; Liu, Z.; Li, T.; Wang, H.; Ge, M.; Zhang, R. Effects of Astragalus polysaccharide on intestinal inflammatory damage in goslings infected with gosling plague. Br. Poult. Sci. 2021, 62, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.M. Recent trends in chemical modification and antioxidant activities of plants-based polysaccharides: A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100045. [Google Scholar] [CrossRef]
- Long, L.N.; Zhang, H.H.; Wang, F.; Yin, Y.X.; Yang, L.Y.; Chen, J.S. Research Note: Effects of polysaccharide-enriched Acanthopanax senticosus extract on growth performance, immune function, antioxidation, and ileal microbial populations in broiler chickens. Poult. Sci. 2021, 100, 101028. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhou, X.; Huang, G. Preparation, structure, and properties of tea polysaccharide. Chem. Biol. Drug Des. 2022, 99, 75–82. [Google Scholar] [CrossRef]
- Xing, Y.Y.; Zheng, Y.K.; Yang, S.; Zhang, L.H.; Guo, S.W.; Shi, L.L.; Xu, Y.Q.; Jin, X.; Yan, S.M.; Shi, B.L. Artemisia ordosica polysaccharide alleviated Lipopolysaccharide-induced oxidative stress of broilers via Nrf2/Keap1 and TLR4/NF-κB pathway. Ecotox. Environ. Saf. 2021, 223, 112566. [Google Scholar] [CrossRef]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar] [CrossRef]
- Wu, Y.; Li, N.; Zhang, T.; Che, Y.; Duan, K.; Wang, Y.; Zhou, H.; Wan, X.; Lei, H.; Nguyen, A.D.; et al. Glycyrrhiza polysaccharides can improve and prolong the response of chickens to the Newcastle disease vaccine. Poult. Sci. 2022, 101, 101549. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Bedford, M.R.; Wu, S.B.; Morgan, N.K. Dietary soluble Non-Starch polysaccharide level influences performance, nutrient utilisation and disappearance of Non-Starch polysaccharides in broiler chickens. Animals 2022, 12, 547. [Google Scholar] [CrossRef]
- Ao, X.; Kim, I.H. Effects of Achyranthes bidentata polysaccharides on performance, immunity, antioxidant capacity, and meat quality in Pekin ducks. Poult. Sci. 2020, 99, 4884–4891. [Google Scholar] [CrossRef]
- Ming, K.; He, M.; Su, L.; Du, H.; Wang, D.; Wu, Y.; Liu, J. The inhibitory effect of phosphorylated Codonopsis pilosula polysaccharide on autophagosomes formation contributes to the inhibition of duck hepatitis A virus replication. Poult. Sci. 2020, 99, 2146–2156. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Zhang, C.; Ni, J.; Luo, Q.; Teng, L.; Liao, S.; Yang, Y.; Chen, H.; Chen, Y. Characterizations of glucose-rich polysaccharides from Amomum longiligulare T.L. Wu fruits and their effects on immunogenicities of infectious bursal disease virus VP2 protein. Int. J. Biol. Macromol. 2021, 183, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, K.; Akhtar, M.; Awais, M.M.; Anwar, M.I. Evaluation of immunotherapeutic effects of Aloe vera polysaccharides against coccidiosis in chicken. Kafkas. Univ. Vet. Fak. Derg. 2017, 23, 895–901. [Google Scholar] [CrossRef]
- Wang, Q.; Miao, Y.; Xu, Y.; Meng, X.; Cui, W.; Wang, Y.; Zhu, L.; Sha, Z.; Wei, K.; Zhu, R. Taishan Pinus Massoniana pollen polysaccharide inhibits the replication of acute tumorigenic ALV-J and its associated tumor growth. Vet. Microbiol. 2019, 236, 108376. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Ashour, E.A.; El-Fakhrany, H.H.H.; Ismail, T.A.; Nasr, M. Early nutrition programming with Astragalus membranaceus polysaccharide: Its effect on growth, carcasses, immunity, antioxidants, lipid profile and liver and kidney functions in broiler chickens. Anim. Biotechnol. 2022, 33, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Li, J.; Li, J.; Huang, Y.; Wu, Y. Effects of Astragalus polysaccharides on intestinal morphology and intestinal immune cells of Muscovy ducklings infected with Muscovy duck reovirus. Poult. Sci. 2021, 100, 64–72. