Early Post-Hatch Nutrition Influences Performance and Muscle Growth in Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sampling
2.3. Genetic Analysis
2.4. Histology
2.5. Morphometry
3. Results
3.1. Histology
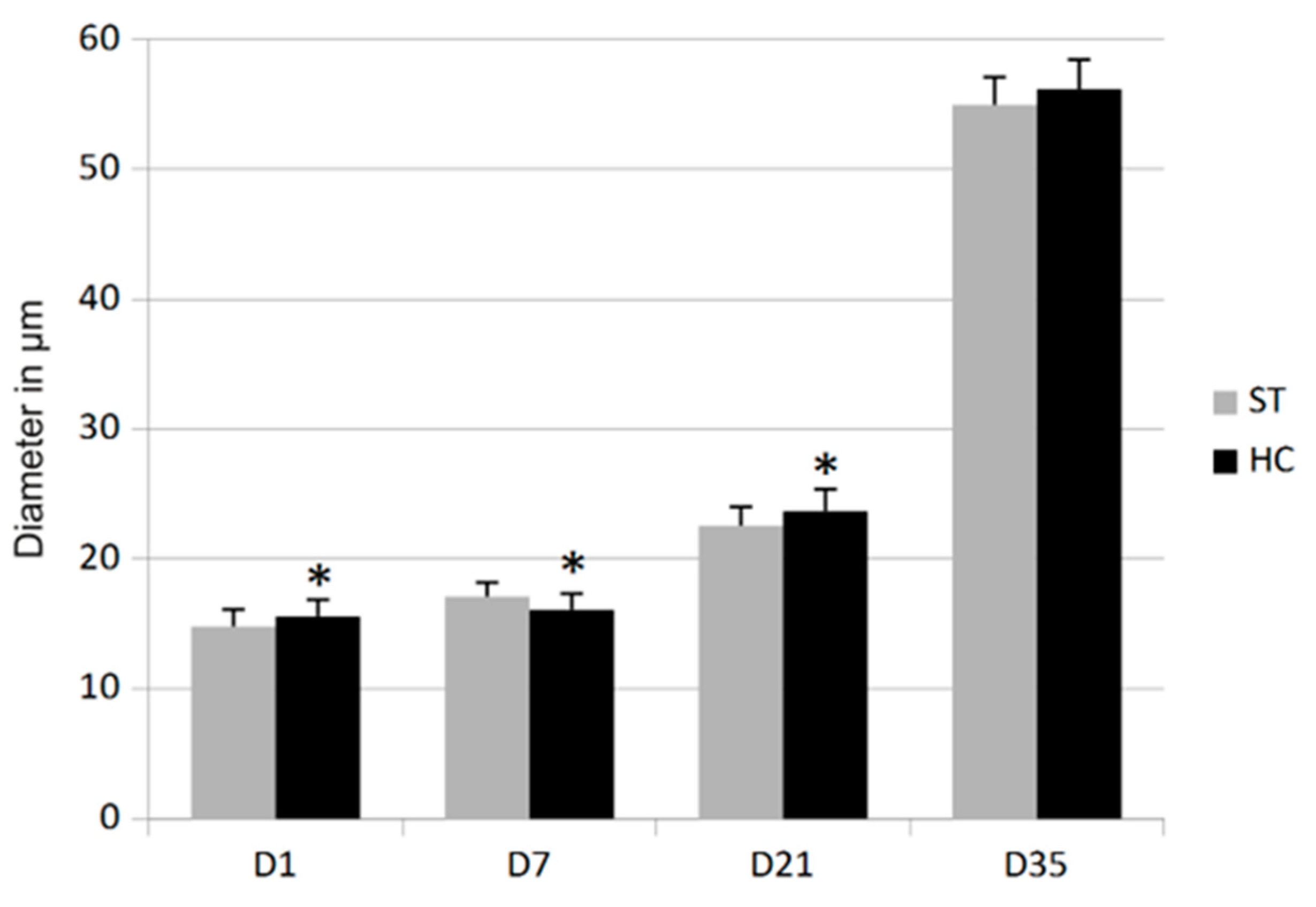
3.2. Morphometry
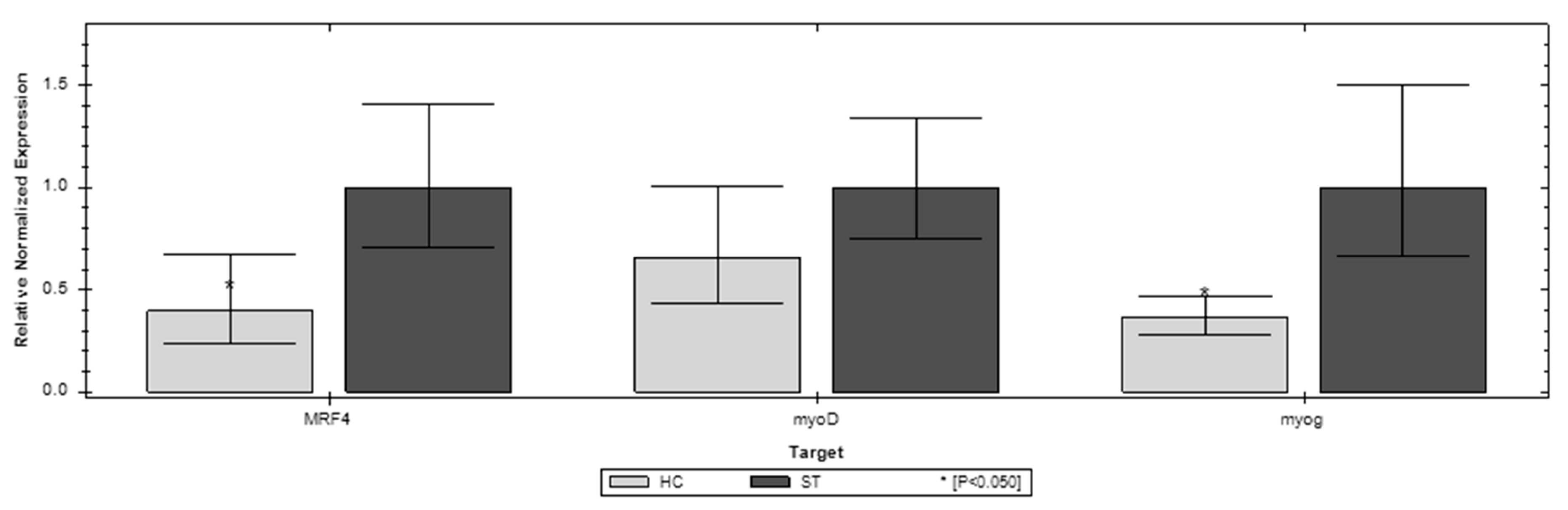
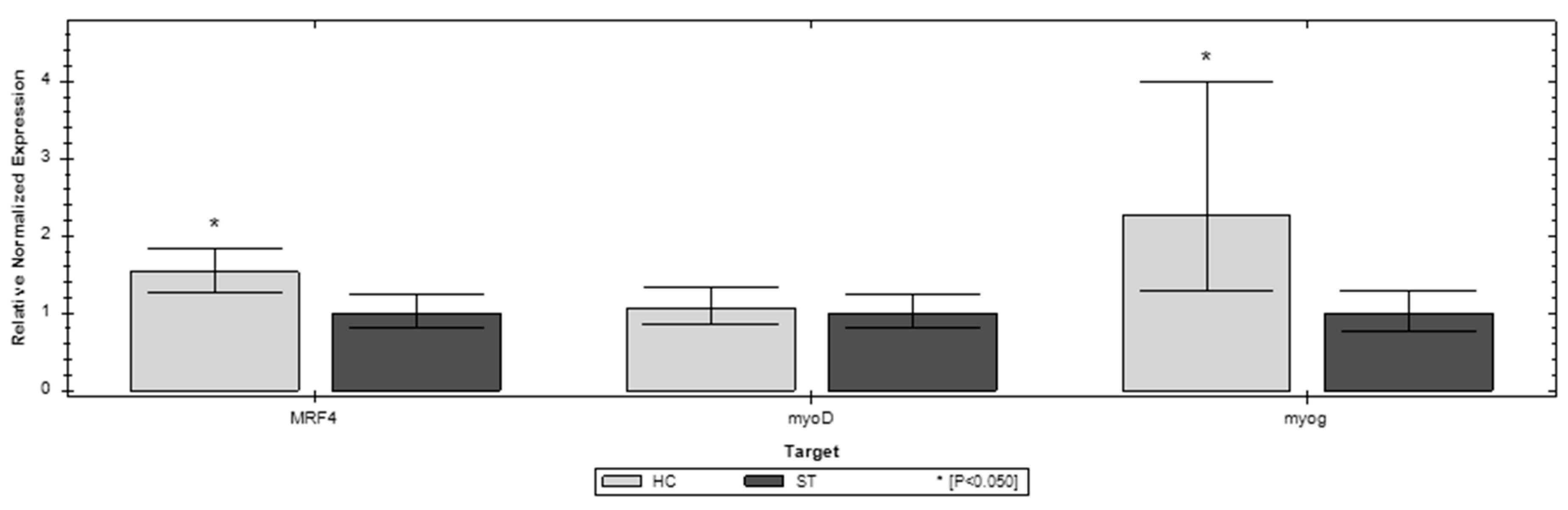
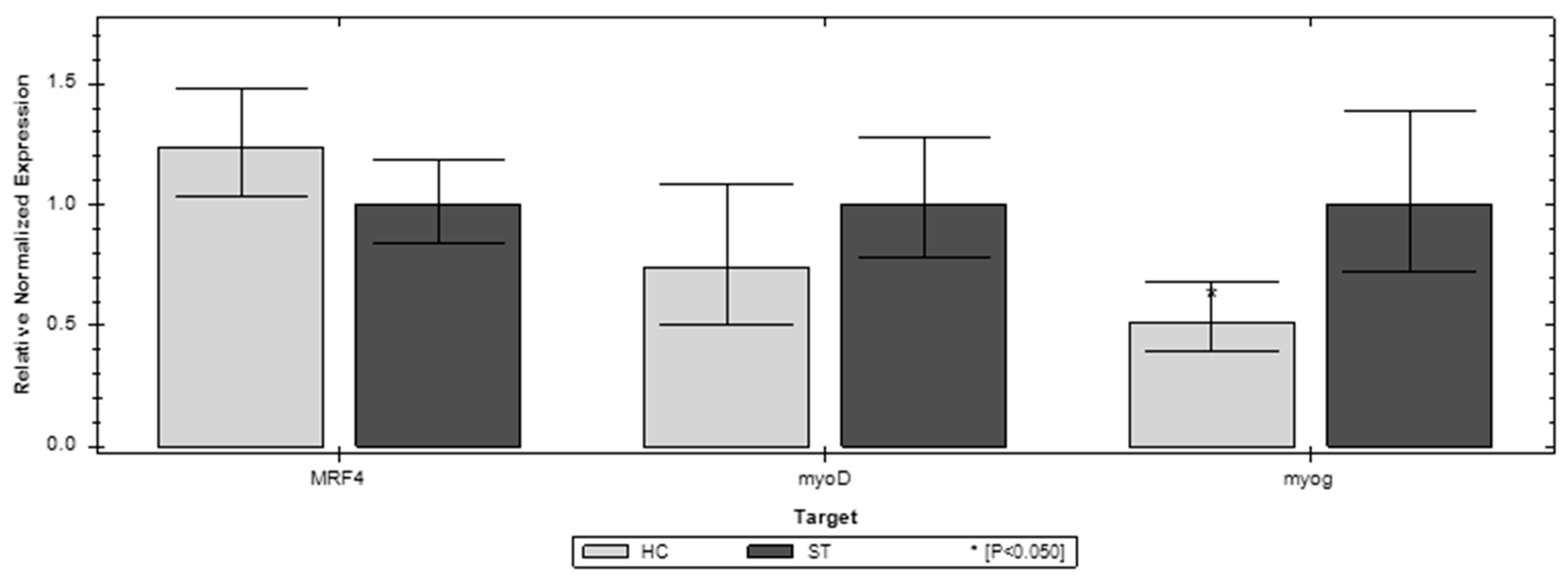
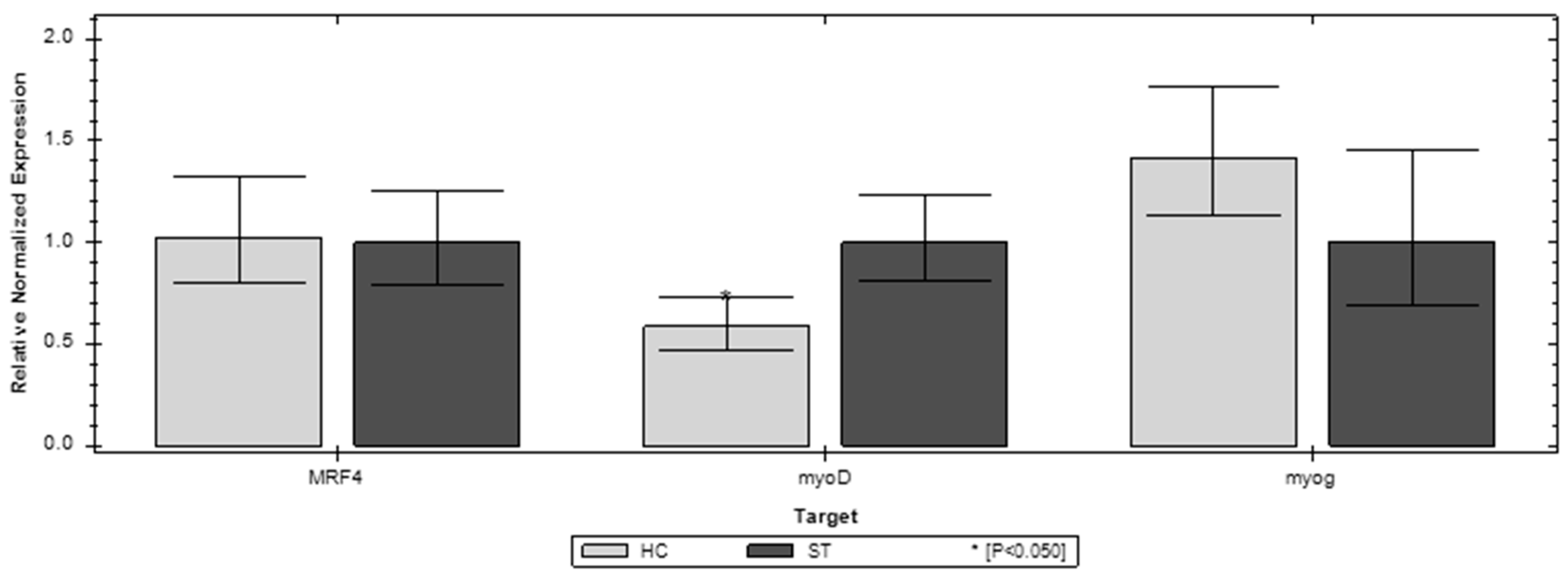
3.3. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sobolewska, A.; Elminowska-Wenda, G.; Bogucka, J.; Dankowiakowska, A.; Kułakowska, A.; Szczerba, A.; Stadnicka, K.; Szpindla, M.; Bednarczyk, M. The influence of in ovo injection with the prebiotic DiNovo® on the development of histomorphological parameters of the duodenum, body mass and productivity in large-scale poultry production conditions. J. Anim. Sci. Biotech. 2017, 8, 45. [Google Scholar] [CrossRef]
- Halevy, O.; Geyra, A.; Barak, M.; Uni, Z.; Sklan, D. Early post-hatch starvation decreases satellite cell proliferation and skeletal muscle growth in chicks. J. Nutr. 2000, 130, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Noy, Y.; Sklan, D. Different types of early feeding and performance in chicks and poults. J. Appl. Poult. Res. 1999, 8, 16–24. [Google Scholar] [CrossRef]
- Bigot, K.; Mignon-Grasteau, S.; Picard, M.; Tesseraud, S. Effects of delayed feed intake on body, intestine, and muscle development in neonate broilers. Poult. Sci. 2003, 82, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.G.; Warriss, P.D.; Brown, S.N.; Edwards, J.E.; Mitchell, M.A. Response of broilers to deprivation of food and water for 24 h. Br. Vet. J. 1995, 151, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Tarvid, I. Effect of early post natal long term fasting on the development of peptide hydrolysis in chicks. Comp. Biochem. Physiol. 1992, 101, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.D.; Dibner, J.J. Nutritional programming in hatching poultry: Why a good start is important? Poult. Dig. 1998, 8, 20–26. [Google Scholar]
- Noy, Y.; Geyra, A.; Sklan, D. The Effect of early feeding on growth and small intestinal development in the posthatch poult. Poult. Sci. 2001, 80, 912–919. [Google Scholar] [CrossRef]
- Batal, A.B.; Parsons, C.M. Effect of fasting versus feeding oasis after hatching on nutrient utilization in chicks. Poult. Sci. 2002, 81, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Juul-Madsen, H.R.; Su, G.; Sorensen, P. Influence of early or late start of first feeding on growth & immune phenotype of broilers. Br. Poult. Sci. 2004, 45, 210–222. [Google Scholar]
- Prabakar, G.; Pavulraj, S.; Shanmuganathan, S.; Kirubakaran, A.; Mohana, N. Early Nutrition and Its Importance in Poultry: A Review. Indian J. Anim. Nutr. 2016, 33, 245–252. [Google Scholar] [CrossRef]
- Uni, Z.; Ferket, R.P. Methods for early nutrition and their potential. World Poult. Sci. J. 2004, 60, 101–111. [Google Scholar] [CrossRef]
- Velleman, S.; Coy, C.; Emmerson, D. Effect of the timing of posthatch feed restrictions on broiler breast muscle development and muscle transcriptional regulatory factor gene expression. Poult. Sci. 2014, 93, 1484–1494. [Google Scholar] [CrossRef]
