Breeding Ewe Lambs: An Australasian Perspective
Abstract
Simple Summary
Abstract
1. Introduction
2. Comparison of Reproductive Traits of Ewe Lambs and Mature Ewes
3. Factors Affecting Ewe Lamb Performance Prior to and during Breeding
3.1. Genetic Factors
Potential for Fetal Programming Effects
3.2. Environmental Factors
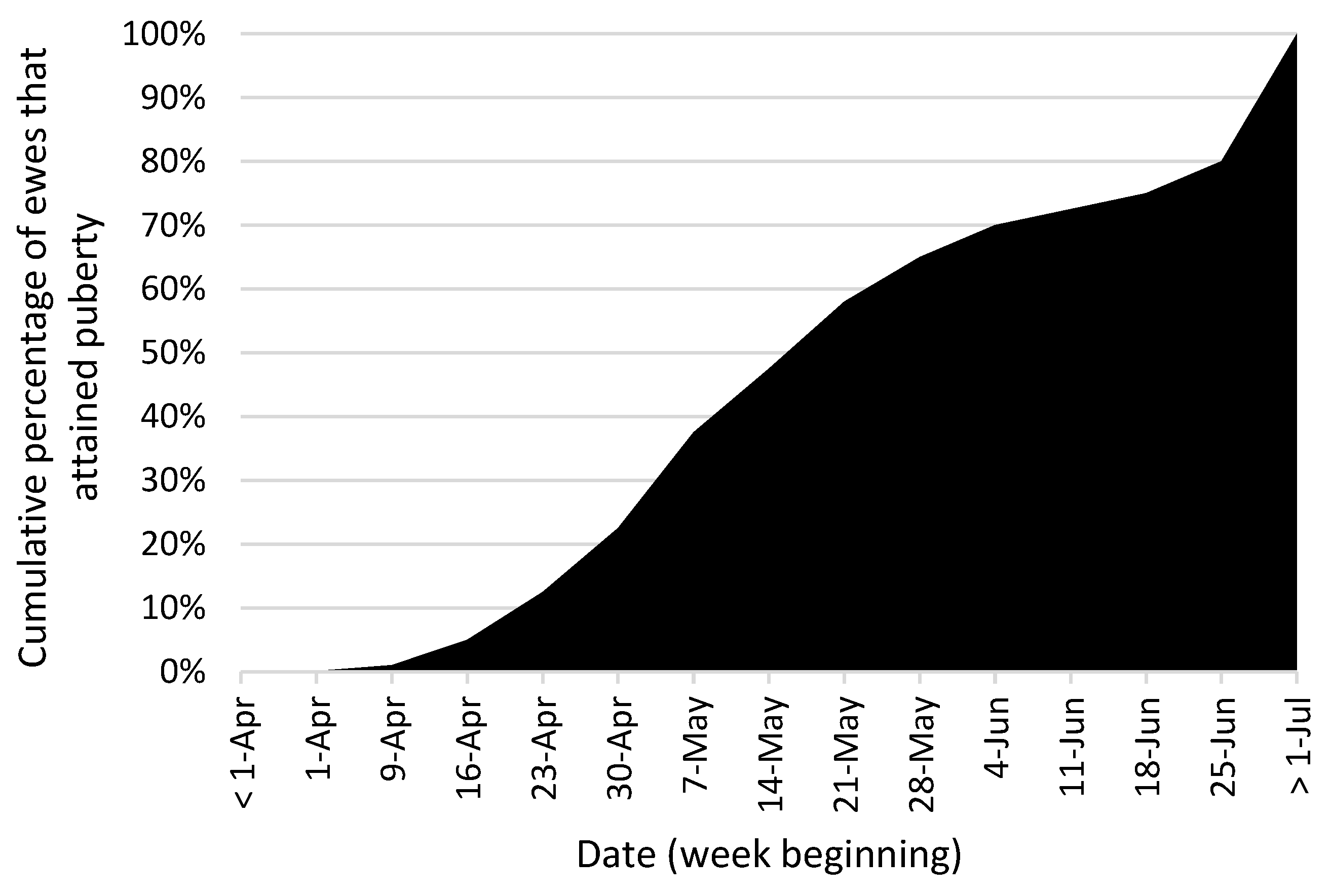
Timing of Puberty and Breeding
3.3. Management Factors
3.3.1. Exogenous Hormones and the Ram Effect
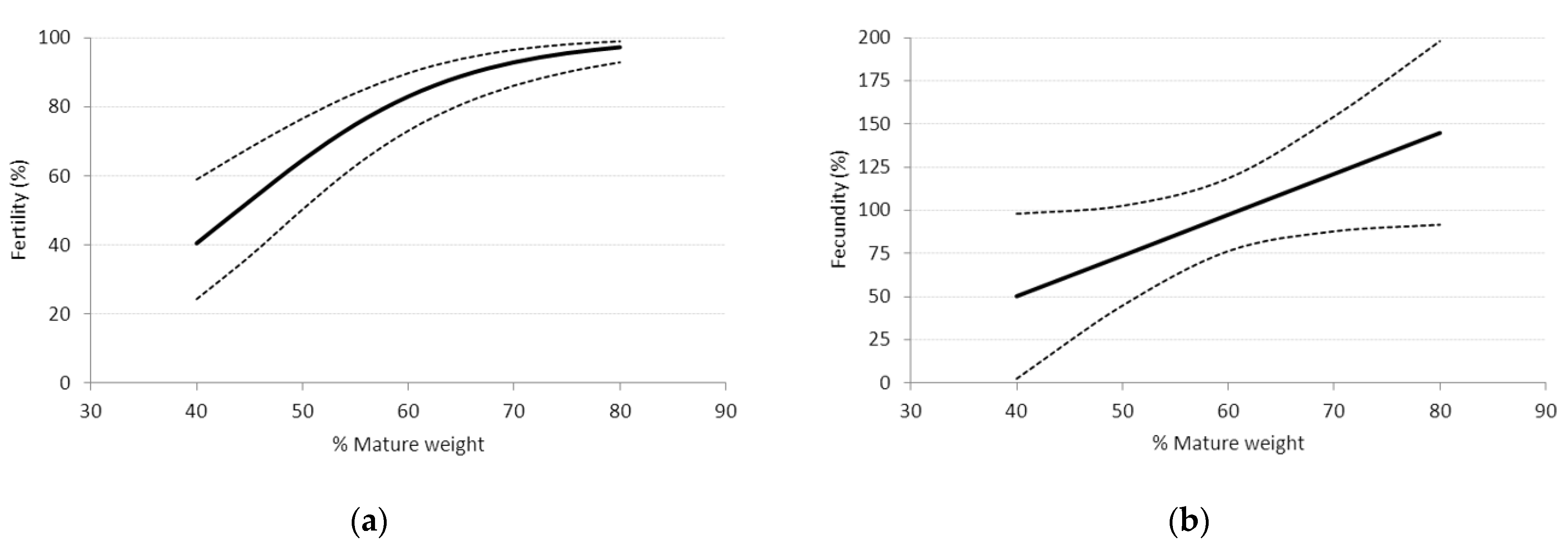
3.3.2. Effect of Liveweight, Liveweight Change, and Body Condition Score (BCS) prior to and during Breeding
3.3.3. Shearing before and during the Breeding Period
3.3.4. Breeding Management
4. Factors Affecting Ewe Lamb Performance during Pregnancy
4.1. Nutritional Management
4.2. Pregnancy and Fetal Loss
4.3. Mid-Pregnancy Shearing
5. Factors Affecting Performance during Lambing and Lactation
5.1. Lamb Survival
5.2. Nutrition
5.3. Lamb Age at Weaning
5.4. Post-Weaning Management
6. Lifetime Impacts of Ewe Lamb Breeding
7. Potential Impacts of Selection of Progeny Born to Ewe Lambs as Replacement Ewes
8. Knowledge Gaps
- Potential long-term impacts in utero experience on ewe lamb reproductive performance (fetal programming),
- Optimal liveweight gain profile for ewe lambs from weaning at 3 months of age until the start of their first breeding period,
- Disease investigation using serial pregnancy scanning to differentiate losses in pregnancy from perinatal loss and causes of these losses,
- Optimal lamb birth weight range for survival of ewe lamb progeny,
- Effect of BCS during pregnancy on ewe lamb progeny weaning weight,
- Optimal nutrition during lactation and the interaction with nutritional levels in pregnancy,
- Management during lactation and post weaning on rebreeding at 18 months of age,
- Lifetime studies or analysis of existing data on ewe lamb lifetime performance,
- Economic analysis of the most cost-effective nutritional regimens in both pregnancy and lactation. This will then allow the identification individual management factors that will help farmers focus their efforts on those that have greater impacts on profitability.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farrell, L.J.; Kenyon, P.R.; Morris, S.T.; Tozer, P.R. The Impact of Hogget and Mature Flock Reproductive Success on Sheep Farm Productivity. Agriculture 2020, 10, 566. [Google Scholar] [CrossRef]
- Farrell, L.J.; Kenyon, P.R.; Tozer, P.R.; Morris, S.T. Determining the Impact of Hogget Breeding Performance on Profitability under a Fixed Feed Supply Scenario in New Zealand. Animals 2021, 11, 1303. [Google Scholar] [CrossRef] [PubMed]
- Young, J.M.; Thompson, A.N.; Kennedy, A.J. Bioeconomic modelling to identify the relative importance of a range of critical control points for prime lamb production systems in south-west Victoria. Anim. Prod. Sci. 2010, 50, 748–756. [Google Scholar] [CrossRef]
- Young, J.M.; Trompf, J.; Thompson, A.N. The critical control points for increasing reproductive performance can be used to inform research priorities. Anim. Prod. Sci. 2014, 54, 645–655. [Google Scholar] [CrossRef]
- Tocker, J.; Behrendt, R.; Raeside, M.; Malcolm, B. The impact of ewe lamb mating and different feeding strategies over summer–autumn on profit and risk: A case study in south-west Victoria. Anim. Prod. Sci. 2020, 61, 1137–1150. [Google Scholar] [CrossRef]
- Court, J.; Hides, S.; Webb-Ware, J. Sheep Farming for Meat and Wool; CSIRO Publishing: Collingwood, Australia, 2010. [Google Scholar]
- Rattray, P.V.; Brookes, I.M.; Nicol, A.M. Pastures and Supplements for Grazing Animals; Rattray, P.V., Brookes, I.M., Nicol, A.M., Eds.; New Zealand Society of Animal Science: Wellington, New Zealand, 2017. [Google Scholar]
- Ferguson, D.M.; Schreurs, N.M.; Kenyon, P.R.; Jacob, R.H. Balancing consumer and societal requirements for sheep meat production: An Australasian perspective. Meat Sci. 2014, 98, 477–483. [Google Scholar] [CrossRef]
- Freer, M.; Dove, H. Sheep Nutrition; CSIRO Publishing: Melbourne, Australia, 2002; p. 385. [Google Scholar]
- Kenyon, P.R.; Thompson, A.N.; Morris, S.T. Breeding ewe lambs successfully to improve lifetime performance. Small Rumin. Res. 2014, 118, 2–15. [Google Scholar] [CrossRef]
- Edwards, S.J.; Juengel, J.L. Limits on hogget lambing: The fertility of the young ewe. N. Z. J. Agric. Res. 2017, 60, 1–22. [Google Scholar] [CrossRef]
- Sloane, R. 2017 Merino Husbandry Practices Survey Final Report; Australian Wool Innovation: Santa Monica, CA, USA, 2018; p. 158. [Google Scholar]
- Statistics New Zealand. Infoshare. Available online: https://www.stats.govt.nz/tools/stats-infoshare (accessed on 14 March 2022).
- Dyrmundsson, O.R. Puberty and early reproductive performance in sheep. I. Ewe lambs. Anim. Breed. Abstr. 1973, 41, 273–289. [Google Scholar]
- Rosales Nieto, C.A.; Thompson, A.N.; Martin, G.B. A new perspective on managing the onset of puberty and early reproductive performance in ewe lambs: A review. Anim. Prod. Sci. 2018, 58, 1967–1975. [Google Scholar] [CrossRef]
- Hutchison, D.; Clarke, B.E.; Hancock, S.; Thompson, A.N.; Bowen, E.; Jacobson, C. Lower Reproductive Rate and Lamb Survival Contribute to Lower Lamb Marking Rate in Maiden Ewes Compared to Multiparous Ewes. Animals 2022, 12, 513. [Google Scholar] [CrossRef] [PubMed]