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, C.; Xie, H.; Wang, L.; Hu, J. Effect of Gan Cao (Glycyrrhiza uralensis Fisch) polysaccharide on growth performance, immune function, and gut microflora of broiler chickens. Poult. Sci. 2022, 101, 102068. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, C.; Guo, Y.; Zhang, W.; Guo, W.; Oleksandr, K.; Wang, Z. Polysaccharides derived from Astragalus membranaceus and Glycyrrhiza uralensis improve growth performance of broilers by enhancing intestinal health and modulating gut microbiota. Poult. Sci. 2022, 101, 101905. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Camara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Li, C.; Duan, S.; Li, Y.; Pan, X.; Han, L. Polysaccharides in natural products that repair the damage to intestinal mucosa caused by cyclophosphamide and their mechanisms: A review. Carbohydr. Polym. 2021, 261, 117876. [Google Scholar] [CrossRef]
- Zheng, L.X.; Chen, X.Q.; Cheong, K.L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020, 151, 344–354. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Pi, F.; Cheng, Y.; Guo, Y.; Qian, H. Extraction, purification, structural characteristics, biological activities and pharmacological applications of Acemannan, a polysaccharide from Aloe vera: A Review. Molecules 2019, 24, 1554. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Cheng, L.; Liu, Y.; Wu, Z.; Zhang, X.; Luo, S. Plant polysaccharides modulate immune function via the gut microbiome and may have potential in COVID-19 Therapy. Molecules 2022, 27, 2773. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Wu, Z.; Sun, W.; Wang, Z.; Wu, J.; Huang, M.; Wang, B.; Sun, B. Protective effects of natural polysaccharides on intestinal barrier injury: A Review. J. Agric. Food Chem. 2022, 70, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, G. Extraction and derivatisation of active polysaccharides. J. Enzyme Inhib. Med. Chem. 2019, 34, 1690–1696. [Google Scholar] [CrossRef]
- Ren, Y.; Bai, Y.; Zhang, Z.; Cai, W.; Del Rio Flores, A. The preparation and structure analysis methods of natural polysaccharides of plants and fungi: A review of recent development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef]
- Shan, C.; Sun, B.; Dalloul, R.A.; Zhai, Z.; Sun, P.; Li, M.; Yang, S.; Luan, W. Effect of the oral administration of Astragalus polysaccharides on jejunum mucosal immunity in chickens vaccinated against Newcastle disease. Microb. Pathog. 2019, 135, 103621. [Google Scholar] [CrossRef]
- Su, L.; Wang, J.; Huang, J.; Zhao, Y.; Jiang, H.; Li, H. Suppresses of Astragalus polysaccharide on E. coli-Induced injured intestinal microvascular through TLR4-NF-κB signal pathways in chickens. Braz. J. Poult. Sci. 2019, 21. [Google Scholar] [CrossRef]
- Yang, S.B.; Qin, Y.J.; Ma, X.; Luan, W.M.; Sun, P.; Ju, A.Q.; Duan, A.Y.; Zhang, Y.N.; Zhao, D.H. Effects of in ovo injection of Astragalus polysaccharide on the intestinal development and mucosal immunity in broiler chickens. Front. Vet. Sci. 2021, 8, 738816. [Google Scholar] [CrossRef]
- Lv, H.; Tang, Y.; Zhang, H.; Li, S.; Fan, Z. Astragalus polysaccharide supplementation improves production performance, egg quality, serum biochemical index and gut microbiota in Chongren hens. Anim. Sci. J. 2021, 92, e13550. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.F.; Xing, T.; Li, J.L.; Zhu, X.D.; Zhang, L.; Gao, F. The combined impact of xylo-oligosaccharides and gamma-irradiated Astragalus polysaccharides on growth performance and intestinal mucosal barrier function of broilers. Poult. Sci. 2021, 100, 100909. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.F.; Ren, L.N.; Li, J.L.; Zhu, X.D.; Xing, T.; Zhang, L.; Gao, F.; Zhou, G.H. Protective effects of gamma-irradiated Astragalus polysaccharides on intestinal development and mucosal immune function of immunosuppressed broilers. Poult. Sci. 2019, 98, 6400–6410. