The Implication of Serum Autoantibodies in Prognosis of Canine Mammary Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Collection
2.2. Preparation of Recombinant Canine Proteins
2.3. Immunoblotting for Serum AAb Detection
2.4. Procedures of an Indirect Enzyme-Linked Immunosorbent Assay (ELISA) for Serum AAb Detection
2.5. Follow-Up of CMT Patients
2.6. Statistical Analysis
3. Results
3.1. Association of Serum AAb Levels with Malignancy and Progression of CMT
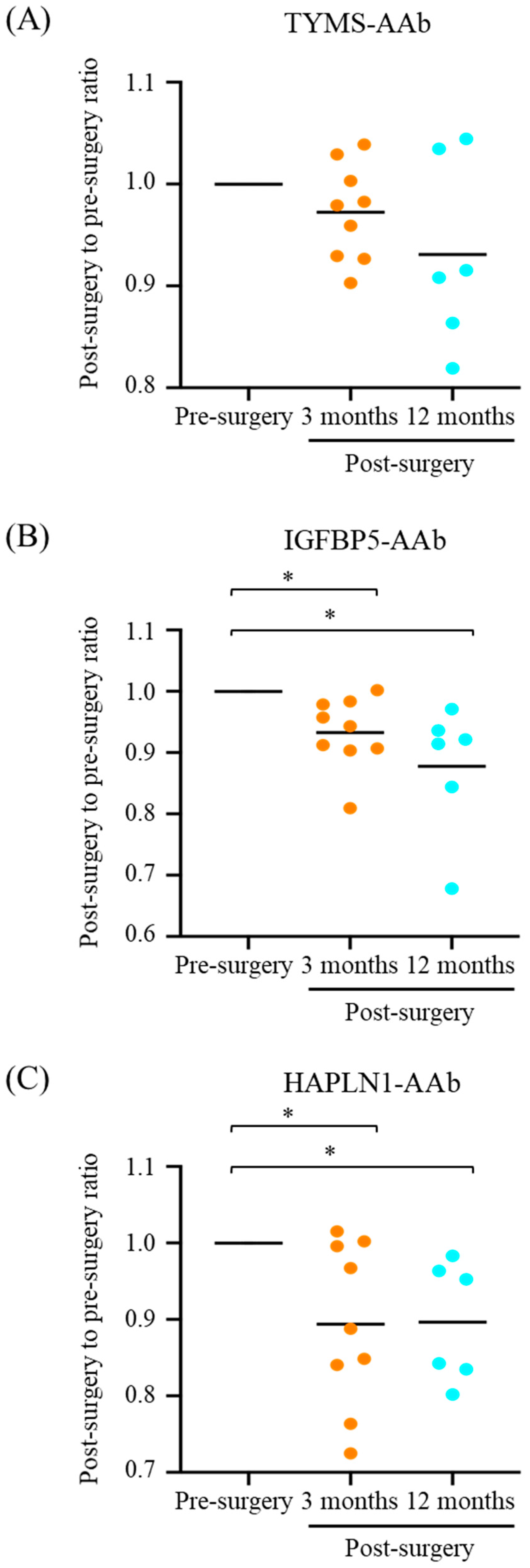
3.2. Declined Serum Levels of CMT-Associated AAbs after CMT Resection
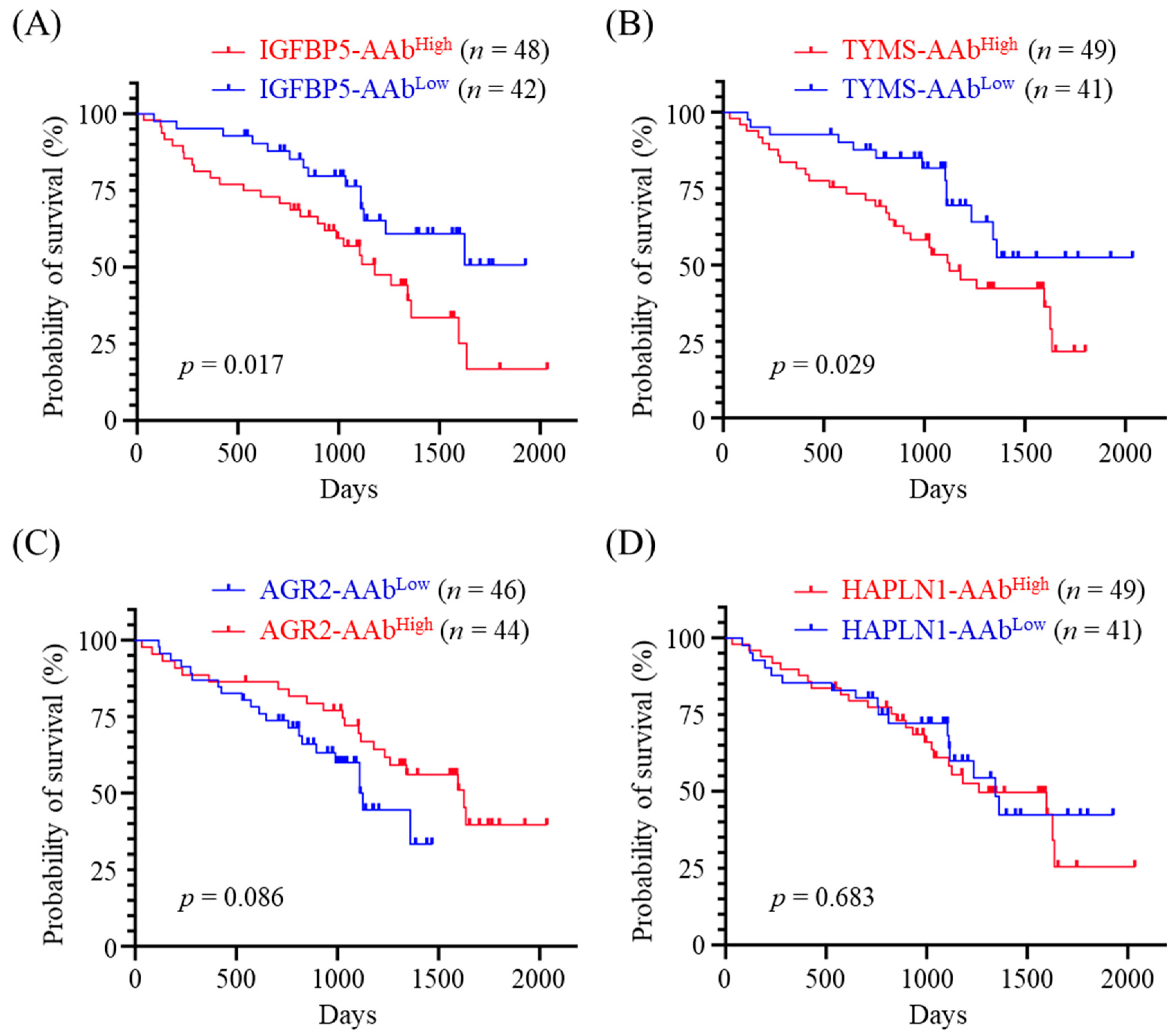
3.3. Correlation between Presurgical Serum AAb Level and Overall Survival Time of CMT Dogs
3.4. Effectiveness of Utilizing the Serum AAb Levels for OS Prediction
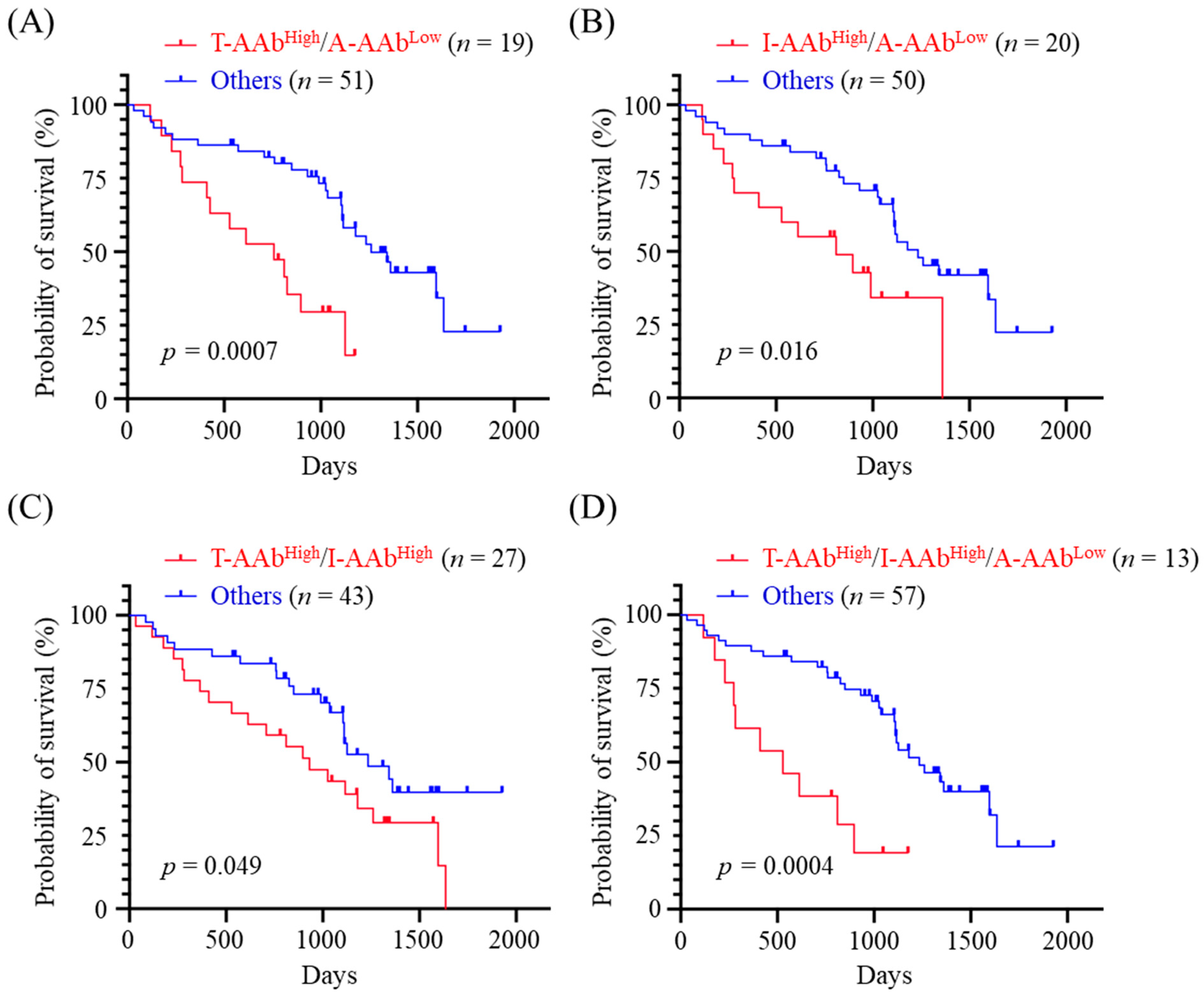
3.5. Correlation between Combined Panels of Serum AAb Levels and OS of CMT Dogs
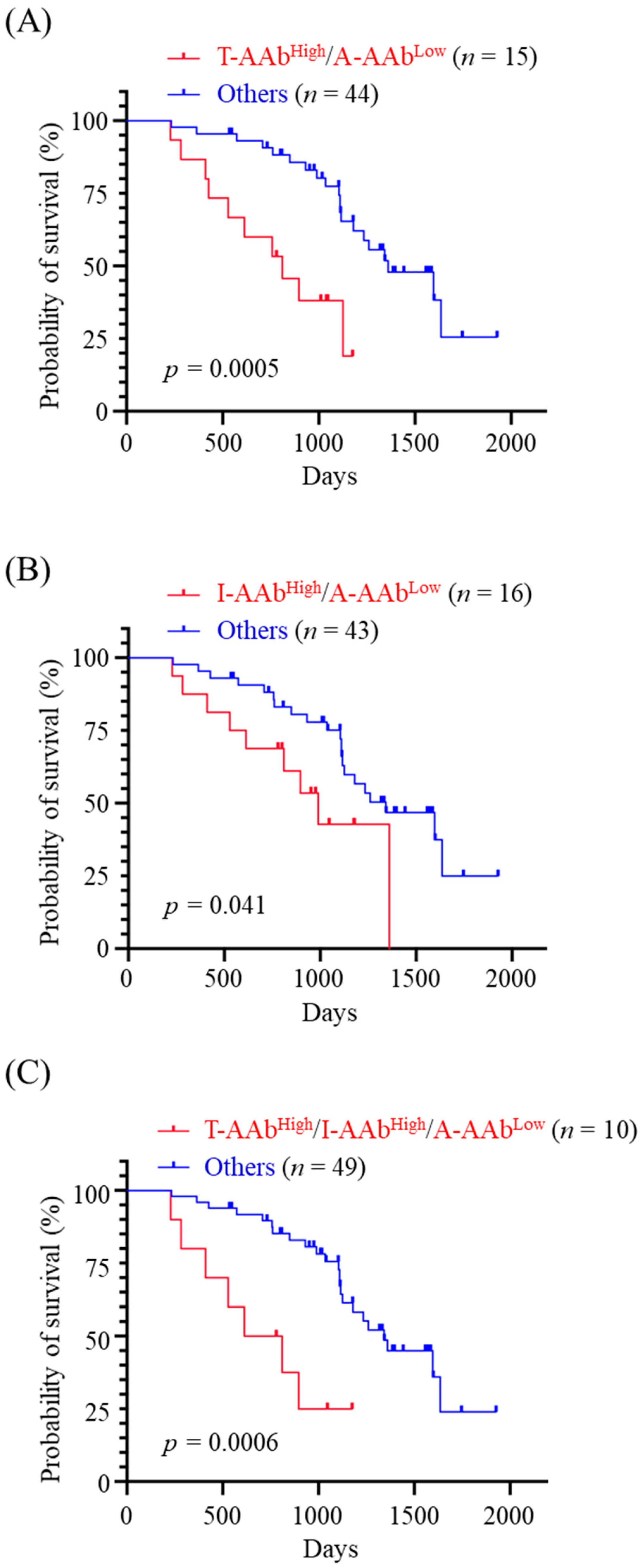
3.6. Correlation between the Combined Panels of Serum AAb Levels and OS of MMT Dogs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldschmidt, M.; Peña, L.; Rasotto, R.; Zappulli, V. Classification and grading of canine mammary tumors. Vet. Pathol. 2011, 48, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Rasotto, R.; Berlato, D.; Goldschmidt, M.H.; Zappulli, V. Prognostic Significance of Canine Mammary Tumor Histologic Subtypes: An Observational Cohort Study of 229 Cases. Vet. Pathol. 2017, 54, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Canadas, A.; Franca, M.; Pereira, C.; Vilaca, R.; Vilhena, H.; Tinoco, F.; Silva, M.J.; Ribeiro, J.; Medeiros, R.; Oliveira, P.; et al. Canine Mammary Tumors: Comparison of Classification and Grading Methods in a Survival Study. Vet. Pathol. 2019, 56, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, F.; Tamburro, R.; Aste, G.; Falerno, I.; Del Signore, F.; Simeoni, F.; Patsikas, M.; Gianfelici, J.; Terragni, R.; Attorri, V.; et al. Lymphatic Drainage Mapping with Indirect Lymphography for Canine Mammary Tumors. Animals 2021, 11, 1115. [Google Scholar] [CrossRef]
