Ventilator-Assisted Inspiratory and Expiratory Breath-Hold Thoracic Computed Tomographic Scans Can Detect Dynamic and Static Airway Collapse in Dogs with Limited Agreement with Tracheobronchoscopy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
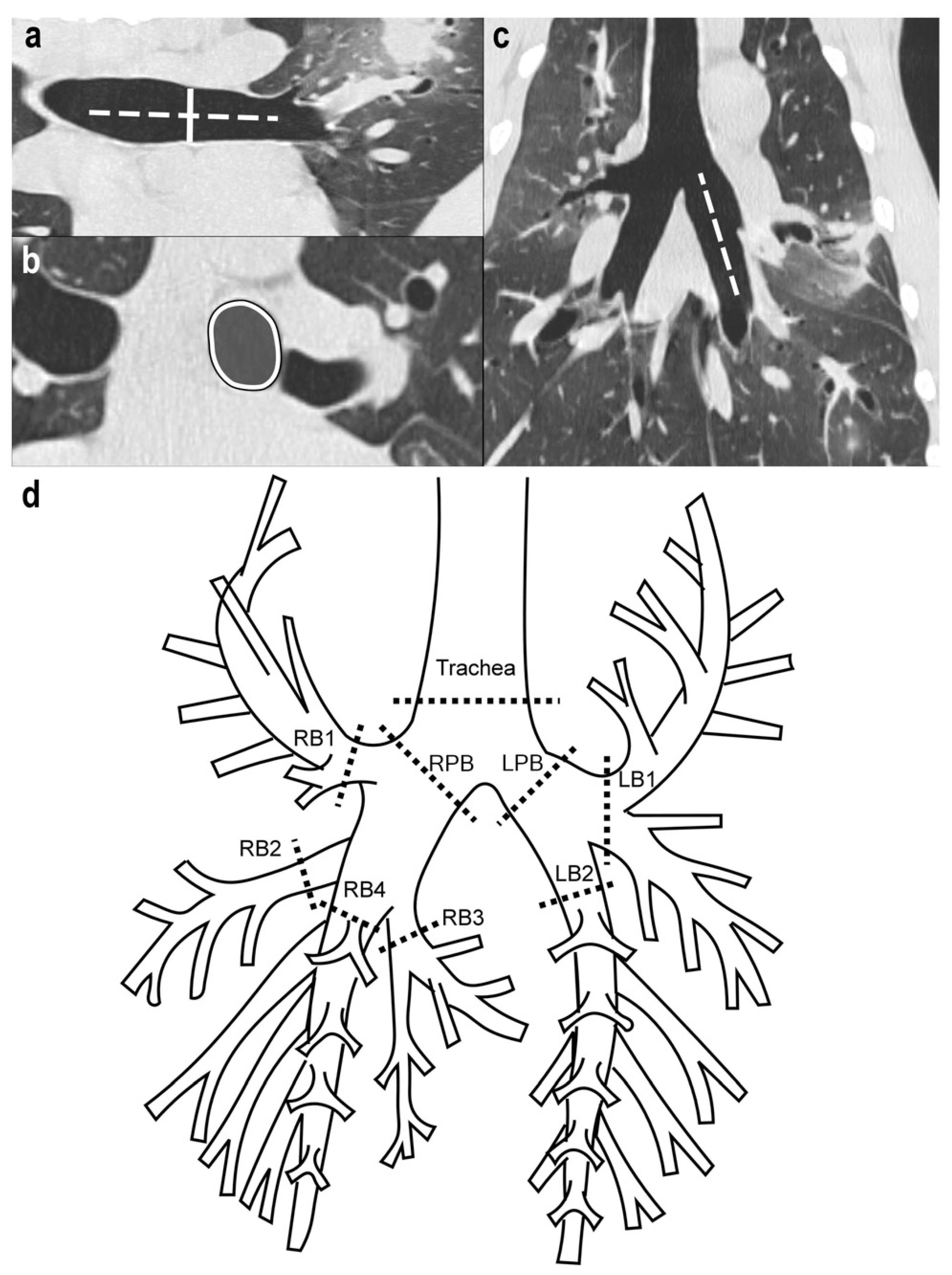
2.2. CT Imaging Protocol
2.3. CT Metrics of AC
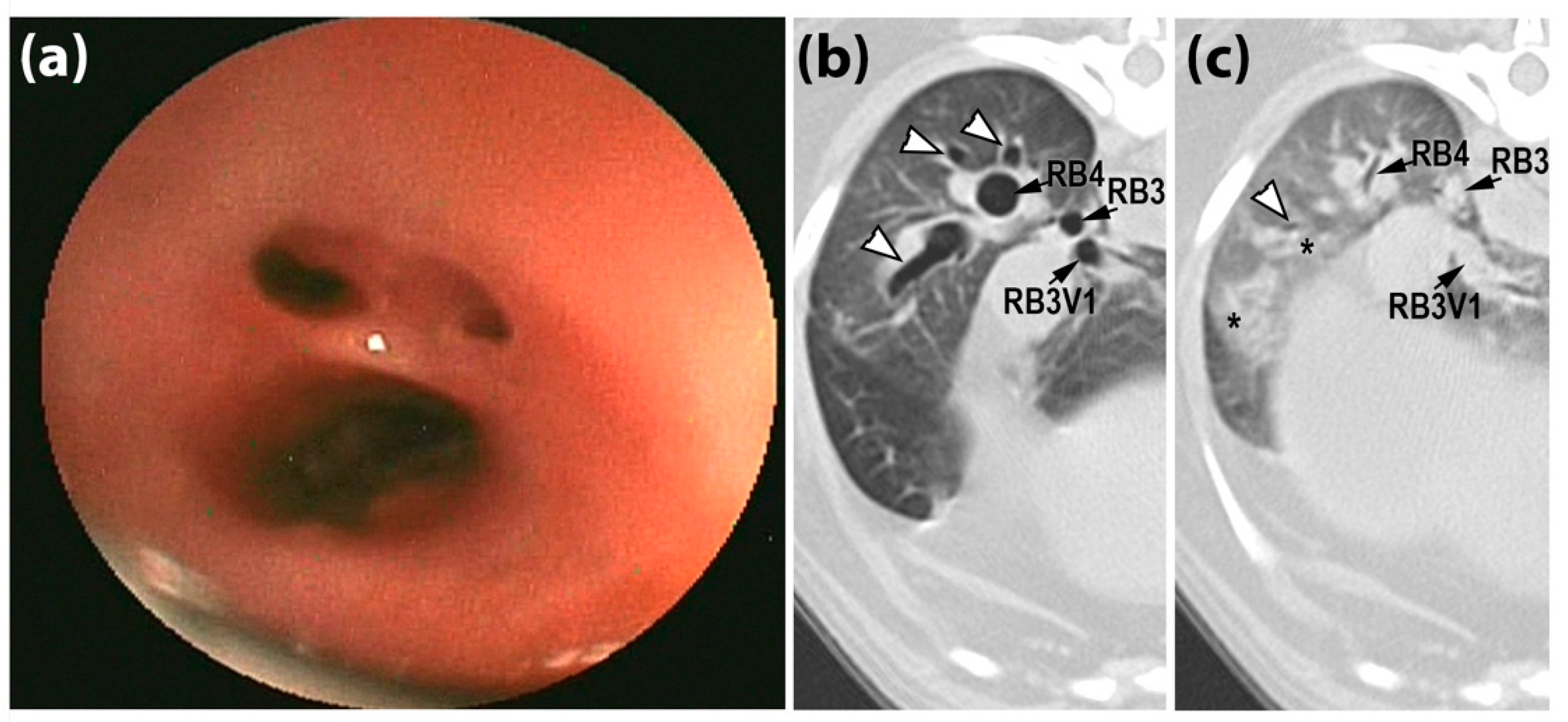
2.4. Tracheobronchoscopic Grading
2.5. Statistical Analyses
3. Results
3.1. Animals
3.2. Dynamic Airway Wall Narrowing from I/E-BH CT Scans
3.3. Comparison of CT to Tracheobronchoscopy Score of AC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reinero, C.R.; Masseau, I. Lower airway collapse: Revisiting the definition and clinicopathologic features of canine bronchomalacia. Veter J. 2021, 273, 105682. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yun, S.; Lee, I.; Choi, M.; Yoon, J. Fluoroscopic characteristics of tracheal collapse and cervical lung herniation in dogs: 222 cases (2012–2015). J. Vet. Sci. 2017, 18, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Gamracy, J.; Wiggen, K.; Vientós-Plotts, A.; Reinero, C. Clinicopathologic features, comorbid diseases, and prevalence of pulmonary hypertension in dogs with bronchomalacia. J. Vet. Intern. Med. 2022, 36, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Tappin, S.W. Canine tracheal collapse. J. Small Anim. Pract. 2016, 57, 9–17. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzi, D.; Bertoncello, D.; Drigo, M. Bronchial abnormalities found in a consecutive series of 40 brachycephalic dogs. J. Am. Vet. Med. Assoc. 2009, 235, 835–840. [Google Scholar] [CrossRef]
- Bottero, E.; Bellino, C.; De Lorenzi, D.; Ruggiero, P.; Tarducci, A.; D’Angelo, A.; Gianella, P. Clinical Evaluation and Endoscopic Classification of Bronchomalacia in Dogs. J. Veter Intern. Med. 2013, 27, 840–846. [Google Scholar] [CrossRef]
- Hall, E.L.; Baines, E.A.; Baines, S. Atypical lateral tracheal collapse in a Yorkshire terrier. J. Small Anim. Pract. 2020, 61, 644–647. [Google Scholar] [CrossRef]
- Della Maggiore, A. An Update on Tracheal and Airway Collapse in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 419–430. [Google Scholar] [CrossRef]
- Herth, F.J.; Kirby, M.; Sieren, J.; Herth, J.; Schirm, J.; Wood, S.; Schuhmann, M. The Modern Art of Reading Computed Tomography Images of the Lungs: Quantitative CT. Respiration 2018, 95, 8–17. [Google Scholar] [CrossRef]
