Exploring the Interactive Effects of Thymol and Thymoquinone: Moving towards an Enhanced Performance, Gross Margin, Immunity and Aeromonas sobria Resistance of Nile Tilapia (Oreochromis niloticus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Thymol and Thymoquinone
2.2. Rearing Conditions of Nile tilapia
2.3. Experimental Design, Diets and Feeding Trial
2.4. Evaluating Growth Performance-Related Parameters and Profitability
2.5. Sampling and Anlalysis Techniques
2.5.1. Blood and Tissue Sampling
2.5.2. Blood Hematological and Serum Biochemical and Immune-Related Indices
2.5.3. Quantitative Reverse Transcription Polymerase Chain Reaction Procedures for Gene Expression Analysis
2.6. Aeromonas sobria Challenge and Expression Analysis of Its Virulence-Related Genes
2.7. Statistical Analysis
3. Results
3.1. Fish Growth Performance and Profitability Variables
3.2. Blood Hematological and Serum Biochemical and Immunological Indicators
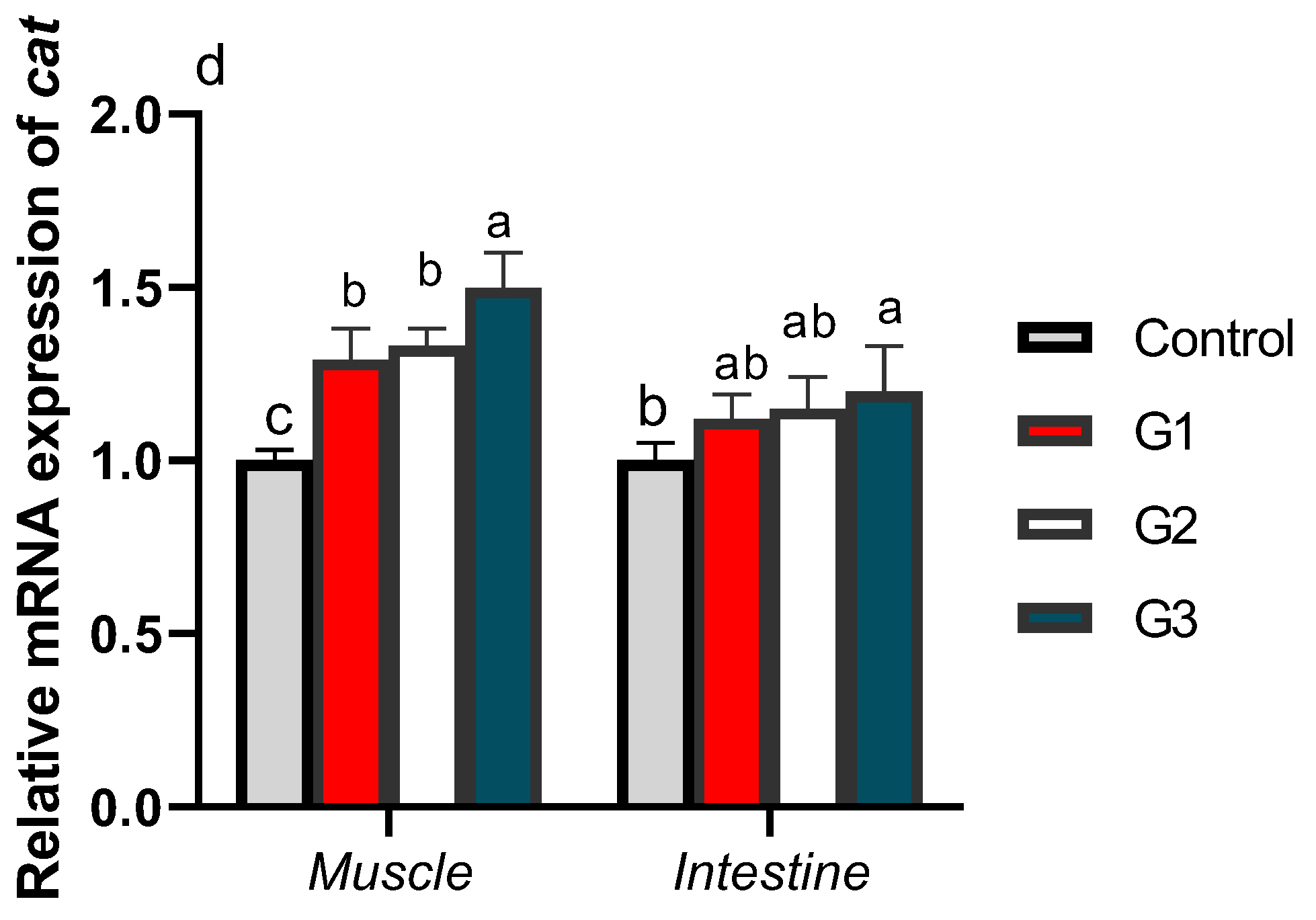
3.3. Gene Expression Profiles of Digestive and Antioxidant Enzymes
3.4. Relative mRNA Expression of Cytokines and Autophagy-Related Genes
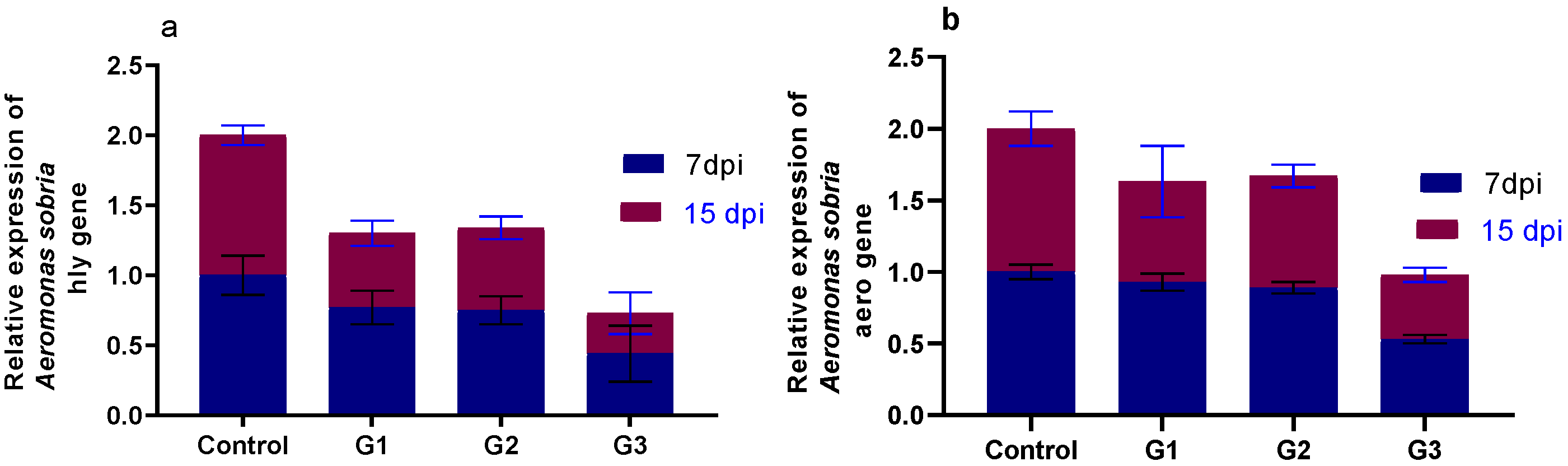
3.5. Effect of Diets Fortified with Thy and/or ThQ on Survival Percentages and Relative Expression of A. sobria Virulence-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, D.; Kishawy, A.T.Y.; Khater, S.I.; Khalifa, E.; Ismail, T.A.; Mohammed, H.A.; Elnahriry, S.S.; Tolba, H.A.; Sherief, W.R.I.A.; Farag, M.F.M.; et al. Interactive effects of dietary quercetin nanoparticles on growth, flesh antioxidant capacity and transcription of cytokines and Aeromonas hydrophila quorum sensing orchestrating genes in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021, 119, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jiang, W.D.; Wu, P.; Liu, Y.; Zeng, Y.Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Wang, S.W.; et al. Soybean isoflavones improve the health benefits, flavour quality indicators and physical properties of grass carp (Ctenopharygodon idella). PLoS ONE 2019, 14, e0209570. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Neamat-Allah, A.N.F.; Ibrahim, S.M.; Eissa, H.M.; Fawzey, M.M.; Mostafa, D.I.A.; El-Kader, S.A.A.; Khater, S.I.; Khater, S.I. Dual effect of selenium loaded chitosan nanoparticles on growth, antioxidant, immune related genes expression, transcriptomics modulation of caspase 1, cytochrome P450 and heat shock protein and Aeromonas hydrophila resistance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021, 110, 91–99. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; Ibrahim, S.M.; Eldemery, F.; El-Mandrawy, S.A.M.; Metwally, A.S.; Khalifa, E.; Elnahriry, S.S.; Ibrahim, D. Dietary cinnamaldehyde nanoemulsion boosts growth and transcriptomes of antioxidant and immune related genes to fight Streptococcus agalactiae infection in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021, 113, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Koshio, S.; Esteban, M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Xu, X.R.; Liu, S.S.; Zhou, G.J.; Sun, K.F.; Zhao, J.L.; Ying, G.G. Antibiotics in typical marine aquaculture farms surrounding Hailing island, South China: Occurrence, bioaccumulation and human dietary exposure. Mar. Pollut. Bull. 2015, 90, 181–187. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Barra Caracciolo, A. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Ibrahim, D.; Arisha, A.H.; Khater, S.I.; Gad, W.M.; Hassan, Z.; Abou-Khadra, S.H.; Mohamed, D.I.; Ismail, T.A.; Gad, S.A.; Eid, S.A.M.; et al. Impact of omega-3 fatty acids nano-formulation on growth, antioxidant potential, fillet quality, immunity, autophagy-related genes and Aeromonas hydrophila resistance in Nile tilapia (Oreochromis niloticus). Antioxidants 2022, 11, 1523. [Google Scholar] [CrossRef]