- Corner-Thomas, R.A.; Ridler, A.L.; Morris, S.T.; Kenyon, P.R. Ewe lamb live weight and body condition scores affect reproductive rates in commercial flocks. N. Z. J. Agric. Res. 2015, 58, 26–34. [Google Scholar] [CrossRef]
- Kenyon, P.R.; Pinchbeck, G.L.; Perkins, N.R.; Morris, S.T.; West, D.M. Identifying factors which maximise the lambing performance of hoggets: A cross sectional study. N. Z. Vet. J. 2004, 52, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Corner, R.A.; Blair, H.T.; Morris, S.T.; Kenyon, P.R. A comparison of aspects of the reproductive success of ewe lambs and mixed age ewes joined over the same period. Proc. N. Z. Soc. Anim. Prod. 2013, 73, 76–78. [Google Scholar]
- Pettigrew, E.J.; Hickson, R.E.; Blair, H.T.; Griffiths, K.J.; Ridler, A.L.; Morris, S.T.; Kenyon, P.R. Differences in lamb production between ewe lambs and mature ewes. N. Z. J. Agric. Res. 2021, 64, 508–521. [Google Scholar]
- Hafez, E.S. Studies on the breeding season and reproduction of the ewe Part I. The breeding season in different environments Part II. The breeding season in one locality. J. Agric. Sci. 1952, 42, 189–231. [Google Scholar] [CrossRef]
- Smith, J.F.; Knight, T.W. Reproductive management of sheep. In Reproductive Management of Grazing Ruminants in New Zealand; Fielden, E.D., Smith, J.F., Eds.; Occasional publication (New Zealand Society of Animal Production); no. 12; New Zealand Society of Animal Production: Hamilton, New Zealand, 1998; pp. 113–134. [Google Scholar]
- McMillan, W.; Parker, W. Mating of Ewe Hoggets—An Appraisal for a Wairarapa Farm; Massey University Riverside Farm Publication: Palmerston, New Zealand, 1981; pp. 35–45. [Google Scholar]
- Williams, S.M. Fertility in Clun Forest sheep. J. Agric. Sci. 1954, 45, 202–228. [Google Scholar] [CrossRef]
- Edey, T.N.; Kilgour, R.; Bremner, K. Sexual-behavior and reproductive-performance of ewe lambs at and after puberty. J. Agric. Sci. 1978, 90, 83–91. [Google Scholar] [CrossRef]
- Hare, L.; Bryant, M.J. Ovulation rate and embryo survival in young ewes mated either at puberty or at the 2nd or 3rd estrus. Anim. Reprod. Sci. 1985, 8, 41–52. [Google Scholar] [CrossRef]
- Schick, G. Hogget mating—Will you follow the trend? Wool Grow. Summer 2001, 2001, 25–26. [Google Scholar]
- Foote, W.; Sefidbakht, N.; Madsen, M. Puberal estrus and ovulation and subsequent estrous cycle patterns in the ewe. J. Anim. Sci. 1970, 30, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Quirke, J. Reproductive performance of Galway, Finnish Landrace and Finn-cross ewe lambs. Ir. J. Agric. Res. 1978, 17, 25–32. [Google Scholar]
- Edey, T.N.; Chu, T.T.; Kilgour, R.; Smith, J.F.; Tervit, H.R. Estrus without ovulation in pubertal ewes. Theriogenology 1977, 7, 11–15. [Google Scholar] [CrossRef]
- Quirke, J.F.; Hanrahan, J.P.; Gosling, J.P. Duration of estrus, ovulation rate, time of ovulation and plasma-lh, total estrogen and progesterone in galway adult ewes and ewe lambs. J. Reprod. Fertil. 1981, 61, 265–272. [Google Scholar] [CrossRef]
- Chu, T.; Edey, T. Reproductive Performance of Ewe Lambs at Puberty; Australian Society of Animal Production: Melbourne, Australia, 1978. [Google Scholar]
- Dyrmundsson, O.R. Natural factors affecting puberty and reproductive-performance in ewe lambs—A review. Livest. Prod. Sci. 1981, 8, 55–65. [Google Scholar]
- Davies, M.C.G.; Beck, N.F.G. A comparison of plasma prolactin, LH and progesterone concentrations during estrus and early-pregnancy in ewe lambs and ewes. Anim. Prod. 1993, 57, 281–286. [Google Scholar]
- Allison, A.J.; Kelly, R.W.; Lewis, J.S.; Binnie, D.B. Preliminary studies on the efficiency of mating on ewe hoggets. Proc. N. Z. Soc. Anim. Prod. 1975, 35, 83–90. [Google Scholar]
- Kenyon, P.R.; Morris, S.T.; West, D.M. Proportion of rams and the condition of ewe lambs at joining influences their breeding performance. Anim. Prod. Sci. 2010, 50, 454–459. [Google Scholar] [CrossRef]
- Stevens, D.; McIntyre, S. Hogget Mating Survey; Internal Report AgResearch Wool Pro: Lincoln, New Zealand, 1999; p. 24. [Google Scholar]
- Quirke, J.F.; Hanrahan, J.P. Comparison of survival in uteri of adult ewes of cleaved ova from adult ewes and ewe lambs. J. Reprod. Fertil. 1977, 51, 487–489. [Google Scholar] [CrossRef]
- Beck, N.F.G.; Davies, M.C.G.; Davies, B. A comparison of ovulation rate and late embryonic mortality in ewe lambs and ewes and the role of late embryo loss in ewe lamb subfertility. Anim. Sci. 1996, 62, 79–83. [Google Scholar] [CrossRef]
- Paganoni, B.L.; Ferguson, M.B.; Ferrio, S.; Jones, C.; Kearney, G.A.; Kenyon, P.R.; Macleay, C.; Vinoles, C.; Thompson, A.N. Early reproductive losses are a major factor contributing to the poor reproductive performance of Merino ewe lambs mated at 8–10 months of age. Anim. Prod. Sci. 2014, 54, 762–772. [Google Scholar] [CrossRef]
- Forrest, P.A.; Bichard, M. Analysis of production records from a lowland sheep flock. 2. Flock statistics and reproductive-performance. Anim. Prod. 1974, 19, 25–32. [Google Scholar] [CrossRef]
- Shorten, P.R.; Edwards, S.J.; Juengel, J.L. The role of reproductive loss on flock performance: A comparison of nine industry flocks. Transl. Anim. Sci. 2021, 5, txab013. [Google Scholar] [CrossRef] [PubMed]