- Santos, A.A.; Lopes, C.C.; Ribeiro, J.R.; Martins, L.R.; Santos, J.C.; Amorim, I.F.; Gartner, F.; Matos, A.J. Identification of prognostic factors in canine mammary malignant tumours: A multivariable survival study. BMC Vet. Res. 2013, 9, 1. [Google Scholar] [CrossRef]
- Kaszak, I.; Ruszczak, A.; Kanafa, S.; Kacprzak, K.; Król, M.; Jurka, P. Current biomarkers of canine mammary tumors. Acta Vet. Scand. 2018, 60, 66. [Google Scholar] [CrossRef]
- Campos, L.C.; Silva, J.O.; Santos, F.S.; Araujo, M.R.; Lavalle, G.E.; Ferreira, E.; Cassali, G.D. Prognostic significance of tissue and serum HER2 and MUC1 in canine mammary cancer. J. Vet. Diagn. Investig. 2015, 27, 531–535. [Google Scholar] [CrossRef]
- Yuan, S.H.; Chang, S.C.; Huang, Y.; Liu, H.P. Serum Level of Tumor-Overexpressed AGR2 Is Significantly Associated with Unfavorable Prognosis of Canine Malignant Mammary Tumors. Animals 2021, 11, 2923. [Google Scholar] [CrossRef]
- Song, D.W.; Ro, W.B.; Park, H.M. Evaluation of circulating PD-1 and PD-L1 as diagnostic biomarkers in dogs with tumors. J. Vet. Sci. 2021, 22, e75. [Google Scholar] [CrossRef]
- Nascimento, C.; Urbano, A.C.; Gameiro, A.; Ferreira, J.; Correia, J.; Ferreira, F. Serum PD-1/PD-L1 Levels, Tumor Expression and PD-L1 Somatic Mutations in HER2-Positive and Triple Negative Normal-Like Feline Mammary Carcinoma Subtypes. Cancers (Basel) 2020, 12, 1386. [Google Scholar] [CrossRef]
- Gameiro, A.; Nascimento, C.; Correia, J.; Ferreira, F. VISTA Is a Diagnostic Biomarker and Immunotherapy Target of Aggressive Feline Mammary Carcinoma Subtypes. Cancers 2021, 13, 5559. [Google Scholar] [CrossRef] [PubMed]