- Johnson, L.; Singh, M.; Pollard, R. Agreement Among Radiographs, Fluoroscopy and Bronchoscopy in Documentation of Airway Collapse in Dogs. J. Vet. Intern. Med. 2015, 29, 1619–1626. [Google Scholar] [CrossRef]
- Oh, D.; Lee, S.; Kim, S.; Choen, S.; Choi, M.; Yoon, J. Computed tomographic bronchial collapsibility values over 50% may be detected in healthy dogs. Vet. Radiol. Ultrasound 2019, 60, 28–37. [Google Scholar] [CrossRef]
- Della Maggiore, A. Tracheal and Airway Collapse in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Murgu, S.; Colt, H. Tracheobronchomalacia and Excessive Dynamic Airway Collapse. Clin. Chest Med. 2013, 34, 527–555. [Google Scholar] [CrossRef] [PubMed]
- Ngerncham, M.; Lee, E.Y.; Zurakowski, D.; Tracy, D.A.; Jennings, R. Tracheobronchomalacia in pediatric patients with esophageal atresia: Comparison of diagnostic laryngoscopy/bronchoscopy and dynamic airway multidetector computed tomography. J. Pediatr. Surg. 2015, 50, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Ciet, P.; Boiselle, P.; Michaud, G.; O’Donnell, C.; Litmanovich, D. Optimal imaging protocol for measuring dynamic expiratory collapse of the central airways. Clin. Radiol. 2016, 71, e49–e55. [Google Scholar] [CrossRef]
- Lee, E.Y.; Boiselle, P.M. Tracheobronchomalacia in Infants and Children: Multidetector CT Evaluation. Radiology 2009, 252, 7–22. [Google Scholar] [CrossRef]
- Lee, E.Y.; Mason, K.P.; Zurakowski, D.; Waltz, D.A.; Ralph, A.; Riaz, F.; Boiselle, P.M. MDCT assessment of tracheomalacia in symptomatic infants with mediastinal aortic vascular anomalies: Preliminary technical experience. Pediatr. Radiol. 2008, 38, 82–88. [Google Scholar] [CrossRef]
- Bianco, Z.; Bukoski, A.; Masseau, I.; Reich, C.; Schultz, L.; Reinero, C. Risk Factors and Outcomes in Dogs with Respiratory Disease Undergoing Diagnostic Airway Lavage. Front. Vet. Sci. 2020, 7, 165. [Google Scholar] [CrossRef]
- Epstein, A. Effects of general anesthesia on respiratory system. Isr. J. Vet. Med. 2011, 66, 9–13. [Google Scholar]
- Carr, S.V.; Reinero, C.; Rishniw, M.; Pritchard, J.C. Specialists’ approach to tracheal collapse: Survey-based opinions on diagnostics, medical management, and comorbid diseases. J. Am. Vet. Med. Assoc. 2022, 1, 1–7. [Google Scholar] [CrossRef]
