Use of a Smartphone-Based Device for Fundus Examination in Birds: A Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Ophthalmic Examination and Pupil Dilation
2.2. Fundus Examination
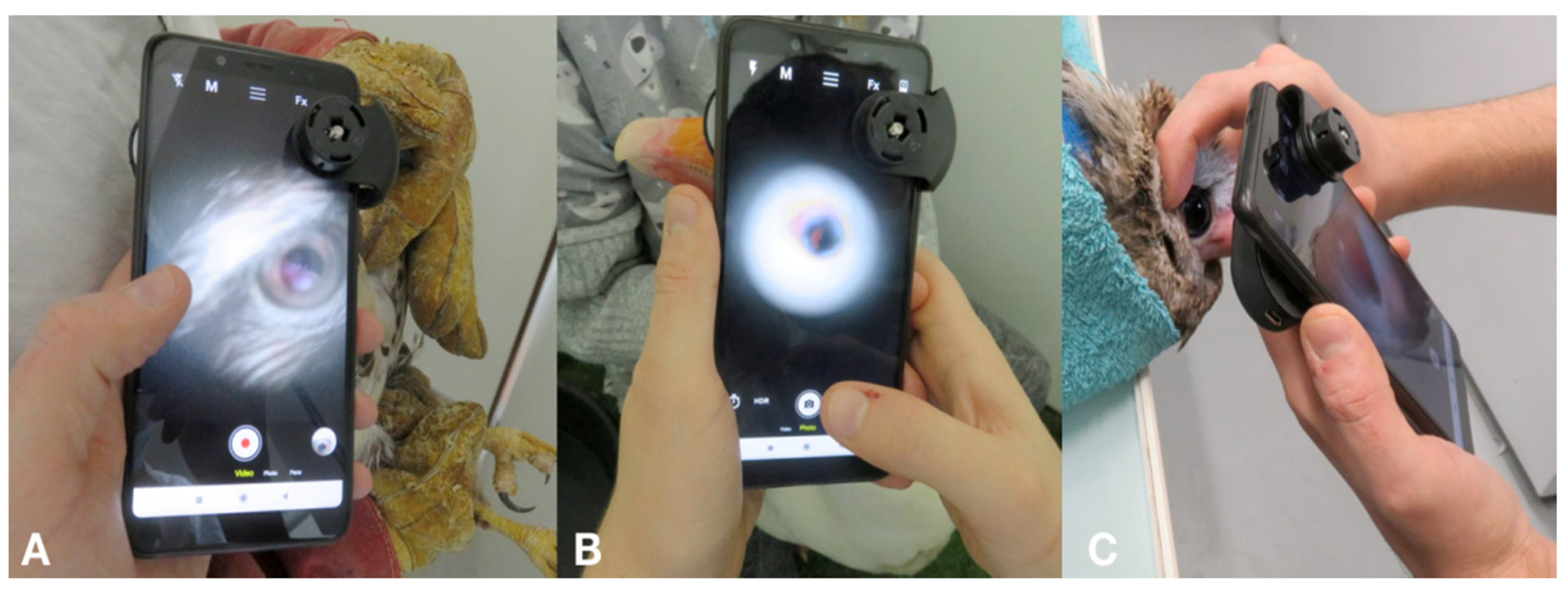
2.3. Photographic Equipment
2.4. Statistical Evaluation
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, C.J.; Kern, T.J.; McKeever, K.; McKeever, L.; MacCoy, D. Ocular lesions in free-living raptors. J. Am. Vet. Med. Assoc. 1982, 181, 1302–1304. [Google Scholar] [PubMed]
- Labelle, A.L.; Whittington, J.K.; Breaux, C.B.; Labelle, P.; Mitchell, M.A.; Zarfoss, M.K.; Schmidt, S.A.; Hamor, R.E. Clinical utility of a complete diagnostic protocol for the ocular evaluation of free-living raptors. Vet. Ophthalmol. 2011, 15, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M. Ocular consequences of trauma in raptors. Semin. Avian Exot. Pet Med. 1997, 6, 121–130. [Google Scholar] [CrossRef]
- Williams, D.L.; Gonzalez Villavincencio, C.M.; Wilson, S. Chronic ocular lesions in tawny owls (Strix aluco) injured by road traffic. Vet. Rec. 2006, 159, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Buyukmihci, N.C. Lesions in the ocular posterior segment of raptors. J. Am. Vet. Med. Assoc. 1985, 187, 1121–1124. [Google Scholar] [PubMed]
- Pauli, A.; Klauss, G.; Diehl, K.; Redig, P. Clinical Techniques: Considerations for Release of Raptors with Ocular Disease. J. Exot. Pet Med. 2007, 16, 101–103. [Google Scholar] [CrossRef]
- Seruca, C.; Molina-López, R.; Peña, T.; Leiva, M. Ocular consequences of blunt trauma in two species of nocturnal raptors (Athene noctua and Otus scops). Vet. Ophthalmol. 2011, 15, 236–244. [Google Scholar] [CrossRef]
- Barsotti, G.; Briganti, A.; Spratte, J.R.; Ceccherelli, R.; Breghi, G. Bilateral mydriasis in common buzzards (Buteo buteo) and little owls (Athene noctua) induced by concurrent topical administration of rocuronium bromide. Vet. Ophthalmol. 2010, 13, 35–40. [Google Scholar] [CrossRef]
- Barsotti, G.; Briganti, A.; Spratte, J.R.; Ceccherelli, R.; Breghi, G. Mydriatic effect of topically applied rocuronium bromide in tawny owls (Strix aluco): Comparison between two protocols. Vet. Ophthalmol. 2010, 13, 9–13. [Google Scholar] [CrossRef]
- Barsotti, G.; Briganti, A.; Spratte, J.R.; Ceccherelli, R.; Breghi, G. Safety and Efficacy of Bilateral Topical Application of Rocuronium Bromide for Mydriasis in European Kestrels (Falco tinnunculus). J. Avian Med. Surg. 2012, 26, 1–5. [Google Scholar] [CrossRef]