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Li, P.; Yan, S.; Liu, Y.; Gao, M.; Lv, H.; Lv, Z.; Guo, Y. Effects of dietary Astragalus polysaccharide supplementation on the Th17/Treg balance and the gut microbiota of broiler chickens challenged with Necrotic Enteritis. Front. Immunol. 2022, 13, 781934. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Tang, Z.; Bai, Y.; Wan, M.; Yu, M.; Chen, J.; Li, G.; Zhang, R.; Ge, M. Astragalus polysaccharide protects against cadmium-induced autophagy injury through reactive oxygen species (ROS) pathway in chicken embryo fibroblast. Biol. Trace Elem. Res. 2022, 200, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, C.; Li, B.; Zhang, S.; Haj, F.G.; Zhang, G.; Lee, Y. The modulatory effects of alfalfa polysaccharide on intestinal microbiota and systemic health of Salmonella serotype (ser.) Enteritidis-challenged broilers. Sci. Rep. 2021, 11, 10910. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Sun, W.; Li, Q.; Wang, K.; Wang, Y.; Lv, F.; Chen, X.; Peng, X.; Wang, Y.; Li, J.; et al. Effects of Caulis Spatholobi polysaccharide on immunity, intestinal mucosal barrier function, and intestinal microbiota in cyclophosphamide-induced immunosuppressive chickens. Front. Vet. Sci. 2022, 9, 833842. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.X.; Shao, Q.; Chen, W.B.; Ma, L.; Xu, W.H.; Li, Y.X.; Huang, S.C.; Ma, Y.B. Effects of Glycyrrhiza polysaccharide in diet on growth performance, serum antioxidant capacity, and biochemistry of broilers. Poult. Sci. 2021, 100, 100927. [Google Scholar] [CrossRef]
- Li, W.; Zhou, X.; Xu, S.; Cao, N.; Li, B.; Chen, W.; Yang, B.; Yuan, M.; Xu, D. Lipopolysaccharide-induced splenic ferroptosis in goslings was alleviated by polysaccharide of atractylodes macrocephala koidz associated with proinflammatory factors. Poult. Sci. 2022, 101, 101725. [Google Scholar] [CrossRef]
- Li, W.; Xiang, X.; Cao, N.; Chen, W.; Tian, Y.; Zhang, X.; Shen, X.; Jiang, D.; Xu, D.; Xu, S. Polysaccharide of Atractylodes Macrocephala Koidz activated T lymphocytes to alleviate cyclophosphamide-induced immunosuppression of geese through novel_mir2/CD28/AP-1 signal pathway. Poult. Sci. 2021, 100, 101129. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, Y.; Shi, L.; Guo, S.; Jin, X.; Xu, Y.; Yan, S.; Shi, B. The effects of dietary supplementation of Artemisia argyi polysaccharide on immune and antioxidative functions in broilers. J. Appl. Anim. Res. 2022, 50, 587–597. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Ou, S.; Arowolo, M.A.; Hou, D.X.; He, J. Effects of Achyranthes bidentata polysaccharides on intestinal morphology, immune response, and gut microbiome in yellow broiler chickens challenged with Escherichia coli K88. Polymers 2018, 10, 1233. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, J.; Wang, H.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. Effects of fermented feeds and ginseng polysaccharides on the intestinal morphology and microbiota composition of Xuefeng black-bone chicken. PLoS ONE 2020, 15, e0237357. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, M.; Gou, Z.; Jiang, S.; Zhang, Y.; Wang, M.; Tang, X.; Xu, B. The effect of Camellia oleifera Cake polysaccharides on growth performance, carcass traits, meat quality, blood profile, and caecum microorganisms in yellow broilers. Animals 2020, 10, 266. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Si, C.; Zhao, Z.T.; Meng, Z.; Yi, H.; Ye, X.M.; Qi, A.; Ouyang, K.H.; Wang, W.J. Effects of polysaccharides from Yingshan Yunwu tea on meat quality, immune status and intestinal microflora in chickens. Int. J. Biol. Macromol. 2020, 155, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Song, Y.; Tian, B.; Qi, C.; Li, L.; Wang, L.; Xing, Y.; Zhao, X.; Liu, J. Platycodon grandifloras polysaccharides inhibit mitophagy injury induced by Cr (VI) in DF-1 cells. Ecotoxicol. Environ. Saf. 2020, 202, 110901. [Google Scholar] [CrossRef]