- Sexauer, D.; Gray, E.; Zaenker, P. Tumour- associated autoantibodies as prognostic cancer biomarkers- a review. Autoimmun. Rev. 2022, 21, 103041. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kashaninejad, N.; Masud, M.K.; Yamauchi, Y.; Nguyen, N.-T.; Shiddiky, M.J.A. Autoantibodies as diagnostic and prognostic cancer biomarker: Detection techniques and approaches. Biosens. Bioelectron. 2019, 139, 111315. [Google Scholar] [CrossRef]
- Cyll, K.; Ersvær, E.; Vlatkovic, L.; Pradhan, M.; Kildal, W.; Avranden Kjær, M.; Kleppe, A.; Hveem, T.S.; Carlsen, B.; Gill, S.; et al. Tumour heterogeneity poses a significant challenge to cancer biomarker research. Br. J. Cancer 2017, 117, 367–375. [Google Scholar] [CrossRef]
- Wu, C.C.; Chang, S.C.; Zeng, G.Y.; Chu, H.W.; Huang, Y.; Liu, H.P. Proteome analyses reveal positive association of COL2A1, MPO, TYMS, and IGFBP5 with canine mammary gland malignancy. Proteom. Clin. Appl. 2019, 13, 1800151. [Google Scholar] [CrossRef]
- Fu, Z.; Jiao, Y.; Li, Y.; Ji, B.; Jia, B.; Liu, B. TYMS presents a novel biomarker for diagnosis and prognosis in patients with pancreatic cancer. Medicine 2019, 98, e18487. [Google Scholar] [CrossRef]
- Song, S.; Tian, B.; Zhang, M.; Gao, X.; Jie, L.; Liu, P.; Li, J. Diagnostic and prognostic value of thymidylate synthase expression in breast cancer. Clin. Exp. Pharmacol. Physiol. 2021, 48, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Shan, F.; Liu, Y.L.; Wang, Q.; Shi, Y.L. Thymidylate synthase predicts poor response to pemetrexed chemotherapy in patients with advanced breast cancer. Oncol. Lett. 2018, 16, 3274–3280. [Google Scholar] [CrossRef]
- Allan, G.J.; Beattie, J.; Flint, D.J. The role of IGFBP-5 in mammary gland development and involution. Domest. Anim. Endocrinol. 2004, 27, 257–266. [Google Scholar] [CrossRef]