- Schreck, C.B.; Tort, L. The concept of stress in fish. Fish Physiol. 2016, 35, 1–34. [Google Scholar] [CrossRef]
- Fernandes, D.C.; Eto, S.F.; Funnicelli, M.I.G.; Fernandes, C.C.; Charlie-Silva, I.; Belo, M.A.A.; Pizauro, J.M. Immunoglobulin Y in the diagnosis of Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus). Aquaculture 2019, 500, 576–585. [Google Scholar] [CrossRef]
- Ahmed, S.A.A.; El-Rahman, G.I.A.; Behairy, A.; Hendam, B.M.; Alsubaie, F.M.; Khalil, S.R. Influence of feeding quinoa (Chenopodium quinoa) seeds and prickly pear fruit (Opuntia ficus indica) peel on the immune response and resistance to Aeromonas sobria infection in Nile tilapia (Oreochromis niloticus). Animals 2020, 10, 2266. [Google Scholar] [CrossRef] [PubMed]
- Kirke, D.F.; Swift, S.; Lynch, M.J.; Williams, P. The Aeromonas hydrophila LuxR homologue AhyR regulates the N-acyl homoserine lactone synthase, AhyI positively and negatively in a growth phase-dependent manner. FEMS Microbiol. Lett. 2004, 241, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hamid, M.I.; Abd El-Aziz, N.K.; Ali, H.A. Protective potency of clove oil and its transcriptional down-regulation of Aeromonas sobria virulence genes in African catfish (Clarias gariepinus L.). Cell. Mol. Biol. 2016, 62, 49–54. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; Bendary, M.M. Comparative phenotypic and genotypic discrimination of methicillin resistant and susceptible Staphylococcus aureus in Egypt. Cell Mol. Biol 2015, 61, 101–112. [Google Scholar] [PubMed]
- Ammar, A.M.; Abd El-Hamid, M.I.; El-Malt, R.M.S.; Azab, D.S.; Albogami, S.; Al-Sanea, M.M.; Soliman, W.E.; Ghoneim, M.M.; Bendary, M.M. Molecular detection of fluoroquinolone resistance among multidrug-, extensively drug-, and pan-drugr resistant Campylobacter species in Egypt. Antibiotics 2021, 10, 1342. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.M.; El-Hamid, M.I.A.; Mohamed, Y.H.; Mohamed, H.M.; Al-khalifah, D.H.M.; Hozzein, W.N.; Selim, S.; El-Neshwy, W.M.; El-Malt, R.M.S. Prevalence and antimicrobial susceptibility of bovine Mycoplasma species in Egypt. Biology 2022, 11, 1083. [Google Scholar] [CrossRef]
- Ammar, A.M.; Abd El-Hamid, M.I.; Eid, S.E.A.; El Oksh, A.S. Insights into antimicrobial resistance and virulence genes of emergent multidrug resistant avian pathogenic Escherichia coli in Egypt: How closely related are they? Rev. Med. Vet. 2015, 166, 304–314. [Google Scholar]
- Ammar, A.M.; Attia, A.M.; Abd El-Aziz, N.K.; Abd El Hamid, M.I.; El-Demerdash, A.S. Class 1 integron and associated gene cassettes mediating multiple-drug resistance in some food borne pathogens. Int. Food Res. J. 2016, 23, 332–339. [Google Scholar]
- Ammar, A.M.; Abd El-Aziz, N.K.; Gharib, A.A.; Ahmed, H.K.; Lameay, A.E. Mutations of domain V in 23S ribosomal RNA of macrolide-resistant Mycoplasma gallisepticum isolates in Egypt. J. Infect. Dev. Ctries. 2016, 10, 807–813. [Google Scholar] [CrossRef]
- Ammar, A.; Abd El-Hamid, M.I.; Hashem, Y.M.; El-Malt, R.M.S.; Mohamed, H.M. Mycoplasma bovis: Taxonomy, characteristics, pathogenesis and antimicrobial resistance. Zagazig Vet. J. 2021, 49, 444–461. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.K.; Abd El-Hamid, M.I.; Bendary, M.M.; El-Azazy, A.A.; Ammar, A.M. Existence of vancomycin resistance among methicillin resistant S. aureus recovered from animal and human sources in Egypt. Slov. Vet. Res. 2018, 55, 221–230. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; Awad, N.F.S.; Hashem, Y.M.; Abdel-Rahman, M.A.; Abdelaziz, A.M.; Mohammed, I.A.A.; Abo-Shama, U.H. In vitro evaluation of various antimicrobials against field Mycoplasma gallisepticum and Mycoplasma synoviae isolates in Egypt. Poult. Sci. 2019, 98, 6281–6288. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; El Sayed, R.; Abdelfattah-Hassan, A.; Morshedy, A.M. Creatine or guanidinoacetic acid? Which is more effective at enhancing growth, tissue creatine stores, quality of meat, and genes controlling growth/myogenesis in Mulard ducks. J. Appl. Anim. Res. 2019, 47, 159–166. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; El-Sayed, M.E.; Ali, A.R.; Abdallah, H.M.; Arnaout, M.I.; El-mowalid, G.A. Marjoram extract down-regulates the expression of Pasteurella multocida adhesion, colonization and toxin genes: A potential mechanism for its antimicrobial activity. Comp. Immunol. Microbiol. Infect. Dis. 2019, 62, 101–108. [Google Scholar] [CrossRef]
- Ammar, A.M.; El-Naenaeey, E.-S.Y.; El-Hamid, M.I.A.; El-Gedawy, A.A.; El-Malt, R.M.S. Campylobacter as a major foodborne pathogen: A review of its characteristics, pathogenesis, antimicrobial resistance and control. J. Microbiol. Biotechnol. Food Sci. 2021, 10, 609–619. [Google Scholar] [CrossRef]
- Hashem, Y.M.; El-Hamid, M.I.A.; Awad, N.F.S.; Ibrahim, D.; Elshater, N.S.; El-Malt, R.M.S.; Hassan, W.H.; Abo-Shama, U.H.; Nassan, M.A.; El-Bahy, S.M.; et al. Insights into growth-promoting, anti-inflammatory, immunostimulant, and antibacterial activities of Toldin CRD as a novel phytobiotic in broiler chickens experimentally infected with Mycoplasma gallisepticum. Poult. Sci. 2022, 102154. [Google Scholar] [CrossRef]
- Bendary, M.M.; Ibrahim, D.; Mosbah, R.A.; Mosallam, F.; Hegazy, W.A.H.; Awad, N.F.S.; Alshareef, W.A.; Alomar, S.Y.; Zaitone, S.A.; Abd El-Hamid, M.I. Thymol nanoemulsion: A new therapeutic option for extensively drug resistant foodborne pathogens. Antibiotics 2021, 10, 25. [Google Scholar] [CrossRef]
- Elmowalid, G.A.; Abd El-Hamid, M.I.; Abd El-Wahab, A.M.; Atta, M.; Abd El-Naser, G.; Attia, A.M. Garlic and ginger extracts modulated broiler chicks innate immune responses and enhanced multidrug resistant Escherichia coli O78 clearance. Comp. Immunol. Microbiol. Infect. Dis. 2019, 66, 101334. [Google Scholar] [CrossRef]
- Caipang, C.M.A. Phytogenics in aquaculture: A short review of their effects on gut health and microflora in fish. Philipp. J. Fish. 2020, 27, 246–259. [Google Scholar] [CrossRef]
- Ammar, A.M.; El-Naenaeey, E.-S.Y.; El-Malt, R.M.S.; El-Gedawy, A.A.; Khalifa, E.; Elnahriry, S.S.; Abd El-Hamid, M.I. Prevalence, antimicrobial susceptibility, virulence and genotyping of Campylobacter jejuni with a special reference to the anti-virulence potential of eugenol and beta-resorcylic acid on some multi-drug resistant isolates in Egypt. Animals 2021, 11, 10003. [Google Scholar] [CrossRef]
- Aljazzar, A.; Abd El-Hamid, M.I.; El-Malt, R.M.S.; Rizk El-Gharreb, W.; Abdel-Raheem, S.M.; Ibrahim, A.M.; Abdelaziz, A.M.; Ibrahim, D. Prevalence and antimicrobial susceptibility of Campylobacter Species with particular focus on the growth promoting, immunostimulant and anti-Campylobacter jejuni activities of eugenol and trans-cinnamaldehyde mixture in broiler chickens. Animals 2022, 12, 905. [Google Scholar] [CrossRef]
- Gou, C.; Wang, J.; Wang, Y.; Dong, W.; Shan, X.; Lou, Y.; Gao, Y. Hericium caput-medusae (Bull.:Fr.) Pers. polysaccharide enhance innate immune response, immune-related genes expression and disease resistance against Aeromonas hydrophila in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 72, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Naiel, M.A.E.; Shehata, A.M.; Negm, S.S.; Abd El-Hack, M.E.; Amer, M.S.; Khafaga, A.F.; Bin-Jumah, M.; Allam, A.A. The new aspects of using some safe feed additives on alleviated imidacloprid toxicity in farmed fish: A review. Rev. Aquac. 2020, 12, 2250–2267. [Google Scholar] [CrossRef]