- Long, L.N.; Kang, B.J.; Jiang, Q.; Chen, J.S. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult. Sci. 2020, 99, 744–751. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Jia, J.; Chen, Y.; Wang, J.; Chen, H.; Jiang, C. Mulberry leaf polysaccharide supplementation contributes to enhancing the respiratory mucosal barrier immune response in Newcastle disease virus-vaccinated chicks. Poult. Sci. 2021, 100, 592–602. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, P.; Xu, X.; Chen, X.; Liu, Q.; Jiang, C. The enhanced immunological activity of Paulownia tomentosa flower polysaccharide on Newcastle disease vaccine in chicken. Biosci. Rep. 2019, 39, BSR20190224. [Google Scholar] [CrossRef]
- Wang, Q.; Meng, X.; Zhu, L.; Xu, Y.; Cui, W.; He, X.; Wei, K.; Zhu, R. A polysaccharide found in Paulownia fortunei flowers can enhance cellular and humoral immunity in chickens. Int. J. Biol. Macromol. 2019, 130, 213–219. [Google Scholar] [CrossRef]
- Yue, C.; Chen, J.; Hou, R.; Tian, W.; Liu, K.; Wang, D.; Lu, Y.; Liu, J.; Wu, Y.; Hu, Y. The antioxidant action and mechanism of selenizing Schisandra chinensis polysaccharide in chicken embryo hepatocyte. Int. J. Biol. Macromol. 2017, 98, 506–514. [Google Scholar] [CrossRef]
- Sha, Z.; Shang, H.; Miao, Y.; Huang, J.; Niu, X.; Chen, R.; Peng, D.; Wei, K.; Zhu, R. Polysaccharides from Pinus massoniana pollen improve intestinal mucosal immunity in chickens. Poult. Sci. 2021, 100, 507–516. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, Z.; Zhang, A.; Jia, F.; Song, M.; Huang, Z.; Fu, J.; Li, G.; Lin, S. Proteomics analysis of chicken peripheral blood lymphocyte in Taishan Pinus massoniana pollen polysaccharide regulation. PLoS ONE 2018, 13, e0208314. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Lan, R.; Wu, Z.; Wang, Z.; Wu, H.; Li, Z.; Yu, H.; Zhao, Z.; Li, H. Yupingfeng polysaccharides enhances growth performance in Qingyuan partridge chicken by up-regulating the mRNA expression of SGLT1, GLUT2 and GLUT5. Vet. Med. Sci. 2019, 5, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Sci. Technol. 2019, 250, 32–40. [Google Scholar] [CrossRef]
- Liu, Y.S.; Li, S.; Wang, X.F.; Xing, T.; Li, J.L.; Zhu, X.D.; Zhang, L.; Gao, F. Microbiota populations and short-chain fatty acids production in cecum of immunosuppressed broilers consuming diets containing gamma-irradiated Astragalus polysaccharides. Poult. Sci. 2021, 100, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Q.; Yang, Y.; Guo, A. Biological function of short-chain fatty acids and its regulation on intestinal health of poultry. Front. Vet. Sci. 2021, 8, 736739. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Bedford, M.R.; Wu, S.B.; Morgan, N.K. Soluble non-starch polysaccharide modulates broiler gastrointestinal tract environment. Poult. Sci. 2021, 100, 101183. [Google Scholar] [CrossRef]
- Sun, Q.; Ho, C.T.; Zhang, X.; Liu, Y.; Zhang, R.; Wu, Z. Strategies for circadian rhythm disturbances and related psychiatric disorders: A new cue based on plant polysaccharides and intestinal microbiota. Food Funct. 2022, 13, 1048–1061. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, N.; Hao, M.; Zhou, J.; Xie, Y.; He, Z. Plant-derived polysaccharides regulated immune status, gut health and microbiota of broilers: A review. Front. Vet. Sci. 2021, 8, 791371. [Google Scholar] [CrossRef]
- Li, S.; Lin, R.; Chen, J.; Hussain, R.; Zhang, S.; Su, Y.; Chan, Y.; Ghaffar, A.; Shi, D. Integrated gut microbiota and metabolomic analysis reveals immunomodulatory effects of Echinacea extract and Astragalus polysaccharides. Front. Vet. Sci. 2022, 9, 971058. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, K.; Mishra, R.; Jha, R. In ovo supplementation of chitooligosaccharide and chlorella polysaccharide affects cecal microbial community, metabolic pathways, and fermentation metabolites in broiler chickens. Poult. Sci. 2020, 99, 4776–4785. [Google Scholar] [CrossRef]