- Gilkeson, R.C.; Ciancibello, L.M.; Hejal, R.B.; Montenegro, H.D.; Lange, P. Tracheobronchomalacia. Am. J. Roentgenol. 2001, 176, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, M.; Minutoli, F.; Girbino, G.; Murabito, A.; Benedetto, C.; Contiguglia, R.; Ruggeri, P.; Privitera, S. Expiratory CT scan in patients with normal inspiratory CT scan: A finding of obliterative bronchiolitis and other causes of bronchiolar obstruction. Multidiscip. Respir. Med. 2013, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Jaffey, J.; Wiggen, K.; Leach, S.; Masseau, I.; Girens, R.; Reinero, C. Pulmonary hypertension secondary to respiratory disease and/or hypoxia in dogs: Clinical features, diagnostic testing and survival. Vet. J. 2019, 251, 105347. [Google Scholar] [CrossRef] [PubMed]
- Reinero, C.R.; Jutkowitz, L.A.; Nelson, N.; Masseau, I.; Jennings, S.; Williams, K. Clinical features of canine pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis. J. Vet. Intern. Med. 2019, 33, 114–123. [Google Scholar] [CrossRef]
- Amis, T.C.; McKiernan, B.C. Systematic identification of endobronchial anatomy during bronchoscopy in the dog. Am. J. Vet. Res. 1986, 47, 2649–2657. [Google Scholar]
- Boiselle, P.M.; O’Donnell, C.R.; Bankier, A.A.; Ernst, A.; Millet, M.E.; Potemkin, A.; Loring, S.H. Tracheal Collapsibility in Healthy Volunteers during Forced Expiration: Assessment with Multidetector CT. Radiology 2009, 252, 255–262. [Google Scholar] [CrossRef]
- Tangner, C.H.; Hobson, H.P. A Retrospective Study of 20 Surgically Managed Cases of Collapsed Trachea. Vet. Surg. 1982, 11, 146–149. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Hara, Y.; Teshima, K.; Yamaya, Y. Arterial blood gas analysis in dogs with bronchomalacia. PLoS ONE 2019, 14, e0227194. [Google Scholar] [CrossRef]
- Monnin, P.; Sfameni, N.; Gianoli, A.; Ding, S. Optimal slice thickness for object detection with longitudinal partial volume effects in computed tomography. J. Appl. Clin. Med. Phys. 2017, 18, 251–259. [Google Scholar] [CrossRef]
- Ullmann, N.; Secinaro, A.; Menchini, L.; Caggiano, S.; Verrillo, E.; Santangelo, T.P.; Cutrera, R.; Tomà, P. Dynamic expiratory CT: An effective non-invasive diagnostic exam for fragile children with suspected tracheo-bronchomalacia. Pediatr. Pulmonol. 2018, 53, 73–80. [Google Scholar] [CrossRef]