- Steiner, T.; Syed, B.; Steiner, T.; Syed, B. Phytogenic Feed additives in animal nutrition. Med. Aromat. Plants World 2015, 20, 403–423. [Google Scholar] [CrossRef]
- Huyghebaert, G.; Ducatelle, R.; Immerseel, F. Van An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef]
- Farahat, M.; Ibrahim, D.; Kishawy, A.T.Y.; Abdallah, H.M.; Hernandez-Santana, A.; Attia, G. Effect of cereal type and plant extract addition on the growth performance, intestinal morphology, caecal microflora, and gut barriers gene expression of broiler chickens. Animal 2021, 15, 100056. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Tartor, Y.H. Carvacrol essential oil stimulates growth performance, immune response, and tolerance of Nile tilapia to Cryptococcus uniguttulatus infection. Dis. Aquat. Organ. 2020, 141, 1–14. [Google Scholar] [CrossRef]
- Firmino, J.P.; Galindo-Villegas, J.; Reyes-López, F.E.; Gisbert, E. Phytogenic bioactive compounds shape fish mucosal immunity. Front. Immunol. 2021, 12, 695973. [Google Scholar] [CrossRef]
- Latif, M.; Faheem, M.; Asmatullah; Hoseinifar, S.H.; Doan, H. Van Dietary black seed effects on growth performance, proximate composition, antioxidant and histo-biochemical parameters of a culturable fish, rohu (Labeo rohita). Animals 2021, 11, 48. [Google Scholar] [CrossRef]
- Ibrahim, D.; Ismail, T.A.; Khalifa, E.; Abd El-Kader, S.A.; Mohamed, D.I.; Mohamed, D.T.; Shahin, S.E.; Abd El-Hamid, M.I. Supplementing garlic nanohydrogel optimized growth, gastrointestinal integrity and economics and ameliorated necrotic enteritis in broiler chickens using a Clostridium perfringens challenge model. Animals 2021, 11, 2027. [Google Scholar] [CrossRef]
- Ibrahim, D.; Abdelfattah-Hassan, A.; Badawi, M.; Ismail, T.A.; Bendary, M.M.; Abdelaziz, A.M.; Mosbah, R.A.; Mohamed, D.I.; Arisha, A.H.; El-Hamid, M.I.A. Thymol nanoemulsion promoted broiler chicken’s growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella Typhimurium. Sci. Rep. 2021, 11, 7742. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Moustafa, A.; Metwally, A.S.; Nassan, M.A.; Abdallah, K.; Eldemery, F.; Tufarelli, V.; Laudadio, V.; Kishawy, A.T. Potential application of Cornelian cherry extract on broiler chickens: Growth, expression of antioxidant biomarker and glucose transport genes, and oxidative stability of frozen meat. Animals 2021, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Magouz, F.I.; Amer, A.A.; Faisal, A.; Sewilam, H.; Aboelenin, S.M.; Dawood, M.A.O. The effects of dietary oregano essential oil on the growth performance, intestinal health, immune, and antioxidative responses of Nile tilapia under acute heat stress. Aquaculture 2022, 548, 737632. [Google Scholar] [CrossRef]
- Amer, S.A.; Metwally, A.E.; Ahmed, S.A.A.A. The influence of dietary supplementation of cinnamaldehyde and thymol on the growth performance, immunity and antioxidant status of monosex Nile tilapia fingerlings (Oreochromis niloticus). Egypt. J. Aquat. Res. 2018, 44, 251–256. [Google Scholar] [CrossRef]
- Khondoker, S.; Hossain, M.M.M.; Hasan-Uj-Jaman, M.; Alam, M.E.; Zaman, M.F.U.; Tabassum, N. Effect of Nigella sativa (Black Cumin Seed) to enhance the immunity of common carp (Cyprinus carpio) against Pseudomonas fluorescens. Am. J. Life Sci. 2016, 4, 87. [Google Scholar] [CrossRef]
- Awad, E.; Austin, D.; Lyndon, A.R. Effect of black cumin seed oil (Nigella sativa) and nettle extract (Quercetin) on enhancement of immunity in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquaculture 2013, 388–391, 193–197. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Falahatkar, B.; Akrami, R. Effects of dietary thymol-carvacrol on growth performance, hematological parameters and tissue composition of juvenile rainbow trout, Oncorhynchus mykiss. Wiley Online Libr. 2011, 27, 1057–1060. [Google Scholar] [CrossRef]