- Baine, K.; Hendrix, D.V.H.; Kuhn, S.E.; Souza, M.J.; Jones, M.P. The Efficacy and Safety of Topical Rocuronium Bromide to Induce Bilateral Mydriasis in Hispaniolan Amazon Parrots (Amazona ventralis). J. Avian Med. Surg. 2016, 30, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Petritz, O.A.; Guzman, D.S.M.; Gustavsen, K.; Wiggans, K.T.; Kass, P.H.; Houck, E.; Murphy, C.J.; Paul-Murphy, J. Evaluation of the mydriatic effects of topical administration of rocuronium bromide in Hispaniolan Amazon parrots (Amazona ventralis). J. Am. Vet. Med. Assoc. 2016, 248, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Susanti, L.; Kang, S.; Lee, E.; Jeong, D.; Jeong, Y.; Park, S.; Seo, K. Efficacy of topical rocuronium bromide as a mydriatic agent in domestic pigeons (Columba livia). J. Vet. Med. Sci. 2021, 83, 501–506. [Google Scholar] [CrossRef]
- Cantero, F.; Ortillés, Á.; Peña, M.T.; Gogova, S.; Molina, R.; Ríos, J.; Leiva, M. Safety and efficacy of unilateral topical application of rocuronium bromide in healthy scops owls (Otus scops). Vet. Ophthalmol. 2021, 24, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M. Smartphone-Based Device in Exotic Pet Medicine. Vet. Clin. N. Am. Exot. Anim. Pract. 2019, 22, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, M.; Hacisoftaoglu, R.E. Comparison of smartphone-based retinal imaging systems for diabetic retinopathy detection using deep learning. BMC Bioinform. 2020, 21 (Suppl. S4), 259. [Google Scholar] [CrossRef]
- Bastawrous, A.; Giardini, M.E.; Bolster, N.M.; Peto, T.; Shah, N.; Livingstone, I.A.T.; Weiss, H.A.; Hu, S.; Rono, H.; Kuper, H.; et al. Clinical Validation of a Smartphone-Based Adapter for Optic Disc Imaging in Kenya. JAMA Ophthalmol. 2016, 134, 151–158. [Google Scholar] [CrossRef]
- Mndeme, F.G.; Mmbaga, B.T.; Kim, M.J.; Sinke, L.; Allen, L.; Mgaya, E.; Bastawrous, A.; MacLeod, D.; Burton, M.J.; Gilbert, C.; et al. Red reflex examination in reproductive and child health clinics for early detection of paediatric cataract and ocular media disorders: Cross-sectional diagnostic accuracy and feasibility studies from Kilimanjaro, Tanzania. Eye 2021, 35, 1347–1353. [Google Scholar] [CrossRef]
- Korbel, R.T. Avian ophthalmology—Principles and application. In Advancing and Promoting Avian Medicine and Stewardship; Institute for Avian Diseases, University Ludwig-Maximilian Munich Oberschleissheim: München, Germany, 2011; p. 37. [Google Scholar]