- Liang, P.-I.; Wang, Y.-H.; Wu, T.-F.; Wu, W.-R.; Liao, A.C.; Shen, K.-H.; Hsing, C.-H.; Shiue, Y.-L.; Huang, H.-Y.; Hsu, H.-P.; et al. IGFBP-5 overexpression as a poor prognostic factor in patients with urothelial carcinomas of upper urinary tracts and urinary bladder. J. Clin. Pathol. 2013, 66, 573–582. [Google Scholar] [CrossRef]
- Baxter, R.C. IGF binding proteins in cancer: Mechanistic and clinical insights. Nat. Rev. Cancer 2014, 14, 329–341. [Google Scholar] [CrossRef]
- Wang, J.; Ding, N.; Li, Y.; Cheng, H.; Wang, D.; Yang, Q.; Deng, Y.; Yang, Y.; Li, Y.; Ruan, X.; et al. Insulin-like growth factor binding protein 5 (IGFBP5) functions as a tumor suppressor in human melanoma cells. Oncotarget 2015, 6, 20636–20649. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Goparaju, C.M.V.; Ivanov, S.V.; Nonaka, D.; Cruz, C.; Beck, A.; Lonardo, F.; Wali, A.; Pass, H.I. Protumorigenic role of HAPLN1 and its IgV domain in malignant pleural mesothelioma. Clin. Cancer Res. 2009, 15, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Ecker, B.L.; Kaur, A.; Douglass, S.M.; Webster, M.R.; Almeida, F.V.; Marino, G.E.; Sinnamon, A.J.; Neuwirth, M.G.; Alicea, G.M.; Ndoye, A.; et al. Age-Related Changes in HAPLN1 Increase Lymphatic Permeability and Affect Routes of Melanoma Metastasis. Cancer Discov. 2019, 9, 82–95. [Google Scholar] [CrossRef]
- Chang, S.C.; Yuan, S.H.; Li, C.Y.; Chang, H.M.; Wang, H.C.; Pan, Y.A.; Hsueh, P.C.; Wu, C.C.; Yang, Y.; Liu, H.P. Significant association of serum autoantibodies to TYMS, HAPLN1 and IGFBP5 with early stage canine malignant mammary tumours. Vet. Comp. Oncol. 2021, 19, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Higa, A.; Mulot, A.; Delom, F.; Bouchecareilh, M.; Nguyên, D.T.; Boismenu, D.; Wise, M.J.; Chevet, E. Role of pro-oncogenic protein disulfide isomerase (PDI) family member anterior gradient 2 (AGR2) in the control of endoplasmic reticulum homeostasis. J. Biol. Chem. 2011, 286, 44855–44868. [Google Scholar] [CrossRef] [PubMed]