- Carden, K.A.; Boiselle, P.M.; Waltz, D.A.; Ernst, A. Tracheomalacia and Tracheobronchomalacia in Children and Adults: An in-depth review. Chest 2005, 127, 984–1005. [Google Scholar] [CrossRef]
- Lee, E.Y.; Boiselle, P.M.; Cleveland, R.H. Multidetector CT Evaluation of Congenital Lung Anomalies. Radiology 2008, 247, 632–648. [Google Scholar] [CrossRef]
- Boiselle, P.M.; Feller-Kopman, D.; Ashiku, S.; Weeks, D.; Ernst, A. Tracheobronchomalacia: Evolving role of dynamic multislice helical CT. Radiol. Clin. N. Am. 2003, 41, 627–636. [Google Scholar] [CrossRef]
- Wright, C.D. Tracheobronchomalacia and Expiratory Collapse of Central Airways. Thorac. Surg. Clin. 2018, 28, 163–166. [Google Scholar] [CrossRef]
| Dynamic Airway Narrowing Magnitude 1 | Score 2 |
|---|---|
| <10% | 0 |
| 10–25% | 1 |
| 26–50% | 2 |
| 51–75% | 3 |
| >75% | 4 |
| Dog | Age (years) | Sex | Weight (kg) | Breed | Clinical Diagnosis | Lobar Bronchial Collapse (Number of Affected Bronchi, Highest Score) 1 |
|---|---|---|---|---|---|---|
| Airway Collapse Group (AC) | ||||||
| 1 | 12.2 | FS | 12.3 | American Cocker Spaniel | Presumptive pulmonary carcinoma, BE, BM, CB | NA |
| 2 | 7.8 | MC | 8.6 | Mixed Breed | TC, MSBC, CB, bronchiolitis, BE | 5, 4 |
| 3 | 10 | MC | 8.3 | Brussels Griffon | TC, MSBC, CB, BE | 6, 3 |
| 4 | 8.7 | MC | 8.1 | Brussels Griffon | Dynamic upper airway obstruction (elongated soft palate), TC, BM | 5, 3 |
| 5 | 12.6 | MC | 3.6 | Chihuahua | TC, MSBC, BM, MVDD, PH, pulmonary fibrosis 2 | 4 (NA = 2), 4 |
| 6 | 16 | FS | 4.5 | Chihuahua | TC, MSBC, BE, BM, PTE 2 | 6, 2 |
| 7 | 11.5 | FS | 5.8 | Chinese Crested | CB, BE, TC, constrictive bronchiolitis obliterans with pulmonary fibrosis 2 | 5, 3 |
| 8 | 13.4 | MC | 6.5 | Dachshund | CB, BE, BM, suspect aspiration pneumonia | 1, 3 |
| 9 | 11 | MC | 6.0 | Dachshund | TC, MSBC, EB, BE, BM, MVDD | 2, 4 |
| 10 | 8.3 | MC | 11.7 | French Bulldog | Aerodigestive disease (megaesophagus, gastroesophageal reflux, and aspiration pneumonia), TC, MSBC, BM | 6, 4 |
| 11 | 12.2 | FS | 5.8 | Jack Russel Terrier | TC, MSBC, BE, BM, paratracheal and paraesophageal chronic inflammation/steatitis with fibrosis2 | 3 (NA = 2), 3 |
| 12 | 8 | FS | 5.1 | Maltese | TC, CB | 1, 1 |