- Huber, N.; Mahr, K.; Tóth, Z.; Szarka, E.Z.; Çınar, Y.U.; Salmón, P.; Lendvai, Á.Z. The stressed bird in the hand: Influence of sampling design on the physiological stress response in a free-living songbird. Physiol. Behav. 2021, 238, 113488. [Google Scholar] [CrossRef]
- King, A.S.; McLelland, J. Birds: Their Structure & Function, 2nd ed.; Baillière Tindall: Eastbourne, UK, 1984; pp. 284–301. [Google Scholar]
- Gelatt, K.N.; Ben-Shlomo, G.; Gilger, B.C.; Hendrix, D.V.H.; Kern, T.J.; Plummer, C.E. Veterinary Ophthalmology Two-Volume Set, 6th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 2055–2084. [Google Scholar]
- David, L. Williams. Ophthalmology of Exotic Pets; John Wiley & Sons, Ltd.: New York, NY, USA, 2012; pp. 283–369. [Google Scholar]
- Barsotti, G.; Asti, M.; Giani, E.; Ceccherelli, R.; Briganti, A. Effect of topical ophthalmic instillation of rocuronium bromide on the intraocular pressure of kestrels (Falco tinnunculus) and little owls (Athene noctuae). J. Am. Vet. Med. Assoc. 2019, 255, 1359–1364. [Google Scholar] [CrossRef]
- Vollath, D. The influence of the scene parameters and of noise on the behaviour of automatic focusing algorithms. J. Microsc. 1988, 151, 133–146. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Tang, J.; Zhang, X.; Liu, X. Robust Automatic Focus Algorithm for Low Contrast Images Using a New Contrast Measure. Sensors 2011, 11, 8281–8294. [Google Scholar] [CrossRef] [PubMed]
- Maclary, E.T.; Phillips, B.; Wauer, R.; Boer, E.F.; Bruders, R.; Gilvarry, T.; Holt, C.; Yandell, M.; Shapiro, M.D. Two Genomic Loci Control Three Eye Colors in the Domestic Pigeon (Columba livia). Mol. Biol. Evol. 2021, 38, 5376–5390. [Google Scholar] [CrossRef]
- Craig, A.; Hulley, P. Iris colour in passerine birds: Why be bright-eyed? S. Afr. J. Sci. 2004, 100, 584–588. [Google Scholar]
- Selek, M. A New Autofocusing Method Based on Brightness and Contrast for Color Cameras. Adv. Electr. Comput. Eng. 2016, 16, 39–44. [Google Scholar] [CrossRef]
- Balland, O.; Russo, A.; Isard, P.F.; Mathieson, I.; Semeraro, F.; Dulaurent, T. Assessment of a smartphone-based camera for fundus imaging in animals. Vet. Ophthalmol. 2016, 20, 89–94. [Google Scholar] [CrossRef]
- Moore, B.A.; Teixeira, L.B.C.; Sponsel, W.E.; Dubielzig, R.R. The consequences of avian ocular trauma: Histopathological evidence and implications of acute and chronic disease. Vet. Ophthalmol. 2017, 20, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Keenan, A.V.; Oster, S.; McMullen, R.J.; Shaw, G.; Dubielzig, R.R.; Teixeira, L.B.C.; Bellah, J.; Moore, P.A.; Boveland, S.D. Clinical and pathologic evaluation of chorioretinal lesions in wild owl species. Vet. Ophthalmol. 2021, 25, 128–139. [Google Scholar] [CrossRef]