- Maurel, M.; Obacz, J.; Avril, T.; Ding, Y.P.; Papadodima, O.; Treton, X.; Daniel, F.; Pilalis, E.; Hörberg, J.; Hou, W.; et al. Control of anterior GRadient 2 (AGR2) dimerization links endoplasmic reticulum proteostasis to inflammation. EMBO Mol. Med. 2019, 11, e10120. [Google Scholar] [CrossRef] [PubMed]
- Fessart, D.; Domblides, C.; Avril, T.; Eriksson, L.A.; Begueret, H.; Pineau, R.; Malrieux, C.; Dugot-Senant, N.; Lucchesi, C.; Chevet, E.; et al. Secretion of protein disulphide isomerase AGR2 confers tumorigenic properties. eLife 2016, 5, e13887. [Google Scholar] [CrossRef]
- Heagerty, P.J.; Lumley, T.; Pepe, M.S. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000, 56, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Wang, B.; Wang, P.; Wang, X.; Ma, Y.; Dai, L.; Shi, J.; Wang, K.; Sun, G.; Ye, H.; et al. Identification of novel autoantibody signatures and evaluation of a panel of autoantibodies in breast cancer. Cancer Sci. 2021, 112, 3388–3400. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.L.; Pottala, J.V.; Nagata, S.; Egland, K.A. Longitudinal autoantibody responses against tumor-associated antigens decrease in breast cancer patients according to treatment modality. BMC Cancer 2018, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashimi, A.A.; Lebeau, P.; Majeed, F.; Polena, E.; Lhotak, Š.; Collins, C.A.F.; Pinthus, J.H.; Gonzalez-Gronow, M.; Hoogenes, J.; Pizzo, S.V.; et al. Autoantibodies against the cell surface-associated chaperone GRP78 stimulate tumor growth via tissue factor. J. Biol. Chem. 2017, 292, 21180–21192. [Google Scholar] [CrossRef]