| 13 | 13.1 | FS | 9.1 | Pekingese | Dynamic pharyngeal collapse, PH, CB, MVDD, TC, MSBC, BM | 6, 4 |
| 14 | 10.6 | MC | 4.3 | Miniature Poodle | TC, MSBC, BM | 6, 4 |
| 15 | 8 | MC | 6.9 | Miniature Poodle | TC, MSBC, BM | 6, 4 |
| 16 | 8 | FS | 7.9 | Pug | TC, BE, BM, bronchiolar disease, pulmonary fibrosis, acute lung injury 2 | 4 (NA = 2), 3 |
| Non-collapsible Inflammatory Airway Disease Group (NCAD) | ||||||
| 17 | 2 | MC | 14.5 | Basenji | EB, BE | NA |
| 18 | 0.8 | FS | 22.4 | Boxer | CB | 0 |
| 19 | 12.3 | MC | 2.8 | Chihuahua | BE, bronchiolar disease | 5, 2 |
| 20 | 10.1 | MC | 6.2 | Dachshund | Pneumocystis bronchopneumonia, BE, CB | 4, 4 |
| 21 | 13 | FS | 7.0 | Dachshund, Longhaired Standard | CB, BE, pulmonary fibrosis2 | 5, 2 |
| 22 | 10 | FS | 20.5 | German Shorthaired Pointer | CB, subtle small nodules on CT (etiology undetermined) | 3, 2 |
| 23 | 1.5 | MC | 60 | Great Pyrenees | Suspect canine hyperreactive airway disease (atelectasis R middle lung lobe, hyperinflation, air trapping, AW wall thickening) | 2, 1 |
| 24 | 11.6 | MC | 27.3 | Hovawart | Laryngeal paralysis, BE | 0 |
| 25 | 11 | M | 35.5 | Labrador Retriever | Bilateral laryngeal paralysis, CB | 4, 2 |
| 26 | 2.5 | FS | 42.4 | Labrador Retriever | Presumptive canine infectious respiratory disease complex (infectious tracheobronchitis) | NA |
| 27 | 4 | FS | 22.5 | Mixed breed | Foreign body pneumonia, BE | 0 |
| 28 | 1.3 | FS | 20.8 | Pointer | Recurrent bacterial pneumonia, BE, CB | NA |
| 29 | 7 | FS | 7.6 | Shetland Sheepdog | EB, BE | NA |
| 30 | 9 | FS | 8.1 | Shih Tzu | CB, BE | 1, 2 |
| 31 | 9 | FS | 14.2 | Welsh Corgi, Pembroke | Lymphoplasmacytic rhinitis2, CB | 2, 1 |
| 32 | 12.6 | FS | 7.8 | West Highland White Terrier | CB, suspect pulmonary fibrosis | NA |
| Non-Lower Airway Respiratory Diseases Group (NLARD) | ||||||
| 33 | 2 | M | 19.0 | Basset Hound | Suspect cryptogenic organizing pneumonia (steroid-responsive) | 0 |
| 34 | 9 | FS | 29.4 | Bernese Mountain Dog | Pulmonary adenocarcinoma | Compression by mass |
| 35 | 12 | FS | 18.2 | Border Collie | Bilateral laryngeal paralysis, aerodigestive disease | 4, 4 |
| 36 | 9.1 | MC | 24.4 | Brittany Spaniel | Mineralized osteomas, epiglottic retroversion | 2, 1 |
| 37 | 12.5 | MC | 5.8 | Dachshund, Miniature | Suspect cryptogenic organizing pneumonia (steroid-responsive) | 3, 2 |