- McMillan, W.; McDonald, M. Survival of fertilized ova from ewe lambs and adult ewes in the uteri of ewe lambs. Anim. Reprod. Sci. 1985, 8, 235–240. [Google Scholar] [CrossRef]
- Keane, M.G. Breeding from ewe lambs. Farm Food Res. 1976, 7, 10–12. [Google Scholar]
- Annett, R.W.; Carson, A.F. Effects of plane of nutrition during the first month of pregnancy on conception rate, foetal development and lamb output of mature and adolescent ewes. Anim. Sci. 2006, 82, 947–954. [Google Scholar] [CrossRef]
- Mulvaney, F.J. Investigating methods to improve the reproductive performance of hoggets: A thesis presented in partial fulfilment of the requirements for the degree of Doctor of Philosophy in Animal Science at Massey University, Palmerston North, New Zealand. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 2011. [Google Scholar]
- O’Connell, A.R.; Demmers, K.J.; Smaill, B.; Reader, K.L.; Juengel, J.L. Early embryo loss, morphology, and effect of previous immunization against androstenedione in the ewe. Theriogenology 2016, 86, 1285–1293. [Google Scholar] [CrossRef]
- Donald, H.P.; Read, J.L.; Russell, W.S. A comparative trial of crossbred ewes by Finnish Landrace and other sires. Anim. Prod. 1968, 10, 413–421. [Google Scholar] [CrossRef]
- Khan, T.H.; Beck, N.F.G.; Khalid, M. The effects of GnRH analogue (buserelin) or hCG (Chorulon) on day 12 of pregnancy on ovarian function, plasma hormone concentrations, conceptus growth and placentation in ewes and ewe lambs. Anim. Reprod. Sci. 2007, 102, 247–257. [Google Scholar] [CrossRef]
- Munoz, C.; Carson, A.F.; McCoy, M.A.; Dawson, L.E.R.; O’Connell, N.E.; Gordon, A.W. Effect of plane of nutrition of 1-and 2-year-old ewes in early and mid-pregnancy on ewe reproduction and offspring performance up to weaning. Animal 2009, 3, 657–669. [Google Scholar] [CrossRef]
- Pattinson, S.; Davies, D.A.R.; Winter, A. Colostrum and lamb production of prolific ewes. BSAP Occas. Publ. 1992, 15, 166–168. [Google Scholar] [CrossRef]
- Baker, R.L.; Clarke, J.N.; Diprose, G.D. Effect of mating Romney ewe hoggets on lifetime production—Preliminary results. N. Z. Soc. Anim. Prod. 1981, 41, 198–203. [Google Scholar]
- Quirke, J.F.; Hanrahan, J.P. Comparison of the survival of fertilized-eggs from adult ewes in the uteri of adult ewes and ewe lambs. J. Reprod. Fertil. 1983, 68, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Gbangboche, A.; Adamou-Ndiaye, M.; Youssao, A.; Farnir, F.; Detilleux, J.; Abiola, F.; Leroy, P. Non-genetic factors affecting the reproduction performance, lamb growth and productivity indices of Djallonke sheep. Small Rumin. Res. 2006, 64, 133–142. [Google Scholar] [CrossRef]
- Gootwine, E.; Rozov, A. Seasonal effects of birth weight of lambs born to prolific ewes maintained under intensive management. Livest. Sci. 2006, 105, 277–283. [Google Scholar] [CrossRef]
- Campion, F.P.; Crosby, T.F.; Creighton, P.; Fahey, A.G.; Boland, T.M. An investigation into the factors associated with ewe colostrum production. Small Rumin. Res. 2019, 178, 55–62. [Google Scholar] [CrossRef]
- Halliday, R. Variations in immunoglobulin concentrations in Finnish × Dorset Horn lambs. Res. Vet. Sci. 1976, 21, 331–334. [Google Scholar] [CrossRef]
- Abd-Allah, M. Effects of parity and nutrition plane during late pregnancy on metabolic responses, colostrum production and lamb output of Rahmani ewes. Egypt. J. Anim. Prod. 2013, 50, 132–142. [Google Scholar]
- Gilbert, R.; Gaskins, C.; Hillers, J.; Parker, C.; McGuire, T. Genetic and environmental factors affecting immunoglobulin G1 concentrations in ewe colostrum and lamb serum. J. Anim. Sci. 1988, 66, 855–863. [Google Scholar] [CrossRef]
- Argüello, A.; Castro, N.; Alvarez, S.; Capote, J. Effects of the number of lactations and litter size on chemical composition and physical characteristics of goat colostrum. Small Rumin. Res. 2006, 64, 53–59. [Google Scholar] [CrossRef]
- Morgan, J.E.; Fogarty, N.M.; Nielsen, S.; Gilmour, A.R. Milk yield and milk composition from grazing primiparous non-dairy crossbred ewes. Aust. J. Agric. Res. 2006, 57, 377–387. [Google Scholar] [CrossRef]
- Afolayan, R.; Fogarty, N.; Morgan, J.; Gaunt, G.; Cummins, L.; Gilmour, A.R.; Nielsen, S. Genetic analysis of milk production and composition in crossbred ewes from different maternal genotypes. Anim. Prod. Sci. 2009, 49, 24–31. [Google Scholar] [CrossRef]
- Peeters, R.; Buys, N.; Robijns, L.; Vanmontfort, D.; Van Isterdael, J. Milk yield and milk composition of Flemish Milksheep, Suffolk and Texel ewes and their crossbreds. Small Rumin. Res. 1992, 7, 279–288. [Google Scholar] [CrossRef]
- Snowder, G.D.; Knight, A.D.; Van Vleck, L.D.; Bromley, C.M.; Kellom, T.R. Usefulness of subjective ovine milk scores: I. Associations with range ewe characteristics and lamb production. J. Anim. Sci. 2001, 79, 811–818. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corner, R.A.; Mulvaney, F.J.; Morris, S.T.; West, D.M.; Morel, P.C.H.; Kenyon, P.R. A comparison of the reproductive performance of ewe lambs and mature ewes. Small Rumin. Res. 2013, 114, 126–133. [Google Scholar] [CrossRef]
- Murphy, P.M.; Purvis, I.W.; Lindsay, D.R.; Le Neindre, P.; Orgeur, P.; Poindron, P. Measures of temperament are highly repeatable in merino sheep and some are related to maternal behaviour. Proc. Aust. Soc. Anim. Prod. 1994, 20, 247–250. [Google Scholar]