- Taheri Mirghaed, A.; Hoseini, S.M.; Hoseinifar, S.H.; Van Doan, H. Effects of dietary thyme (Zataria multiflora) extract on antioxidant and immunological responses and immune-related gene expression of rainbow trout (Oncorhynchus mykiss) juveniles. Fish Shellfish Immunol. 2020, 106, 502–509. [Google Scholar] [CrossRef]
- Abdelwahab, A.M.; El-Bahr, S.M. Influence of black cumin seeds (Nigella sativa) and turmeric (Curcuma longa Linn.) mixture on performance and serum biochemistry of Asian sea bass, Lates calcarifer. World J. Fish Mar. Sci. 2012, 4, 496–503. [Google Scholar]
- Khatun, A.; Hossain, M.; Rahman, M.; Alam, M.; Yasmin, F.; Islam, M.; Islam, M. Effect of black cumin seed oil (Nigella sativa) on enhancement of immunity in the climbing perch, anabas testudineus. Br. Microbiol. Res. J. 2015, 6, 331–339. [Google Scholar] [CrossRef]
- Zheng, Z.L.; Tan, J.Y.W.; Liu, H.Y.; Zhou, X.H.; Xiang, X.; Wang, K.Y. Evaluation of oregano essential oil (Origanum heracleoticum L.) on growth, antioxidant effect and resistance against Aeromonas hydrophila in channel catfish (Ictalurus punctatus). Aquaculture 2009, 292, 214–218. [Google Scholar] [CrossRef]
- Nutritional Research Council (NRC). Nutrient Requirements of Fish Nutrient Requirements of Domestic Animal Series; National Academy Press: Washigton, DC, USA, 1993. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 19th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2012; ISBN 9780935584837. [Google Scholar]
- Kishawy, A.T.Y.; Sewid, A.H.; Nada, H.S.; Kamel, M.A.; El-Mandrawy, S.A.M.; Abdelhakim, T.M.N.; El-Murr, A.E.I.; El Nahhas, N.; Hozzein, W.N.; Ibrahim, D. Mannanoligosaccharides as a carbon source in biofloc boost dietary plant protein and water quality, growth, immunity and Aeromonas hydrophila resistance in Nile tilapia (Oreochromis niloticus). Animals 2020, 10, 1724. [Google Scholar] [CrossRef] [PubMed]
- Goldenfarb, P.B.; Bowyer, F.P.; Hall, E.; Brosious, E. Reproducibility in the hematology laboratory: The microhematocrit determination. Am. J. Clin. Pathol. 1971, 56, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M. Complement and complement fixation. In Experimental Immunochemistry, 2nd ed.; C. C. Thomas Publisher: Springfield, IL, USA, 1961. [Google Scholar]
- Taoka, Y.; Okajima, K.; Uchiba, M.; Murakami, K.; Kushimoto, S.; Johno, M.; Naruo, M.; Okabe, H.; Takatsuki, K. Role of neutrophils in spinal cord injury in the rat. Neuroscience 1997, 79, 1177–1182. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Borrell, N.; Acinas, S.G.; Figueras, M.J.; Martínez-Murcia, A.J. Identification of Aeromonas clinical isolates by restriction fragment length polymorphism of PCR-amplified 16S rRNA genes. J. Clin. Microbiol. 1997, 35, 1671–1674. [Google Scholar] [CrossRef]
- Castro-Escarpulli, G.; Figueras, M.J.; Aguilera-Arreola, G.; Soler, L.; Fernández-Rendón, E.; Aparicio, G.O.; Guarro, J.; Chacón, M.R. Characterisation of Aeromonas spp. isolated from frozen fish intended for human consumption in Mexico. Int. J. Food Microbiol. 2003, 84, 41–49. [Google Scholar] [CrossRef]
- Singh, V.; Rathore, G.; Kapoor, D.; Mishra, B.N.; Lakra, W.S. Detection of aerolysin gene in Aeromonas hydrophila isolated from fish and pond water. Indian J. Microbiol. 2009, 48, 453–458. [Google Scholar] [CrossRef]
- Orsi, R.O.; dos Santos, V.G.; Pezzato, L.E.; de Carvalho, P.L.P.F.; Teixeira, C.P.; Freitas, J.M.A.; Padovani, C.R.; Sartori, M.M.P.; Barros, M.M. Activity of Brazilian propolis against Aeromonas hydrophila and its effect on Nile tilapia growth, hematological and non-specific immune response under bacterial infection. An. Acad. Bras. Cienc. 2017, 89, 1785–1799. [Google Scholar] [CrossRef]