| 38 | 13 | FS | 6.9 | Dachshund | Chronic lung congestion, suppurative interstitial pneumonia 2 | 4, 2 |
| 39 | 7 | MC | 10.5 | Fox Terrier, Smooth | Foreign body pneumonia | NA |
| 40 | 1.5 | FS | 10.7 | German Shepherd Dog | Bacterial pneumonia, pyothorax | NA |
| 41 | 3 | MC | 26.4 | German Shepherd Dog | Structural disease of nose and nasopharynx, constrictive bronchiolitis obliterans 2 | NA |
| 42 | 10 | FS | 59.8 | German Shepherd Dog | No cause found for panting, morbid obesity? | NA |
| 43 | 1.5 | FS | 24.9 | Giant Schnauzer | Streptococcus canis bronchopneumonia/necrotizing bronchiolitis 2 | 2, 1 |
| 44 | 9.6 | F | 37.5 | Labrador Retriever | Histiocytic sarcoma | 2, 1 |
| 45 | 6.1 | FS | 26.8 | Mixed Breed | Emphysema 2 | 0 |
| 46 | 9.8 | MC | 40.9 | Mixed Breed | Pulmonary neoplasia | NA |
| 47 | 3.5 | M | 31.0 | Pointer | Histiocytic sarcoma (pulmonary involvement) 2 | NA |
| 48 | 5 | M | 6.3 | Shih Tzu | Pulmonary blastomycosis | NA |
| 49 | 8 | FS | 18.7 | Siberian Husky | Histiocytic sarcoma (pulmonary involvement) 2 | 4, 4 |
| 50 | 9.5 | MC | 28.2 | Welsh Springer Spaniel | Rhinitis, laryngeal dysfunction, aerodigestive disorder (dysphagia, aspiration pneumonia) | 0 |
| 51 | 10.3 | FS | 37.2 | Weimaraner | Pulmonary papillary adenocarcinoma | 2, 1 |
| Site | AC | NCAD | NLARD |
|---|---|---|---|
| Based upon dorsoventral luminal diameter | |||
| Trachea | 17.9 (10.1, 45.4) 1 | 7.2 (0.9, 13) | 8.1 (3.2, 11.3) |
| Right Principal Bronchus | 24.1 (14.4, 36.6) 1 | 9.3 (1.6, 14.9) | 9 (4.1, 16.9) |
| Left Principal Bronchus | 17.3 (5.3, 49.7) | 12.6 (7.6, 24) | 14.2 (3.7, 17.9) |
| Right cranial lobar RB1 | 43.5 (5.5, 58.5) | 16.2 (1.1, 26.1) | 16.4 (10.9, 21) |
| Right middle lobar RB2 | 37.2 (6.6, 52) 2 | 2.2 (0, 22.3) | 13.3 (6.5, 27.3) |
| Right accessory lobar RB3 | 14.9 (0.6, 27.8) | 11.3 (0, 17.2) | 14.3 (7.1, 25) |
| Right caudal lobar RB4 | 24.1 (0.3, 33.5) | 9.1 (0, 18.7) | 16.4 (5.4, 18.9) |
| Left cranial lobar LB1 | 28.1 (11.3, 38.2) | 12.3 (2.7, 32.7) | 12.0 (4.5, 22.2) |
| Left caudal lobar LB2 | 22.5 (5.5, 36.9) | 12.6 (6.4, 24.9) | 15.8 (6.4, 24.9) |
| Based upon cross-sectional area | |||
| Trachea | 16.2 (9.8, 36) 1 | 7.6 (1.8, 14) | 4.8 (-0.2, 15.6) |
| Right Principal Bronchus | 35.2 (21.6, 57.3) 1 | 9.9 (4.6, 18.6) | 14.9 (6.1, 24.7) |