- Fogarty, N.M.; Ingham, V.M.; Gilmour, A.R.; Afolayan, R.A.; Cummins, L.J.; Edwards, J.E.H.; Gaunt, G.M. Genetic evaluation of crossbred lamb production. 5. Age of puberty and lambing performance of yearling crossbred ewes. Aust. J. Agric. Res. 2007, 58, 928–934. [Google Scholar] [CrossRef]
- Ch’ang, T.; Rae, A. The genetic basis of growth, reproduction, and maternal environment in Romney ewes. Aust. J. Agric. Res. 1970, 21, 115–129. [Google Scholar] [CrossRef]
- Ch’Ang, T.; Rae, A. The genetic basis of growth, reproduction, and maternal environment in Romney ewes. II.* Genetic covariation between hogget characters, fertility, and maternal environment of the ewe. Aust. J. Agric. Res. 1972, 23, 149–165. [Google Scholar] [CrossRef]
- Alkass, J.E.; Aziz, D.A.; Al-Nidawi, K.A. Genetic aspects of puberty in Awassi ewes. Small Rumin. Res. 1994, 14, 249–252. [Google Scholar] [CrossRef]
- Baker, R.; Morris, C. Selection for early puberty and increased fertility at first mating. In Proceedings of the 2nd World Congress on Genetics Applied to Livestock Production, Madrid, Spain, 4–8 October 1982; pp. 282–293. [Google Scholar]
- Meyer, H.H.; Bigham, M.L.; Baker, R.L.; Harvey, T.G.; Hickey, S.M. Effects of Booroola Merino breeding and the FecB gene on performance of crosses with longwool breeds. 1. Effects on growth, onset of puberty, wool production and wool traits. Livest. Prod. Sci. 1994, 39, 183–190. [Google Scholar] [CrossRef]
- Fossceco, S.L.; Notter, D.R. Heritabilities and genetic correlations of body weight, testis growth and ewe lamb reproductive traits in crossbred sheep. Anim. Sci. 1995, 60, 185–195. [Google Scholar] [CrossRef]
- Fogarty, N.; Brash, L.; Gilmour, A. Genetic parameters for reproduction and lamb production and their components and liveweight, fat depth and wool production in Hyfer sheep. Aust. J. Agric. Res. 1994, 45, 443–457. [Google Scholar] [CrossRef]
- Newton, J.E.; Brown, D.J.; Dominik, S.; van der Werf, J.H.J. Genetic and phenotypic parameters between yearling, hoggetand adult reproductive performance and age of first oestrus in sheep. Anim. Prod. Sci. 2014, 54, 753–761. [Google Scholar] [CrossRef]
- Rosales Nieto, C.A.; Ferguson, M.B.; Macleay, C.A.; Briegel, J.R.; Martin, G.B.; Thompson, A.N. Selection for superior growth advances the onset of puberty and increases reproductive performance in ewe lambs. Anim. Int. J. Anim. Biosci. 2013, 7, 990–997. [Google Scholar] [CrossRef]
- Rosales Nieto, C.A.; Ferguson, M.B.; Macleay, C.A.; Briegel, J.R.; Wood, D.A.; Martin, G.B.; Thompson, A.N. Ewe lambs with higher breeding values for growth achieve higher reproductive performance when mated at age 8 months. Theriogenology 2013, 80, 427–435. [Google Scholar] [CrossRef]
- Thompson, A.; Bairstow, C.; Ferguson, M.; Kearney, G.; Macleay, C.; Thompson, H.; Paganoni, B. Growth pattern to the end of the mating period influences the reproductive performance of merino ewe lambs mated at 7 to 8 months of age. Small Rumin. Res. 2019, 179, 1–6. [Google Scholar] [CrossRef]
- Rosales Nieto, C.A.; Ferguson, M.B.; Macleay, C.A.; Briegel, J.R.; Wood, D.A.; Martin, G.B.; Bencini, R.; Thompson, A.N. Milk production and composition, and progeny performance in young ewes with high merit for rapid growth and muscle and fat accumulation. Animal 2018, 12, 2292–2299. [Google Scholar] [CrossRef]
- Newton, J.E.; Brown, D.J.; Dominik, S.; van der Werf, J.H.J. Impact of young ewe fertility rate on risk and genetic gain in sheep-breeding programs using genomic selection. Anim. Prod. Sci. 2017, 57, 1653–1664. [Google Scholar] [CrossRef]
- Moore, R.W.; McMillan, W.H.; Dockrill, G.; Dow, B.W. Lambing of Romney and Booroola cross hoggets with and without the F gene under different pasture allowances. N. Z. Soc. Anim. Prod. 1989, 49, 281–284. [Google Scholar]
- Kenyon, P.; Blair, H. Foetal programming in sheep–effects on production. Small Rumin. Res. 2014, 118, 16–30. [Google Scholar] [CrossRef]
- Walsh, S.W.; Mossa, F.; Butler, S.T.; Berry, D.P.; Scheetz, D.; Jimenez-Krassel, F.; Tempelman, R.J.; Carter, F.; Lonergan, P.; Evans, A.C.O.; et al. Heritability and impact of environmental effects during pregnancy on antral follicle count in cattle. J. Dairy Sci. 2014, 97, 4503–4511. [Google Scholar] [CrossRef]
- Bollwein, H.; Kawashima, C.; Shimizu, T.; Miyamoto, A.; Kaske, M. Impact of metabolism and production diseases on reproductive function in dairy cows. In Reproduction in Domestic Ruminants VIII: Proceedings of the Ninth International Symposium on Reproduction in Domestic Ruminants; Juengel, J.L., Miyamoto, A., Price, C., Reynolds, L.P., Smith, M.F., Webb, R., Eds.; Nottingham University Press: Chicago, IL, USA, 2019. [Google Scholar]
- Dýrmundsson, Ø.R. Studies on the breeding season of Icelandic ewes and ewe lambs. J. Agric. Sci. 1978, 90, 275–281. [Google Scholar] [CrossRef]
- Fitzgerald, J.; Butler, W.R. Seasonal Effects and Hormonal Patterns Related to Puberty in Ewe Lambs. Biol. Reprod. 1982, 27, 853–863. [Google Scholar] [CrossRef]