- Anderson, R.C.; Dalziel, J.E.; Gopal, P.K.; Bassett, S.; Ellis, A.; Roy, N.C. The role of intestinal barrier function in early life in the development of Colitis. In Colitis; InTechOpen: London, UK, 2012; pp. 1–30. [Google Scholar] [CrossRef][Green Version]
- Wu, S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018, 97, 3489–3493. [Google Scholar] [CrossRef] [PubMed]
- Yaghobfar, A.; Kalantar, M. Effect of Non-Starch Polysaccharide (NSP) of wheat and barley supplemented with exogenous enzyme blend on growth performance, gut microbial, pancreatic enzyme activities, expression of glucose transporter (SGLT1) and mucin producer (MUC2) genes of broiler chickens. Braz. J. Poult. Sci. 2017, 19, 629–638. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, L.; Zhao, J.-L.; Xu, W.; Guo, Z.; Zhang, A.-Z.; Li, M.-Y. Dietary Taraxacum mongolicum polysaccharide ameliorates the growth, immune response, and antioxidant status in association with NF-κB, Nrf2 and TOR in Jian carp (Cyprinus carpio var. Jian). Aquaculture 2022, 547, 737522. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Patra, A.K.; Amasheh, S.; Aschenbach, J.R. Modulation of gastrointestinal barrier and nutrient transport function in farm animals by natural plant bioactive compounds—A comprehensive review. Crit. Rev. Food Sci. 2019, 59, 3237–3266. [Google Scholar] [CrossRef]
- Patra, A.K. Influence of Plant bioactive compounds on intestinal epithelial barrier in poultry. Mini Rev. Med. Chem. 2020, 20, 566–577. [Google Scholar] [CrossRef]
- Zheng, Z.; Pan, X.; Wang, H.; Wu, Z.; Sullivan, M.A.; Liu, Y.; Liu, J.; Wang, K.; Zhang, Y. Mechanism of Lentinan intestinal absorption: Clathrin-mediated endocytosis and macropinocytosis. J Agric. Food Chem. 2021, 69, 7344–7352. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Qin, T.; Ren, Z.; Yi, L.; Liu, X.; Luo, Y.; Long, Y.; Peng, S.; Li, J.; Ma, Y.; Wu, Y.; et al. Immunological modulation effects of an acid Epimedium polysaccharide on immune response in chickens. Int. Immunopharmacol. 2019, 70, 56–66. [Google Scholar] [CrossRef]
- Li, Y.; Lei, X.; Guo, W.; Wu, S.; Duan, Y.; Yang, X.; Yang, X. Transgenerational endotoxin tolerance-like effect caused by paternal dietary Astragalus polysaccharides in broilers’ jejunum. Int. J. Biol. Macromol. 2018, 111, 769–779. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, B.; Li, T.; Xu, Y.; Jin, X.; Guo, X.; Yan, S. Immunomodulatory effect of Artemisia argyi polysaccharide on peripheral blood leucocyte of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2018, 102, 939–946. [Google Scholar] [CrossRef] [PubMed]

| Source | Application Form | Experiment Object | Main Function | Reference |
|---|---|---|---|---|
| Astragalus polysaccharide, 70% content | At dosage of 0.6 g/L in drinking water | Muscovy ducklings | Improved intestinal mucosal immune function and morphology | [15] |
| Astragalus polysaccharides, net content 70% | Orally gavaged daily with 0.5 mL (at doses of 1, 2, 4 mg/mL for four consecutive days) | Hy-Line male chickens | Enhanced the jejunum mucosal immune function | [26] |
| Astragalus membranaceus polysaccharide | Oral 1.5 mL daily (10 mg/mL) | Male Gushi chickens | Alleviated intestinal inflammatory processes and vascular dysfunction | [27] |
| Astragalus polysaccharide | Injected with 0.5 mL of 3 different concentrations solution (1, 2, 4 mg in 0.5 mL physiological saline) | Fertilized eggs | Promoted intestinal development and mucosal immunity | [28] |
| Astragalus membranaceus polysaccharide, purity 87.81% | Intramuscular injected with 0.3 mL | Goslings | Enhanced intestinal antioxidant function and reduced inflammatory damage | [1] |
| Astragalus polysaccharides | Basal diet supplemented with 100, 200, 400 mg/kg | Chongren hens | Improved production performance, egg quality, serum biochemical index and gut microbiota | [29] |