| Left Principal Bronchus | 33.5 (7.6, 32.4) | 16.9 (7.6, 32.4) | 17.4 (9.4, 30.3) |
| Right cranial lobar RB1 | 52 (9.7, 75.4) | 24.5 (8.8, 43.1) | 26.7 (16.1, 38.6) |
| Right middle lobar RB2 | 58.8 (33.1, 78.4) 1 | 27 (6, 39.1) | 33 (8.3, 43.4) |
| Right accessory lobar RB3 | 29.4 (20.7, 33) | 20.6 (5.1, 34.0) | 23.6 (11.2, 36.7) |
| Right caudal lobar RB4 | 46.2 (6.8, 51.3) | 18.6 (1.7, 31.5) | 26.3 (12.9, 32.4) |
| Left cranial lobar LB1 | 31.9 (11, 55.5) | 24.9 (15.7, 45.6) | 29.6 (12.7, 43) |
| Left caudal lobar LB2 | 27.8 (14, 58.3) | 27.7 (8.7, 39.9) | 26.4 (8.5, 40.2) |
| Site | AC | NCAD | NLARD |
|---|---|---|---|
| Trachea | 0.95 (0.90, 0.96) * | 0.97 (0.96, 0.98) | 0.97 (0.97, 0.98) |
| Right Principal Bronchus | 0.96 (0.93, 0.97) | 0.96 (0.95, 0.97) | 0.97 (0.96, 0.97) |
| Left Principal Bronchus | 0.94 (0.91, 0.97) | 0.96 (0.93, 0.97) | 0.97 (0.96, 0.98) |
| Right cranial lobar RB1 | 0.96 (0.96, 0.97) | 0.96 (0.96, 0.97) | 0.96 (0.94, 0.97) |
| Right middle lobar RB2 | 0.95 (0.94, 0.97) | 0.96 (0.94, 0.97) | 0.95 (0.94, 0.96) |
| Right accessory lobar RB3 | 0.97 (0.96, 0.97) | 0.95 (0.94, 0.97) | 0.97 (0.94, 0.97) |
| Right caudal lobar RB4 | 0.97 (0.96, 0.97) | 0.97 (0.96, 0.97) | 0.97 (0.96, 0.98) |
| Left cranial lobar LB1 | 0.95 (0.93, 0.96) | 0.96 (0.95, 0.97) | 0.95 (0.95, 0.96) |
| Left caudal lobar LB2 | 0.97 (0.96, 0.97) | 0.97 (0.96, 0.97) | 0.97 (0.97, 0.98) |
| CT | |||||||
|---|---|---|---|---|---|---|---|
| Grade of Collapse | 0 | 1 | 2 | 3 | 4 | Total | |
| 0 | 4 | 61 | 6 | 0 | 0 | 71 | |
| 1 | 2 | 6 | 5 | 0 | 0 | 13 | |
| TB | 2 | 0 | 5 | 6 | 2 | 0 | 13 |
| 3 | 2 | 3 | 1 | 2 | 1 | 9 | |
| 4 | 0 | 3 | 2 | 3 | 1 | 9 | |
| Total | 8 | 78 | 20 | 7 | 2 | 115 |
| CT | |||||||
|---|---|---|---|---|---|---|---|
| Grade of Collapse | 0 | 1 | 2 | 3 | 4 | Total | |
| 0 | 11 | 54 | 36 | 3 | 0 | 104 | |
| 1 | 8 | 21 | 12 | 2 | 0 | 43 | |
| B | 2 | 3 | 13 | 11 | 7 | 1 | 35 |
| 3 | 3 | 6 | 7 | 5 | 1 | 22 | |
| 4 | 1 | 4 | 6 | 2 | 0 | 13 | |
| Total | 26 | 98 | 72 | 19 | 2 | 217 |
| CT | ||||||
|---|---|---|---|---|---|---|
| Grade of Collapse | 0 | 1 | 2 | 3 | Total | |
| 0 | 11 | 0 | 0 | 0 | 11 | |
| 1 | 6 | 4 | 3 | 0 | 13 | |
| B | 2 | 1 | 2 | 2 | 2 | 7 |
| 3 | 0 | 0 | 6 | 1 | 7 | |
| Total | 18 | 6 | 11 | 3 | 38 |
| Weighted Kappa Coefficient 1 | Interpretation 2 | |
|---|---|---|