- Tabuchi, Y.; Shimoda, M.; Kagara, N.; Naoi, Y.; Tanei, T.; Shimomura, A.; Shimazu, K.; Kim, S.J.; Noguchi, S. Protective effect of naturally occurring anti-HER2 autoantibodies on breast cancer. Breast Cancer Res. Treat. 2016, 157, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, C.Q.; Guo, S.J.; Guo, W.; Jiang, H.W.; Li, H.C.; Tao, S.C. Longitudinal serum autoantibody repertoire profiling identifies surgery-associated biomarkers in lung adenocarcinoma. EBioMedicine 2020, 53, 102674. [Google Scholar] [CrossRef] [PubMed]


| CMT | Benign Mammary Tumor (BMT) | Malignant Mammary Tumor (MMT) | ||||
|---|---|---|---|---|---|---|
| Group | Case Number (%) | Median (Range) Age, Year | Median (Range) B.W., kg | Case Number (%) | Median (Range) Age, Year | Median (Range) B.W., kg |
| Total | 20 (100) | 8.4 (3–16) | 5.4 (2.45–38) | 70 (100) | 10 (1–16) | 7.4 (1.7–44.6) |
| Breed | ||||||
| Pedigree | 18 (90) | 8.2 (3–15) | 5.4 (2.45–38) | 55 (78.6) | 9 (1–16) | 5.9 (1.7–38) |
| Mixed | 2 (10) | 14.5 (13–16) | 18.4 (13.4–23.4) | 15 (21.4) | 12 (8–16) | 15.3 (2.4–44.6) |
| Neuter status | ||||||
| Yes | 6 (30) | 10 (9–15) | 7.2 (2.45–19.4) | 29 (41.4) | 12 (3.5–16) | 11.4 (2.1–44.6) |
| No | 14 (70) | 7.9 (3–16) | 5.5 (2.7–38) | 41 (58.6) | 8.5 (1–13) | 5.6 (1.7–30.4) |
| Clinical stage 1 | ||||||
| I | - | - | 34 (48.6) | 9 (1–16) | 5.05 (1.7–20.6) | |
| II | - | - | - | 9 (12.9) | 11 (8–16) | 8.5 (2.1–44.6) |
| III | - | - | 16 (22.8) | 12 (8–16) | 11.8 (2.36–29.8) | |
| IV | 7 (10) | 13 (8–15) | 10.8 (4.2–38) | |||
| V | - | - | 4 (5.7) | 11 (3.5–11) | 19.7 (12.9–26.4) | |
| Metastasis | - | - | ||||
| No (I/II/III) | - | - | 59 (84.3) | 10 (1–16) | 6.9 (1.7–44.6) | |
| Yes (IV/V) | - | - | 11 (15.7) | 11 (3.5–15) | 15.6 (4.2–38) | |