- Foster, D.; Yellon, S.; Olster, D.H. Internal and external determinants of the timing of puberty in the female. Reproduction 1985, 75, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Rosales Nieto, C.A.; Ferguson, M.B.; Briegel, J.R.; Hedger, M.P.; Martin, G.B.; Thompson, A.N. Pre-pubertal growth, muscle and fat accumulation in male and female sheep—Relationships with metabolic hormone concentrations, timing of puberty and reproductive outcomes. Reprod. Domest. Anim. 2019, 54, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.J.; Juengel, J.L.; O’Connell, A.R.; Johnstone, P.D.; Farquhar, P.A.; Davis, G.H. Attainment of puberty by ewes in the first year of life is associated with improved reproductive performance at 2 years of age. Small Rumin. Res. 2015, 123, 118–123. [Google Scholar] [CrossRef]
- Wall, A.; Juengel, J.; Edwards, S.; Rendel, J. The economic value of replacement breeding ewes attaining puberty within their first year of life on New Zealand sheep farms. Agric. Syst. 2018, 164, 38–46. [Google Scholar] [CrossRef]
- Quirke, J.F. Regulation of puberty and reproduction in female lambs: A review. Livest. Prod. Sci. 1981, 8, 37–53. [Google Scholar]
- Thompson, A.N.; Bowen, E.; Keiller, J.; Pegler, D.; Kearney, G.; Rosales-Nieto, C.A. The Number of Offspring Weaned from Ewe Lambs Is Affected Differently by Liveweight and Age at Breeding. Animals 2021, 11, 2733. [Google Scholar] [CrossRef]
- Bunge, R.; Thomas, D.L.; Nash, T.G. Performance of hair breeds and prolific wool breeds of sheep in southern Illinois: Lamb production of F1 ewe lambs. J. Anim. Sci. 1993, 71, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Knights, M.; Baptiste, Q.S.; Lewis, P.E. Ability of ram introduction to induce LH secretion, estrus and ovulation in fall-born ewe lambs during anestrus. Anim. Reprod. Sci. 2002, 69, 199–209. [Google Scholar] [CrossRef]
- Kenyon, P.R.; Vinoles, C.; Morris, S.T. Effect of teasing by the ram on the onset of puberty in Romney ewe lambs. N. Z. J. Agric. Res. 2012, 55, 283–291. [Google Scholar] [CrossRef]
- Dýrmundsson, Ó.R.; Lees, J.L. Effect of rams on the onset of breeding activity in Clun Forest ewe lambs. J. Agric. Sci. 1972, 79, 269–271. [Google Scholar] [CrossRef]
- Cave, L.M.; Kenyon, P.R.; Morris, S.T. Effect of timing of exposure to vasectomised rams and ewe lamb body condition score on the breeding performance of ewe lambs. Anim. Prod. Sci. 2012, 52, 471–477. [Google Scholar] [CrossRef]
- Kenyon, P.R.; Morel, P.C.H.; Morris, S.T.; Burnham, D.L.; West, D.M. Effect of the ratio of teaser rams used prior to breeding on the reproductive performance of ewe hoggets. N. Z. Vet. J. 2007, 55, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, P.R.; Morel, P.C.H.; Morris, S.T.; Burnham, D.L.; West, D.M. The effect of length of use of teaser rams prior to mating and individual liveweight on the reproductive performance of ewe hoggets. N. Z. Vet. J. 2006, 54, 91–95. [Google Scholar] [CrossRef]
- Kenyon, P.R.; Morris, S.T.; West, D.M. Can Romney ram lambs whose scrotums had been shortened by the use of a rubber ring be used as an alternative to vasectomised Perendale rams for inducing early breeding activity in Romney ewe lambs? N. Z. Vet. J. 2008, 56, 326–329. [Google Scholar] [CrossRef]
- Corner-Thomas, R.A.; Hickson, R.E.; Morris, S.T.; Kenyon, P.R. The influences of live weight and body condition score of ewe lambs from breeding to lambing on the live weight of their singleton lambs to weaning. Small Rumin. Res. 2014, 119, 16–21. [Google Scholar] [CrossRef]
- Haslin, E.; Corner-Thomas, R.A.; Kenyon, P.R.; Pettigrew, E.J.; Hickson, R.E.; Morris, S.T.; Blair, H.T. Effect of Breeding Heavier Romney Ewe Lambs at Seven Months of Age on Lamb Production and Efficiency over Their First Three Breeding Seasons. Animals 2022, 11, 3486. [Google Scholar] [CrossRef]
- Kenyon, P.R.; Maloney, S.K.; Blache, D. Review of sheep body condition in relation to production characteristics. N. Z. J. Agric. Res. 2014, 57, 38–64. [Google Scholar] [CrossRef]
- Owens, F.N.; Dubeski, P.; Hanson, C.F. Factors that alter the growth and development of ruminants. J. Anim. Sci. 1993, 71, 3138–3150. [Google Scholar] [CrossRef] [PubMed]
- Rosales Nieto, C.; Ferguson, M.; Thompson, H.; Briegel, J.; Macleay, C.; Martin, G.; Thompson, A. Relationships among Puberty, Muscle and Fat, and Liveweight Gain during Mating in Young Female Sheep. Reprod. Domest. Anim. 2015, 50, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, P.R. Hogget Performance: Unlocking the Potential; Beef & Lamb New Zealand: Auckland, New Zealand, 2012; p. 50. [Google Scholar]
- Kenyon, P.R.; Corner-Thomas, R.A.; Paganoni, B.L.; Morris, S.T. Percentage of Mature Liveweight Affects Reproductive Performance in Ewe Lambs. Proc. Aust. Soc. Anim. Prod. 2014, 30, 255. [Google Scholar]
- Evans, A.C.O. Ovarian follicle growth and consequences for fertility in sheep. Anim. Reprod. Sci. 2003, 78, 289–306. [Google Scholar] [CrossRef]
- Bichard, M.; Younis, A.A.; Forrest, P.A.; Cumberla, P. Analysis of production records from a lowland sheep flock. 4. Factors influencing incidence of successful pregnancy in young females. Anim. Prod. 1974, 19, 177–191. [Google Scholar]
- Edwards, S.J.; Smaill, B.; O’Connell, A.R.; Johnstone, P.D.; Stevens, D.R.; Quirke, L.D.; Farquhar, P.A.; Juengel, J.L. Reduced ovulation rate, failure to be mated and fertilization failure/embryo loss are the underlying causes of poor reproductive performance in juvenile ewes. Anim. Reprod. Sci. 2016, 167, 125–132. [Google Scholar] [CrossRef]