| 1. Trachea, mainstem bronchi and lobar bronchi | ||
| Trachea (DVd) 3 | 0.2895 | Fair |
| Trachea (CSA) 4 | 0.2519 | Fair |
| Right Principal bronchus (DVd) 3 | 0.1674 | Slight |
| Right Principal bronchus (CSA) 4 | 0.1502 | Slight |
| Left Principal bronchus (DVd) 3 | 0.2550 | Fair |
| Left Principal bronchus (CSA) 4 | 0.3184 | Fair |
| Right cranial lobar RB1 (DVd) 3 | 0.2977 | Fair |
| Right cranial lobar RB1 (CSA) 4 | 0.1303 | Slight |
| Right middle lobar RB2 (DVd) 3 | 0.2382 | Fair |
| Right middle lobar RB2 (CSA) 4 | −0.1250 | None |
| Right accessory lobar RB3 (DVd) 3 | 0.1529 | Slight |
| Right accessory lobar RB3 (CSA) 4 | 0.0166 | Slight |
| Right caudal lobar RB4 (DVd) 3 | 0.1866 | Slight |
| Right caudal lobar RB4 (CSA) 4 | 0.0899 | Slight |
| Left cranial lobar LB1 (DVd) 3 | 0.0517 | Slight |
| Left cranial lobar LB1 (CSA) 4 | −0.0756 | None |
| Left caudal lobar LB2 (DVd) 3 | 0.0565 | Slight |
| Left caudal lobar LB2 (CSA) 4 | 0.0585 | Slight |
| 2. Segmental and subsegmental bronchi | 0.52 | Moderate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy, A.; Reinero, C.; Masseau, I. Ventilator-Assisted Inspiratory and Expiratory Breath-Hold Thoracic Computed Tomographic Scans Can Detect Dynamic and Static Airway Collapse in Dogs with Limited Agreement with Tracheobronchoscopy. Animals 2022, 12, 3091. https://doi.org/10.3390/ani12223091
Levy A, Reinero C, Masseau I. Ventilator-Assisted Inspiratory and Expiratory Breath-Hold Thoracic Computed Tomographic Scans Can Detect Dynamic and Static Airway Collapse in Dogs with Limited Agreement with Tracheobronchoscopy. Animals. 2022; 12(22):3091. https://doi.org/10.3390/ani12223091
Chicago/Turabian StyleLevy, Alice, Carol Reinero, and Isabelle Masseau. 2022. "Ventilator-Assisted Inspiratory and Expiratory Breath-Hold Thoracic Computed Tomographic Scans Can Detect Dynamic and Static Airway Collapse in Dogs with Limited Agreement with Tracheobronchoscopy" Animals 12, no. 22: 3091. https://doi.org/10.3390/ani12223091
APA StyleLevy, A., Reinero, C., & Masseau, I. (2022). Ventilator-Assisted Inspiratory and Expiratory Breath-Hold Thoracic Computed Tomographic Scans Can Detect Dynamic and Static Airway Collapse in Dogs with Limited Agreement with Tracheobronchoscopy. Animals, 12(22), 3091. https://doi.org/10.3390/ani12223091