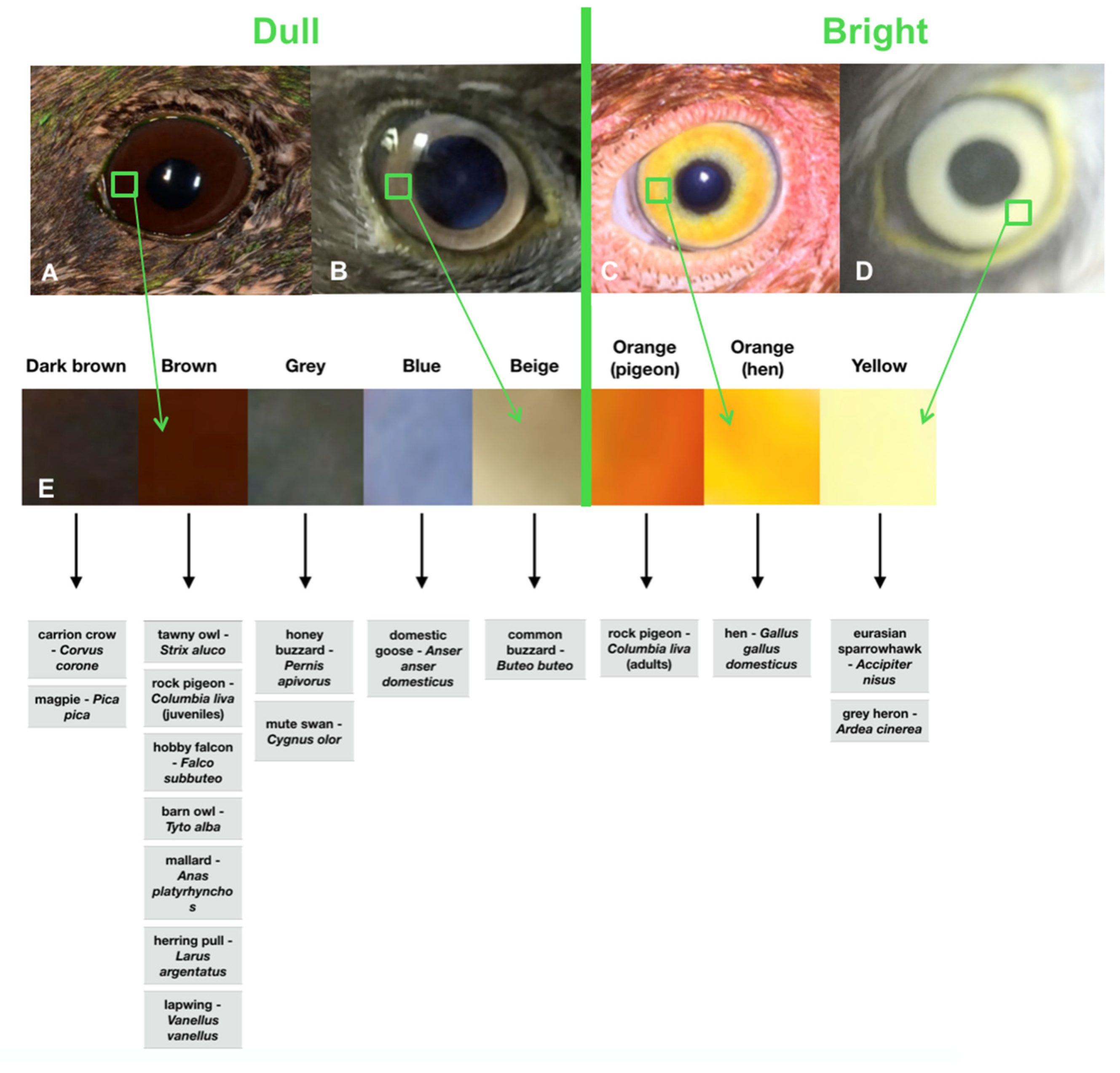



| Species (Number of Animals) | Order | Family | Shape of the Globe | Iris Coloration |
|---|---|---|---|---|
| tawny owl—Strix aluco (12) | Strigiformes | Strigidae | Tubular | Brown |
| rock pigeon—Columbia liva (6) | Columbiformes | Columbidae | Flat | Bright orange (Adults); Brown (Juveniles) |
| hen—Gallus gallus domesticus (4) | Galliformes | Phasianidae | Flat | Bright orange |
| common buzzard—Buteo buteo (3) | Acciptriformes | Accipitridae | Globoid | Beige |
| carrion crow—Corvus corone (2) | Passeriformes | Corvidae | Globoid | Dark brown |
| hobby falcon—Falco subbuteo (2) | Falconiformes | Falconidae | Globoid | Brown |
| barn owl—Tyto alba (1) | Strigiformes | Tytonidae | Tubular | Brown |
| honey buzzard—Pernis apivorus (1) | Acciptriformes | Accipitridae | Globoid | Grey |
| mallard—Anas platyrhynchos (1) | Anseriformes | Anatidae | Flat | Brown |
| eurasian sparrowhawk—Accipiter nisus (1) | Acciptriformes | Accipitridae | Globoid | Yellow |
| herring pull—Larus argentatus (1) | Charadriformes | Laridae | Flat | Brown |
| grey heron—Ardea cinerea (1) | Pelecaniformes | Ardeidae | Flat | Yellow |
| domestic goose—Anser anser domesticus (1) | Anseriformes | Anatidae | Flat | Blue |
| lapwing—Vanellus vanellus (1) | Charadriformes | Charadridae | Flat | Brown |
| magpie—Pica pica (1) | Passeriformes | Corvidae | Globoid | Dark brown |
| mute swan—Cygnus olor (1) | Anseriformes | Anatidae | Flat | Grey |
| Species (Number of Animals) | Satisfactory (n/Total Eye Number per Species) | Acceptable (n/Total Eye Number per Species | Absent (n/Total Eye Number per Species |
|---|---|---|---|
| tawny owl—Strix aluco (12) | 7/24 eyes | 10/24 eyes | 7/24 eyes |
| rock pigeon—Columbia livia (6) | 1/12 eyes | 5/12 eyes | 6/12 eyes |
| hen—Gallus gallus domesticus (4) | - | 4/8 eyes | 4/8 eyes |
| common buzzard—Buteo buteo (3) | 2/6 eyes | 2/6 eyes | 2/6 eyes |
| carrion crow—Corvus corone (2) | - | - | 4/4 eyes |
| hobby falcon—Falco subbuteo (2) | 2/4 eyes | 2/4 eyes | - |
| barn owl—Tyto alba (1) | - | 2/2 eyes | - |
| honey buzzard—Pernis apivorus (1) | - | - | 2/2 eyes |
| mallard—Anas platyrhynchos (1) | 1/2 eyes | - | 1/2 eyes |
| eurasian sparrowhawk—Accipiter nisus (1) | 1/2 eyes | 1/2 eyes | - |