- dos Santos, A.C.; Sutili, F.J.; Heinzmann, B.M.; Cunha, M.A.; Brusque, I.C.M.; Baldisserotto, B.; Zeppenfeld, C.C. Aloysia triphylla essential oil as additive in silver catfish diet: Blood response and resistance against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2017, 62, 213–216. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Kim, I.H. Efficacy of phytogenic feed additive on performance, production and health status of monogastric animals—A review. Ann. Anim. Sci. 2017, 17, 929–948. [Google Scholar] [CrossRef]
- Aanyu, M.; Betancor, M.B.; Monroig, Ó. The effects of combined phytogenics on growth and nutritional physiology of Nile tilapia Oreochromis niloticus. Aquaculture 2020, 519, 734867. [Google Scholar] [CrossRef]
- El-hawarry, W.N.; Mohamed, R.A.; Ibrahim, S.A. Collaborating effects of rearing density and oregano oil supplementation on growth, behavioral and stress response of Nile tilapia (Oreochromis niloticus). Egypt. J. Aquat. Res. 2018, 44, 173–178. [Google Scholar] [CrossRef]
- Peterson, B.C.; Bosworth, B.G.; Li, M.H.; Beltran, R.; Santos, G.A. Assessment of a phytogenic feed additive (digestarom P.E.P. MGE) on growth performance, processing yield, fillet composition, and survival of channel catfish. J. World Aquac. Soc. 2014, 45, 206–212. [Google Scholar] [CrossRef]
- El-naby, A.S.A.; Al-sagheer, A.A.; Negm, S.S.; Naiel, M.A.E. Dietary combination of chitosan nanoparticle and thymol affects feed utilization, digestive enzymes, antioxidant status, and intestinal morphology of Oreochromis niloticus. Aquaculture 2020, 515, 734577. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, N.; Yu, Y.; Wu, S.; Li, S.; Wang, W. Expression profile of the digestive enzymes of manis javanica reveals its adaptation to diet specialization. ACS Omega 2019, 4, 19925–19933. [Google Scholar] [CrossRef]
- Ibrahim, D.; Eldemery, F.; Metwally, A.S.; Abd-Allah, E.M.; Mohamed, D.T.; Ismail, T.A.; Hamed, T.A.; Al Sadik, G.M.; Neamat-Allah, A.N.F.; Abd El-Hamid, M.I. Dietary eugenol nanoemulsion potentiated performance of broiler chickens: Orchestration of digestive enzymes, intestinal barrier functions and cytokines related gene expression with a consequence of attenuating the severity of E. coli O78 infection. Front. Vet. Sci. 2022, 9, 847580. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Samir, F.; Abd El-Naby, A.S.; Monier, M.N. Antioxidative and immunostimulatory effect of dietary cinnamon nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.) and its susceptibility to hypoxia stress and Aeromonas hydrophila infection. Fish Shellfish Immunol. 2018, 74, 19–25. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 1–22. [Google Scholar] [CrossRef]
- Zahran, E.; Risha, E.; Awadin, W.; Palić, D. Acute exposure to chlorpyrifos induces reversible changes in health parameters of Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2018, 197, 47–59. [Google Scholar] [CrossRef]
- Valladão, M.G.R.; Gallani, S.U.; Kotzent, S. Effects of dietary thyme essential oil on hemato-immunological indices, intestinal morphology, and microbiota of Nile tilapia. Aquac. Int. 2019, 27, 399–411. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; El-Sabagh, M.; Esteban, M.A.; Zaineldin, A.I. Probiotics as an environment-friendly approach to enhance red sea bream, pagrus major growth, immune response and oxidative status. Fish Shellfish Immunol. 2016, 57, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Acar, Ü.; Karabayır, A.; Kesbİç, O.S.; Yılmaz, S.; Zemherİ, F. Effects on some immunological parameters and gene expression levels of lupin meal (Lupinus albus) replaced with fish meal in rainbow trout (Oncorhynchus mykiss). COMU J. Agric. Fac. 2018, 6, 81–89. [Google Scholar]