- Corner-Thomas, R.A.; Panns, J.M.; Kemp, P.D.; Morris, S.T.; Kenyon, P.R. The effect of grazing ewe lambs on lucerne (Medicago sativa) prior to breeding on aspects of reproductive performance. Proc. N. Z. Soc. Anim. Prod. 2017, 77, 55–60. [Google Scholar]
- Mulvaney, F.J.; Morris, S.T.; Kenyon, P.R.; West, D.M.; Morel, P.C.H. Effect of liveweight at the start of the breeding period and liveweight gain during the breeding period and pregnancy on reproductive performance of hoggets and the liveweight of their lambs. N. Z. J. Agric. Res. 2010, 53, 355–364. [Google Scholar] [CrossRef]
- Dyrmundsson, O.R.; Lees, J.L. Effect of autumn shearing on breeding activity in Clun Forest ewe lambs. J. Agric. Sci. 1972, 79, 431–433. [Google Scholar] [CrossRef]
- Dwyer, C. Reproductive management (including impacts of prenatal stress on offspring development). In Advances in Sheep Welfare; Ferguson, D.M., Lee, C., Fisher, A., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 131–152. [Google Scholar]
- Kenyon, P.R.; Morel, P.C.H.; Morris, S.T.; West, D.M. Effect of the age of rams on reproductive performance of ewe hoggets. N. Z. Vet. J. 2007, 55, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, P.R.; Smith, S.L.; Morel, P.C.H.; Morris, S.T.; West, D.M. The effect of the maturity and prior breeding activity of rams and body condition score of ewe hoggets on the reproductive performance of ewe hoggets. N. Z. Vet. J. 2009, 57, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.M.; Aitken, R.P.; Cheyne, M.A. Nutrient partitioning and fetal growth in rapidly growing adolescent ewes. J. Reprod. Fertil. 1996, 107, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.M. Nutrient partitioning during pregnancy: Adverse gestational outcome in overnourished adolescent dams. Proc. Nutr. Soc. 2000, 59, 107–117. [Google Scholar] [CrossRef]
- Wallace, J.M.; Milne, J.S.; Aitken, R.P. Effect of Weight and Adiposity at Conception and Wide Variations in Gestational Dietary Intake on Pregnancy Outcome and Early Postnatal Performance in Young Adolescent Sheep1. Biol. Reprod. 2010, 82, 320–330. [Google Scholar] [CrossRef]
- Wallace, J.M.; Aitken, R.P.; Milne, J.S.; Hay, W.W. Nutritionally mediated placental growth restriction in the growing adolescent: Consequences for the fetus. Biol. Reprod. 2004, 71, 1055–1062. [Google Scholar] [CrossRef]
- Wallace, J.M.; Bourke, D.A.; Aitken, R.P.; Milne, J.S.; Hay, W.W. Placental glucose transport in growth-restricted pregnancies induced by overnourishing adolescent sheep. J. Physiol. Lond. 2003, 547, 85–94. [Google Scholar] [CrossRef]
- Wallace, J.M. Competition for nutrients in pregnant adolescents: Consequences for maternal, conceptus and offspring endocrine systems. J. Endocrinol. 2019, 242, T1–T19. [Google Scholar] [CrossRef]
- Kenyon, P.R.; Morris, S.T.; Burnham, D.L.; West, D.M. Effect of nutrition during pregnancy on hogget pregnancy outcome and birthweight and liveweight of lambs. N. Z. J. Agric. Res. 2008, 51, 77–83. [Google Scholar] [CrossRef]
- Morris, S.T.; Kenyon, P.R.; West, D.M. Effect of hogget nutrition in pregnancy on lamb birthweight and survival to weaning. N. Z. J. Agric. Res. 2005, 48, 165–175. [Google Scholar] [CrossRef][Green Version]
- Mulvaney, F.; Morris, S.; Kenyon, P.; West, D.; Morel, P. Effect of nutrition around the time of breeding and during pregnancy on yearling liveweight change, pregnancy loss and live weight and survival of their offspring. Proc. N. Z. Soc. Anim. Prod. 2010, 70, 91–95. [Google Scholar]
- Clune, T.; Lockwood, A.; Hancock, S.; Thompson, A.N.; Beetson, S.; Campbell, A.J.D.; Glanville, E.; Brookes, D.; Trengove, C.; O’Handley, R.; et al. Abortion and Lamb Mortality between Pregnancy Scanning and Lamb Marking for Maiden Ewes in Southern Australia. Animals 2022, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, F.J.; Kenyon, P.R.; Morris, S.T.; West, D.M. Ewe lamb nutrition during pregnancy affects pregnancy outcome. Aust. J. Exp. Agric. 2008, 48, 1085–1089. [Google Scholar] [CrossRef]
- Swanson, T.J.; Hammer, C.J.; Luther, J.S.; Carlson, D.B.; Taylor, J.B.; Redmer, D.A.; Neville, T.L.; Reed, J.J.; Reynolds, L.P.; Caton, J.S.; et al. Effects of gestational plane of nutrition and selenium supplementation on mammary development and colostrum quality in pregnant ewe lambs. J. Anim. Sci. 2008, 86, 2415–2423. [Google Scholar] [CrossRef]
- Ridler, A.L.; Vallee, E.; Corner, R.A.; Kenyon, P.R.; Heuer, C. Factors associated with fetal losses in ewe lambs on a New Zealand sheep farm. N. Z. Vet. J. 2015, 63, 330–334. [Google Scholar] [CrossRef]
- Ridler, A.; Corner-Thomas, R.; Kenyon, P.; Griffiths, K. Investigation of fetal loss in ewe lambs in relation to liveweight changes and progesterone concentrations in early to mid gestation. N. Z. Vet. J. 2017, 65, 34–38. [Google Scholar] [CrossRef]
- Griffiths, K.J.; Ridler, A.L.; Heuer, C.; Corner-Thomas, R.A.; Kenyon, P.R. The effect of liveweight and body condition score on the ability of ewe lambs to successfully rear their offspring. Small Rumin. Res. 2016, 145, 130–135. [Google Scholar] [CrossRef]