| gamma-irradiated Astragalus polysaccharides | Basal diet supplemented with 600 mg/kg | Ross-308 chicks | Improved growth performance and intestinal mucosal barrier function | [30,31] |
| Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao. polysaccharides | Basal diet supplemented with 200 ppm | Arbor Acres broiler chicks | Improved production performance, immune function and gut microbiota | [32] |
| Astragalus membranaceus and Glycyrrhiza uralensis polysaccharides | Basal diet supplemented with 300 mg/kg Astragalus membranaceus polysaccharides, 150 mg/kg Glycyrrhiza uralensis polysaccharides | Male Arbor Acres broiler chickens | Improved growth performance, intestinal health and gut microbiota | [17] |
| Astragalus membranaceus polysaccharide | Dissolved in 0.5 mL saline and injected into the air sac of the 7-day-old embryos through the wide end of the eggs | Eggs | Improved antioxidant activity, immune function, and liver and kidney functions | [14] |
| Astragalus | Cell supernatant 40 mg/L | Chicken embryo fibroblast Hailan white egg hen embryos | Attenuated chicken embryo fibroblast autophagy damage | [33] |
| alfalfa polysaccharide | Basal diet supplemented with 500 mg/kg | Arbor Acres broiler chicks (mixed sex) | Improved intestinal microbiota and systemic health | [34] |
| Caulis Spatholobi polysaccharides | Basal diet supplemented with 0.2, 0.4, 0.6% | Sanhuang cocks | Improved immunity, intestinal mucosal barrier function, and intestinal microbiota | [35] |
| Glycyrrhiza uralensis root polysaccharides | Administered by gavage at 3 different concentrations: 600, 450, 300 mg/kg for 14 d | Male Hy-Line brown chickens | Boosted immune function | [7] |
| Gan Cao (Glycyrrhiza uralensis Fisch) polysaccharides | Basal diet supplemented with 0.5, 1.0, 1.5% | Avian commercial female broilers | Enhanced growth performance, immune function, and gut microflora | [16] |
| Licorice (Glycyrrhiza glabra) polysaccharides | Basal diet supplemented with 200, 500, 1000, 1500 mg/kg | Male Arbor Acres broilers | Improved growth performance, serum antioxidant capacity, and biochemistry | [36] |
| Atractylodes macrocephala Koidz polysaccharides | Basal diet supplemented with 400 mg/kg | Magang goslings | Reduced oxidative stress and inflammatory response | [37] |
| Atractylodes Macrocephala Koidz Polysaccharide, purity 95% | Basal diet supplemented with 400 mg (kg body weight) | Specific pathogen-free goose | Promoted T lymphocytes activation and proliferation, restored the thymus cells morphology, alleviated immune suppression | [38] |
| Artemisia ordosica polysaccharide | Basal diet supplemented with 750 mg/kg | Arbor Acres broilers | Improved immune and antioxidative function | [5] |
| Artemisia argyi polysaccharide | Basal diet supplemented with 250, 500, 750, 1000 mg/kg | Arbor Acres broilers | Improved immune and antioxidative function | [39] |
| Achyranthes bidentata polysaccharides | Basal diet supplemented with 0, 0.02, 0.04% | Female Pekin ducks | Improved growth performance, immunity, antioxidant capacity, and meat quality | [9] |
| Achyranthes bidentata polysaccharides | Basal diet supplemented with 500 mg/kg | Female yellow-feathered broiler chickens | Promoted intestinal morphology, immune response, and gut microbiome | [40] |
| Acanthopanax senticosus polysaccharides | Basal diet supplemented with 0, 1, 2, 4 g/kg | Male Arbor Acres broiler chicks | Improved growth performance, immune function, antioxidation, and ileal microbial populations | [3] |
| ginseng polysaccharides | Basal diet supplemented with 200 g/t | Xuefeng blackbone chickens | Improved intestinal morphology and microbiota composition | [41] |
| Camellia oleifera cake polysaccharides | Basal diet supplemented with 0, 200, 800 mg/kg | Lingnan yellow broiler | Improved growth performance, carcass traits, meat quality, blood profile, and caecum microorganisms | [42] |