- Zhang, C.; Gao, H.; Yang, Z.; Jiang, Y.; Li, Z.; Wang, X.; Xiao, B.; Su, X.-Z.; Cui, H.; Yuan, J. CRISPR/Cas9 mediated sequential editing of genes critical for ookinete motility in Plasmodium yoelii. Mol. Biochem. Parasitol. 2017, 212, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rymuszka, A.; Adaszek, Ł. Pro- and anti-inflammatory cytokine expression in carp blood and head kidney leukocytes exposed to cyanotoxin stress—An in vitro study. Fish Shellfish Immunol. 2012, 33, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Hal, A.M.; El-barbary, M.I. Effect of Nigella sativa oil and ciprofloxacin against bacterial infection on gene expression in Nile tilapia (Oreochromis niloticus) blood. Aquaculture 2021, 532, 736071. [Google Scholar] [CrossRef]
- Spits, H.; Lanier, L.L. Natural killer or dendritic: What’s in a name? Immunity 2007, 26, 11–16. [Google Scholar] [CrossRef]
- Paquette, M.; El-Houjeiri, L.; Pause, A. mTOR pathways in cancer and autophagy. Cancers 2018, 10, 18. [Google Scholar] [CrossRef]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abeliovich, H.; Agostinis, P.; Agrawal, D.K.; Aliev, G.; Askew, D.S.; Baba, M.; Baehrecke, E.H.; Bahr, B.A.; Ballabio, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 2008, 4, 151–175. [Google Scholar] [CrossRef]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, N.K.; Abd El-Hamid, M.I.; El-Naenaeey, E.S.Y. A complex hierarchical quorum-sensing circuitry modulates phenazine gene expression in Pseudomonas aeruginosa. J. Infect. Dev. Ctries. 2018, 11, 919–925. [Google Scholar] [CrossRef] [PubMed][Green Version]




| Ingredient | % |
|---|---|
| Fish meal | 27 |
| Yellow corn | 27.5 |
| Soybean meal | 28.5 |
| Rice bran | 9 |
| Corn gluten | 2.5 |
| Lysine | 0.1 |
| DL-Methionine, 98% | 0.2 |
| Fish oil | 2.8 |
| Di-calcium phosphate | 0.3 |
| Threonine | 0.1 |
| * Vitamins and minerals premix | 2 |
| Chemical analysis | |
| Digestible energy (kcal/kg) | 2922 |
| Nitrogen free extract % | 42.06 |
| Methionine, % | 0.93 |
| Ether extract, % | 5.17 |
| Crude protein, % | 35.00 |
| Lysine, % | 2.33 |
| Available P, % | 0.58 |
| Ca, % | 1.05 |
| Target Gene | Primer Sequence (5′-3′) | Accession No. (GeneBank/Ensemble) |
|---|---|---|
| β-actin | F-TGGCATCACACCTTCTATAACGA R-TGGCAGGAGTGTTGAAGGTCT | XM_003455949.2 |
| Pepsinogen | F-TGACCAATGACGCTGACTTG R-GGAGGAACCGGTGTCAAAAATG | JQ043215.1 |
| Chymotrypsinogen | F-TTCTGCCTTCGCTTCTCATC R-TTCAACGCCATCTGCTACTG | ENSONIG00000003237 |
| Lipase | F-TCGGTGGATGGCATGATGGAGA R-GCGACTGGATAGTGCTGCTGAG | ENSONIG00000005832 |
| α-amylase | F-GCGACTGGATAGTGCTGCTGAG R-TGGCGTTGGGCTGACATTGC | ENSONIG00000018530 |
| gsh-px | F-CCAAGAGAACTGCAAGAACGA R-CAGGACACGTCATTCCTACAC | NM_001279711.1 |
| cat | F-TCAGCACAGAAGACACAGACA R-GACCATTCCTCCACTCCAGAT | XM_031754288.1 |
| sod | F-GACGTGACAACACAGGTTGC R-TACAGCCACCGTAACAGCAG | XM_003449940.5 |
| il-10 | F-CTGCTAGATCAGTCCGTCGAA R-GCAGAACCGTGTCCAGGTAA | XM_013269189.3 |
| il-8 | F-GCACTGCCGCTGCATTAAG R-GCAGTGGGAGTTGGGAAGAA | XM_031747075.1 |
| il-β | F-TGCTGAGCACAGAATTCCAG R-GCTGTGGAGAAGAACCAAGC | XM_019365841.2 |
| tnf-α | F-GAGGTCGGCGTGCCAAGA R-TGGTTTCCGTCCACAGCGT | NM_001279533.1 |
| mtor | F-TGCGGAGTATGTGGAGTT R-CATCTCTTTGGTCTCTCTCTGG | XM_019108641.1 |
| bcln-1 | F-TCTGTTTGATATCATGTCTGG R-TAATTCTGGCACTCATTTTCT | XM_019068185.1 |
| lc3-II | F-GGAACAGCATCCAAGCAAGA R-TCAGAAATGGCGGTGGACA | NM199604.1 |
| atg12 | F-ACAGTACAGTCACTCGCTCA R-AAAACACTCGAAAAGCACACC | XM_019125508.1 |
| atg5 | F-ATTGGCGTTTTGTTTGATCTT R-TTTGAGTGCATCCGCCTCTTT | XM_019082404.1 |
| Parameter | Experimental Group | p Value | SEM | |||