- Mulvaney, F.J.; Morris, S.T.; Kenyon, P.R.; Morel, P.C.H.; West, D.M. Effect of nutrition from mid-pregnancy to parturition on the live weight of twin-bearing hoggets and the live weight and survival of their lambs. N. Z. J. Agric. Res. 2012, 55, 385–392. [Google Scholar] [CrossRef][Green Version]
- Schreurs, N.M.; Kenyon, P.R.; Mulvaney, F.J.; Morel, P.C.H.; West, D.M.; Morris, S.T. Response of additional ewe lamb liveweight during gestation on birth and weaning weight of offspring and liveweight of the ewe lamb at weaning. Anim. Prod. Sci. 2010, 50, 528–532. [Google Scholar] [CrossRef]
- West, D.M.; Pomroy, W.E.; Collett, M.G.; Hill, F.I.; Ridler, A.L.; Kenyon, P.R.; Morris, S.T.; Pattison, R.S. A possible role for Neospora caninum in ovine abortion in New Zealand. Small Rumin. Res. 2006, 62, 135–138. [Google Scholar] [CrossRef]
- Howe, L.; West, D.M.; Collett, M.G.; Tattersfield, G.; Pattison, R.S.; Pomroy, W.E.; Kenyon, P.R.; Morris, S.T.; Williamson, N.B. The role of Neospora caninum in three cases of unexplained ewe abortions in the southern North Island of New Zealand. Small Rumin. Res. 2008, 75, 115–122. [Google Scholar] [CrossRef]
- Mulvaney, F.J.; Morris, S.T.; Kenyon, P.R.; Morel, P.C.H.; West, D.M. Effect of nutrition pre-breeding and during pregnancy on breeding performance of ewe lambs. Anim. Prod. Sci. 2010, 50, 953–960. [Google Scholar] [CrossRef]
- Clune, T.; Lockwood, A.; Hancock, S.; Thompson, A.; Beetson, S.; Campbell, A. On-farm investigation of foetal and lamb losses in maiden ewes. In Proceedings of the Sheep, Camelid and Goat Veterinarians Conference (Australian Veternary Association), Onilne, 5 October 2021; pp. 18–26. [Google Scholar]
- Ridler, A. Fetal Loss in Maiden Ewes—An Update; Proceedings of the Society of Sheep and Beef Veterinarians of the NZVA and Cervetec Conference, 309; Sheep and Beef Cattle Veterinarians of the New Zealand Veterinary Association: Wellington, New Zealand; pp. 169–174.
- West, D. Ovine abortion in New Zealand. N. Z. Vet. J. 2002, 50, 93–95. [Google Scholar] [CrossRef]
- Kenyon, P.R.; Morris, S.T.; Revell, D.K.; McCutcheon, S.N. Shearing during pregnancy—Review of a policy to increase birthweight and survival of lambs in New Zealand pastoral farming systems. N. Z. Vet. J. 2003, 51, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, P.R.; Sherlock, R.G.; Morris, S.T.; Morel, P.C.H. The effect of mid- and late-pregnancy shearing of hoggets on lamb birthweight, weaning weight, survival rate, and wool follicle and fibre characteristics. Aust. J. Agric. Res. 2006, 57, 877–882. [Google Scholar] [CrossRef]
- Mulvaney, F.J.; Morris, S.T.; Kenyon, P.R.; Morel, P.C.H.; West, D.M.; Vinoles, C.; Glover, K.M.M. Comparison between the reproductive performance of ewe hoggets and mature ewes following a progesterone based oestrus synchronization protocol. N. Z. J. Agric. Res. 2013, 56, 288–296. [Google Scholar] [CrossRef][Green Version]
- Ridler, A.L.; Flay, K.J.; Kenyon, P.R.; Blair, H.T.; Corner-Thomas, R.A.; Pettigrew, E.J. Factors Associated with Mortality of Lambs Born to Ewe Hoggets. Animals 2022, 12, 319. [Google Scholar] [CrossRef]
- Hinch, G.N.; Brien, F. Lamb survival in Australian flocks: A review. Anim. Prod. Sci. 2014, 54, 656–666. [Google Scholar] [CrossRef]
- McMillan, W.H. Hogget lamb mortality. Proc. N. Z. Soc. Anim. Prod. 1983, 43, 33–36. [Google Scholar] [CrossRef]
- Stevens, D.R. On-farm ewe lamb mating outcomes from feeding practices before mating and during pregnancy. Proc. N. Z. Soc. Anim. Prod. 2010, 70, 113–117. [Google Scholar]
- Jacobson, C.; Bruce, M.; Kenyon, P.R.; Lockwood, A.; Miller, D.; Refshauge, G.; Masters, D.G. A review of dystocia in sheep. Small Rumin. Res. 2020, 192, 106209. [Google Scholar] [CrossRef]
- Dwyer, C.M.; Conington, J.; Corbiere, F.; Holmøy, I.H.; Muri, K.; Nowak, R.; Rooke, J.; Vipond, J.; Gautier, J.M. Invited review: Improving neonatal survival in small ruminants: Science into practice. Animal 2016, 10, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Poindron, P. From birth to colostrum: Early steps leading to lamb survival. Reprod. Nutr. Dev. 2006, 46, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Corner-Thomas, R.A.; Cranston, L.M.; Kemp, P.D.; Morris, S.T.; Kenyon, P.R. The influence of three herbage types on the liveweight change of twin-bearing hoggets and their lambs. N. Z. J. Agric. Res. 2018, 63, 365–378. [Google Scholar] [CrossRef]
- Corner-Thomas, R.A.; Kemp, P.D.; Morris, S.T.; Kenyon, P.R. Grazing alternative herbages in lactation increases the liveweight of both ewe lambs and their progeny at weaning. Anim. Prod. Sci. 2014, 54, 1741–1746. [Google Scholar] [CrossRef]
- Mulvaney, F.; Morris, S.; Kenyon, P.; West, D.; Morel, P. The effect of weaning at 10 or 14 weeks of age on liveweight changes in the hogget and her lambs. Proc. N. Z. Soc. Anim. Prod. 2009, 69, 68–70. [Google Scholar]
- Thomson, B.C.; Smith, N.B.; Muir, P.D. Effect of birth rank and age at first lambing on lifetime performance and ewe efficiency. N. Z. J. Agric. Res. 2021, 64, 529–539. [Google Scholar] [CrossRef]
- Tyrrell, R.N. Some effects of pregnancy in 8-month-old Merino ewes. Aust. J. Exp. Agric. 1976, 16, 458–461. [Google Scholar]