| Pre-Surgery | Post-Surgery | ||||
|---|---|---|---|---|---|
| 3 Months | p Value 1,2 | 12 Months | p Value 1,2 | ||
| Case number | 11 | 9 | - | 6 | - |
| Serum AAb levels (mean ± SD) | |||||
| TYMS-AAb | 0.534 ± 0.109 | 0.487 ± 0.087 | 0.203 | 0.499 ± 0.100 | 0.156 |
| IGFBP5-AAb | 0.281 ± 0.057 | 0.245 ± 0.033 | 0.008 ** | 0.253 ± 0.038 | 0.031 * |
| HAPLN1-AAb | 0.248 ± 0.043 | 0.225 ± 0.057 | 0.027 * | 0.230 ± 0.037 | 0.031 * |
| CMT (BMT, MMT) | MMT (Stage I–V) | Non-Metastasized CMT (Benign, I/II/III) | Non-Metastasized MMT (I/II/III) | |
|---|---|---|---|---|
| OST (days) | ||||
| Median | 1037.5 | 1014.5 | 1103 | 1047 |
| Range | 33–2035 | 22–1927 | 229–2035 | 229–1927 |
| AUC for OS prediction | ||||
| TYMS-AAb | 0.600 | 0.611 | 0.623 | 0.664 |
| IGFBP5-AAb | 0.639 | 0.616 | 0.671 | 0.661 |
| HAPLN1-AAb | 0.500 | 0.489 | 0.591 | 0.614 |
| AGR2-AAb | 0.491 | 0.467 | 0.491 | 0.458 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.H.-C.; Chang, S.-C.; Chou, P.-Y.; Yang, Y.; Liu, H.-P. The Implication of Serum Autoantibodies in Prognosis of Canine Mammary Tumors. Animals 2022, 12, 2463. https://doi.org/10.3390/ani12182463
Yuan SH-C, Chang S-C, Chou P-Y, Yang Y, Liu H-P. The Implication of Serum Autoantibodies in Prognosis of Canine Mammary Tumors. Animals. 2022; 12(18):2463. https://doi.org/10.3390/ani12182463
Chicago/Turabian StyleYuan, Stephen Hsien-Chi, Shih-Chieh Chang, Pei-Yi Chou, Youngsen Yang, and Hao-Ping Liu. 2022. "The Implication of Serum Autoantibodies in Prognosis of Canine Mammary Tumors" Animals 12, no. 18: 2463. https://doi.org/10.3390/ani12182463
APA StyleYuan, S. H.-C., Chang, S.-C., Chou, P.-Y., Yang, Y., & Liu, H.-P. (2022). The Implication of Serum Autoantibodies in Prognosis of Canine Mammary Tumors. Animals, 12(18), 2463. https://doi.org/10.3390/ani12182463