| Yingshan Yunwu tea polysaccharides | Basal diet supplemented with 200, 400, 800 mg/kg | Chongren chickens | Improved meat quality, immune status and intestinal microflora | [43] |
| Codonopsis pilosula polysaccharide | Orally treated with CPPS (3 mg per feather), pCPPS (2.5 mg per feather) for 3 consecutive days | Ducklings | Promoted immune ability | [10] |
| Platycodon grandifloras polysaccharides | Cell supernatant incubated in DMEM containing 200 μg/mL | Chicken embryo fibroblast cell lines | Attenuated chicken embryo fibroblast cell lines mitochondrial damage | [44] |
| Lycium barbarum polysaccharides | Basal diet supplemented with 1000, 2000, 4000 mg/kg | Male Arbor Acres broiler chicks | Improved growth performance, digestive enzyme activities, antioxidant status, and immunity | [45] |
| Mulberry leaf polysaccharide, purity 95% | Oral: at doses of 8, 4, 2 mg continuously for 7 d | Male chicks | Enhanced the respiratory mucosal barrier immune response | [46] |
| Paulownia tomentosa flower polysaccharide | Oral: at different doses of 50, 25, 12.5, 6.25, 3.125 mg/kg for 3 successive days | White Roman chickens | Enhanced immunological activity | [47] |
| Paulownia fortunei flowers polysaccharide | Subcutaneously injected in the neck with 0.25 mL at concentrations of 40, 20, 10 mg/mL for 3 successive days | Specific pathogen-free chickens | Enhanced cellular and humoral immunity | [48] |
| selenizing Schisandra chinensis polysaccharide | Cell supernatant SCP and sSCP were respectively twofold diluted from 1600 μg/mL to 1.563 μg/mL with DMEM | Chicken embryo hepatocytes | Attenuated hepatocyte oxidative damage | [49] |
| Aloe vera polysaccharides | Oral: at the dose rates of 100, 200, 300 mg/kg body weight | Broiler chicks | Enhanced immunotherapeutic effects and anti-coccidial | [12] |
| Amomum longiligulare T.L. Wu fruits polysaccharide | Injection: 30 mg ALP1 (glucose) with 0.25 mL normal saline; 30 mg ALP2 (glucose, glucuronic acid and galacturonic acid with the ratio of 91.43:5.23:3.34.) with 0.25 mL normal saline | Chickens | Promoted bursa of Fabricius immune ability | [11] |
| Taishan Pinus massoniana pollen polysaccharides | Orally administered with 10, 20, 40 mg/mL (0.2 mL/chicken) for 21 consecutive days | Specific pathogen–free chickens | Enhanced intestinal mucosal immunity and intestinal villi development | [50] |
| Taishan Pinus Massoniana pollen polysaccharide | Oral: received 5.0 mg for 7 consecutive days | Specific pathogen-free chickens | Enhanced antiviral and antitumor activity | [13] |
| Taishan Pinus massoniana pollen polysaccharide | Cell supernatant concentrations, including 0, 12.5, 50, 100, 200, 400, 800, 1600 μg/mL | Chicken peripheral blood lymphocytes | Enhanced host immune response | [51] |
| Yupingfeng polysaccharides | Basal diet supplemented with 0.5, 1, 2, 4 g/kg | Qingyuan partridge chickens | Enhanced growth performance and small intestinal digestion and absorption | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Xing, Y.; Xu, Y.; Jin, X.; Yan, S.; Shi, B. Progress of Studies on Plant-Derived Polysaccharides Affecting Intestinal Barrier Function in Poultry. Animals 2022, 12, 3205. https://doi.org/10.3390/ani12223205
Guo S, Xing Y, Xu Y, Jin X, Yan S, Shi B. Progress of Studies on Plant-Derived Polysaccharides Affecting Intestinal Barrier Function in Poultry. Animals. 2022; 12(22):3205. https://doi.org/10.3390/ani12223205
Chicago/Turabian StyleGuo, Shiwei, Yuanyuan Xing, Yuanqing Xu, Xiao Jin, Sumei Yan, and Binlin Shi. 2022. "Progress of Studies on Plant-Derived Polysaccharides Affecting Intestinal Barrier Function in Poultry" Animals 12, no. 22: 3205. https://doi.org/10.3390/ani12223205
APA StyleGuo, S., Xing, Y., Xu, Y., Jin, X., Yan, S., & Shi, B. (2022). Progress of Studies on Plant-Derived Polysaccharides Affecting Intestinal Barrier Function in Poultry. Animals, 12(22), 3205. https://doi.org/10.3390/ani12223205