- McCall, D.G.; Hight, G.K. Environmental influences on hogget lambing performance and the relationship between hogget and two-tooth lambing performance. N. Z. J. Agric. Res. 1981, 24, 145–152. [Google Scholar] [CrossRef]
- Flay, K.J.; Ridler, A.L.; Compton, C.W.R.; Kenyon, P.R. Ewe Wastage in New Zealand Commercial Flocks: Extent, Timing, Association with Hogget Reproductive Outcomes and BCS. Animals 2021, 11, 779. [Google Scholar] [CrossRef]
- Kenyon, P.R.; van der Linden, D.S.; West, D.M.; Morris, S.T. The effect of breeding hoggets on lifetime performance. N. Z. J. Agric. Res. 2011, 54, 321–330. [Google Scholar] [CrossRef]
- Haslin, E.; Corner-Thomas, R.A.; Kenyon, P.R.; Pettigrew, E.J.; Hickson, R.E.; Morris, S.T.; Blair, H.T. Effects of heavier live weight of ewe lambs at mating on fertility, lambing percentage, subsequent live weight and the performance of their progeny. N. Z. J. Agric. Res. 2022, 65, 114–128. [Google Scholar] [CrossRef]
- Haslin, E.; Corner-Thomas, R.A.; Kenyon, P.R.; Pettigrew, E.J.; Hickson, R.E.; Morris, S.T.; Blair, H.T. Breeding heavier ewe lambs at seven months of age did not impact their subsequent two and three-year-old ewe live weight and reproductive performance. N. Z. J. Agric. Res. 2022, 65, 129–144. [Google Scholar] [CrossRef]
- Craig, R.L. Breeding from Romney ewe hoggets in the Waihora group breeding scheme. N. Z. J. Agric. Sci. 1982, 16, 101–104. [Google Scholar]
- Loureiro, M.F.P.; Pain, S.J.; Kenyon, P.R.; Blair, H.T. Do fetuses from primiparous one-year-old ewes differ from those of multiparous mature ewes? Proc. N. Z. Soc. Anim. Prod. 2010, 70, 118–120. [Google Scholar]
- Loureiro, M.F.P.; Pain, S.J.; Kenyon, P.R.; Peterson, S.W.; Blair, H.T. Single female offspring born to primiparous ewe-lambs are lighter than those born to adult multiparous ewes but their reproduction and milk production are unaffected. Anim. Prod. Sci. 2012, 52, 552–556. [Google Scholar] [CrossRef]
- Pettigrew, E.; Hickson, R.; Morris, S.; Lopez-Villalobos, N.; Pain, S.; Kenyon, P.; Blair, H. The effects of birth rank (single or twin) and dam age on the lifetime productive performance of female dual purpose sheep (Ovis aries) offspring in New Zealand. PLoS ONE 2019, 14, e0214021. [Google Scholar] [CrossRef]
- Pettigrew, E.; Hickson, R.; Morris, S.; Kenyon, P.; Corner-Thomas, R.; Haslin, E.; Blair, H. The Effect of Age of Dam and Birth Rank on the Reproductive Performance of Ewes as One- and Two-Year-Olds. Animals 2021, 11, 770. [Google Scholar] [CrossRef]

| Trait | General Comments in Comparison with Mature Ewes | References |
|---|---|---|
| Onset of breeding activity within breeding season | Later due the need to achieve puberty, which is driven by achieving appropriate live weight prior to cyclic activity | [14,21,22,23,24] |
| Length of breeding season | Shorter due to delayed onset of start of breeding activity within the breeding season window | [14,23,25,26,27,28,29] |
| Regularity of estrus length | More likely to be irregular | [26] |
| Length of estrus period and chance of silent estrus | Shorter estrus period. More likely to have estrus without ovulation or ovulation without estrus. | [30,31,32] |
| Mating behavior | Less likely to seek and stand appropriately for the ram. It has also been suggested that rams prefer mature ewes. | [22,33,34] |
| Suggested ram-to-ewe ratio | A lower ram-to-ewe ratio required, at least half | [18,22,35,36,37] |
| Ovulation rate | Lower | [11,34,38,39,40,41,42] |
| Ovum/ova quality | Lower | [31,38,43] |
| Conception rate | Lower due to the combined effects of the above factors | [16,19,22,44,45,46] |
| Early pregnancy loss | Higher due to lower embryo quality and impaired uterine environment | [11,39,40,42,47] |
| Pregnancy rate (ewes pregnant/ewes bred) | Lower due to the combined effects of the above factors | [16,45,46,48,49] |
| Number of lambs born per ewe bred | Lower due to the combined effects of the above factors | [11,16,19,41,45,46,50,51] |
| Gestation length | Shorter | [52,53] |
| Lamb birth weight | Lower | [19,20,45,51,54,55] |
| Colostrum production | Lower and composition differs | [51,56,57,58,59,60] |
| Milk production | Lower | [61,62,63,64] |
| Milk composition | Lower fat and protein percentage | [62,63] |
| Mothering ability | Some studies suggest poorer but not all | [50,65,66] |
| Lamb vigor at birth | Lower | [20] |
| Lamb survival | Lower due to above factors from birth | [16,20,42,45,65] |
| Lamb growth to weaning and weaning weight | Lower due to above factors from birth | [20,42,45,54,65] |
| Lambs weaned per ewe presented for breeding | Lower due to the combined effects of the above factors | [14,46,48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenyon, P.R.; Corner-Thomas, R.A. Breeding Ewe Lambs: An Australasian Perspective. Animals 2022, 12, 3207. https://doi.org/10.3390/ani12223207
Kenyon PR, Corner-Thomas RA. Breeding Ewe Lambs: An Australasian Perspective. Animals. 2022; 12(22):3207. https://doi.org/10.3390/ani12223207
Chicago/Turabian StyleKenyon, Paul R., and Rene A. Corner-Thomas. 2022. "Breeding Ewe Lambs: An Australasian Perspective" Animals 12, no. 22: 3207. https://doi.org/10.3390/ani12223207
APA StyleKenyon, P. R., & Corner-Thomas, R. A. (2022). Breeding Ewe Lambs: An Australasian Perspective. Animals, 12(22), 3207. https://doi.org/10.3390/ani12223207