|---|---|---|---|---|---|---|
| Control | G1 | G2 | G3 | |||
| Initial body weight (g/fish) | 12.61 | 12.43 | 12.80 | 12.65 | 0.136 | 0.06 |
| Final body weight (g/fish) | 68.07 c | 93.47 b | 91.65 b | 99.67 a | <0.02 | 15.63 |
| Final weight gain (g/fish) | 55.45 c | 81.03 b | 78.85 b | 87.01 a | <0.03 | 11.20 |
| Total feed intake (g/fish) | 81.83 | 96.80 | 92.17 | 82.80 | 0.09 | 14.60 |
| Feed conversion ratio | 1.48 a | 1.19 b | 1.17 b | 0.95 c | <0.02 | 0.003 |
| Specific growth rate (%) | 2.01 c | 2.40 ab | 2.34 b | 2.46 a | <0.001 | 0.03 |
| Protein efficiency ratio | 2.12 c | 2.62 b | 2.67 b | 3.28 a | <0.001 | 0.019 |
| Survival (%) | 92 c | 94 b | 95 b | 97 a | 0.02 | 4.65 |
| Net profit | 0.08 c | 0.14 ab | 0.13 b | 0.14 a | 0.04 | 0.01 |
| Economic efficiency | 1.21 b | 1.58 a | 1.45 a | 1.45 a | <0.001 | 0.16 |
| Feed cost/kg gain | 1.18 a | 1.05 b | 1.11 ab | 1.12 ab | 0.04 | 1.33 |
| Parameter | Experimental Group | p-Value | SEM | |||
|---|---|---|---|---|---|---|
| Control | G1 | G2 | G3 | |||
| Ht (%) | 28.88 | 28.38 | 29.10 | 28.70 | 0.49 | 0.09 |
| Hb (g/dL) | 8.80 | 9.27 | 10.01 | 10.17 | 0.345 | 0.34 |
| RBCs (×106/μL) | 1.93 | 2.20 | 2.10 | 2.70 | 0.06 | 0.08 |
| ALT (U/L) | 64.97 | 62.96 | 65.80 | 63.80 | 0.86 | 3.25 |
| AST(U/L) | 21.27 | 21.53 | 21.00 | 21.16 | 0.09 | 1.96 |
| Creatinine (mg/dL) | 0.39 | 0.37 | 0.38 | 0.39 | 0.93 | 0.002 |
| Urea (mg/dL) | 6.33 | 6.03 | 6.16 | 6.03 | 0.069 | 0.06 |
| Cholesterol (mg/dL) | 77.87 a | 79.03 a | 72.13 b | 67.20 c | 0.02 | 15.30 |
| Triacylglycerol (mg/dL) | 52.60 a | 53.40 a | 47.06 ab | 42.70 b | 0.03 | 10.25 |
| Serum lysozyme (μg/mL) | 1.03 d | 1.32 c | 1.57 b | 1.98 a | <0.001 | 0.01 |
| Serum alternative complementary (u/mL) | 259 c | 271 b | 272 b | 283 a | 0.03 | 19.36 |
| MPO (μmoL/L, OD 450 nm) | 0.51 c | 0.50 c | 0.75 b | 0.90 a | <0.001 | 0.003 |
| IgM (μg/mL) | 29.49 d | 30.20 c | 35.10 b | 37.90 a | <0.001 | 2.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, D.; Shahin, S.E.; Alqahtani, L.S.; Hassan, Z.; Althobaiti, F.; Albogami, S.; Soliman, M.M.; El-Malt, R.M.S.; Al-Harthi, H.F.; Alqadri, N.; et al. Exploring the Interactive Effects of Thymol and Thymoquinone: Moving towards an Enhanced Performance, Gross Margin, Immunity and Aeromonas sobria Resistance of Nile Tilapia (Oreochromis niloticus). Animals 2022, 12, 3034. https://doi.org/10.3390/ani12213034
Ibrahim D, Shahin SE, Alqahtani LS, Hassan Z, Althobaiti F, Albogami S, Soliman MM, El-Malt RMS, Al-Harthi HF, Alqadri N, et al. Exploring the Interactive Effects of Thymol and Thymoquinone: Moving towards an Enhanced Performance, Gross Margin, Immunity and Aeromonas sobria Resistance of Nile Tilapia (Oreochromis niloticus). Animals. 2022; 12(21):3034. https://doi.org/10.3390/ani12213034
Chicago/Turabian StyleIbrahim, Doaa, Sara E. Shahin, Leena S. Alqahtani, Zeinab Hassan, Fayez Althobaiti, Sarah Albogami, Mohamed Mohamed Soliman, Rania M. S. El-Malt, Helal F. Al-Harthi, Nada Alqadri, and et al. 2022. "Exploring the Interactive Effects of Thymol and Thymoquinone: Moving towards an Enhanced Performance, Gross Margin, Immunity and Aeromonas sobria Resistance of Nile Tilapia (Oreochromis niloticus)" Animals 12, no. 21: 3034. https://doi.org/10.3390/ani12213034
APA StyleIbrahim, D., Shahin, S. E., Alqahtani, L. S., Hassan, Z., Althobaiti, F., Albogami, S., Soliman, M. M., El-Malt, R. M. S., Al-Harthi, H. F., Alqadri, N., Elabbasy, M. T., & El-Hamid, M. I. A. (2022). Exploring the Interactive Effects of Thymol and Thymoquinone: Moving towards an Enhanced Performance, Gross Margin, Immunity and Aeromonas sobria Resistance of Nile Tilapia (Oreochromis niloticus). Animals, 12(21), 3034. https://doi.org/10.3390/ani12213034