| herring pull—Larus argentatus (1) | - | 2/2 eyes | - |
| grey heron—Ardea cinerea (1) | 2/2 eyes | - | - |
| domestic goose—Anser anser domesticus (1) | 2/2 eyes | - | - |
| lapwing—Vanellus vanellus (1) | - | - | 2/2 eyes |
| magpie—Pica pica (1) | - | - | 1/1 eye |
| mute swan—Cygnus olor (1) | 1/2 eyes | 1/2 eyes | - |
| 39 | 19 | 29 | 29 |
| Species (Number of Animals) | Satisfactory (n/Total Eye Number per Species) | Acceptable (n/Total Eye Number per Species) | Unsatisfactory (n/Total Eye Number per Species) |
|---|---|---|---|
| tawny owl—Strix aluco (12) | 24/24 eyes | - | - |
| rock pigeon—Columbia livia (6) | 6/12 eyes | - | 6/12 eyes |
| hen—Gallus gallus domesticus (4) | - | - | 8/8 eyes |
| common buzzard—Buteo buteo (3) | 6/6 eyes | - | - |
| carrion crow—Corvus corone (2) | - | - | 4/4 eyes |
| hobby falcon—Falco subbuteo (2) | - | 4/4 eyes | - |
| barn owl—Tyto alba (1) | 2/2 eyes | - | - |
| honey buzzard —Pernis apivorus (1) | 2/2 eyes | - | - |
| mallard—Anas platyrhynchos (1) | 2/2 eyes | - | - |
| eurasian sparrowhawk—Accipiter nisus (1) | 2/2 eyes | - | - |
| herring pull—Larus argentatus (1) | - | 2/2 eyes | - |
| grey heron—Ardea cinerea (1) | - | 2/2 eyes | - |
| domestic goose—Anser anser domesticus (1) | 2/2 eyes | - | - |
| lapwing—Vanellus vanellus (1) | - | 2/2 eyes | - |
| magpie—Pica pica (1) | - | 1/1 eye | - |
| mute swan—Cygnus olor (1) | 2/2 eyes | - | - |
| 39 | 48 | 11 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grillot, A.-E.; Coutant, T.; Louste, E.; Le Barzic, C.; Arné, P.; Payen, G.; Huynh, M. Use of a Smartphone-Based Device for Fundus Examination in Birds: A Pilot Study. Animals 2022, 12, 2429. https://doi.org/10.3390/ani12182429
Grillot A-E, Coutant T, Louste E, Le Barzic C, Arné P, Payen G, Huynh M. Use of a Smartphone-Based Device for Fundus Examination in Birds: A Pilot Study. Animals. 2022; 12(18):2429. https://doi.org/10.3390/ani12182429
Chicago/Turabian StyleGrillot, Aure-Eline, Thomas Coutant, Eva Louste, Cécile Le Barzic, Pascal Arné, Guillaume Payen, and Minh Huynh. 2022. "Use of a Smartphone-Based Device for Fundus Examination in Birds: A Pilot Study" Animals 12, no. 18: 2429. https://doi.org/10.3390/ani12182429
APA StyleGrillot, A.-E., Coutant, T., Louste, E., Le Barzic, C., Arné, P., Payen, G., & Huynh, M. (2022). Use of a Smartphone-Based Device for Fundus Examination in Birds: A Pilot Study. Animals, 12(18), 2429. https://doi.org/10.3390/ani12182429