- Mozdziak, P.E.; Walsh, T.J.; McCoy, D.W. The effect of early posthatch nutrition on satellite cell mitotic activity. Poult. Sci. 2002, 81, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Halevy, O.; Nadel, Y.; Barak, M.; Rozenboim, I.; Sklan, D. Early posthatch feeding stimulates satellite cell proliferation and skeletal muscle growth in turkey poults. J. Nutr. 2003, 133, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Incubation Technology that Delivers Consistently Superior Chick Quality. 2022. Available online: https://hatchtech.com (accessed on 10 May 2022).
- Mok, G.F.; Mohammed, R.H.; Sweetman, D. Expression of myogenic regulatory factors in chicken embryos during somite and limb development. J. Anat. 2015, 227, 352–360. [Google Scholar] [CrossRef]
- Berkes, C.A.; Tapscott, S.J. MyoD and the transcriptional control of myogenesis. Semin. Cell. Dev. Biol. 2005, 16, 585–595. [Google Scholar] [CrossRef]
- Moore, D.T.; Ferket, P.R.; Mozdziak, P.E. Early post-hatch fasting induces satellite cell self-renewal. Comp. Biochem. Physiol. Part A 2005, 142, 331–339. [Google Scholar] [CrossRef]
- Wright, W.E.; Sassoon, D.A.; Lin, V.K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 1989, 56, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Daumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr Physiol. 2015, 5, 1027–1059. [Google Scholar]
- Lamot, D.M.; van de Linde, I.B.; Molenaar, R.; van der Pol, C.W.; Wijtten, P.J.; Kemp, B.; van den Brand, H. Effects of moment of hatch and feed access on chicken development. Poult. Sci. 2014, 93, 2604–2614. [Google Scholar] [CrossRef] [PubMed]
- Lingens, J.B.; Abd El-Wahab, A.; Ahmed, M.F.E.; Schubert, D.C.; Sürie, C.; Visscher, C. Effects of Early Nutrition of Hatched Chicks on Welfare and Growth Performance: A Pilot Study. Animals 2021, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L.; Wang, J.S.; Liu, L.J.; Li, K.; Xu, Y.B.; Ding, X.Q.; Wang, Y.Y.; Zhang, Y.F.; Xie, L.Y.; Liang, S.; et al. Effects of early post-hatch feeding on the growth performance, hormone secretion, intestinal morphology, and intestinal microbiota structure in broilers. Poult Sci. 2022, 101, 102–133. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, E.; Kondo, N.; Saldanha, E.S.P.B.; Loddy, M.M.; Careghi, C.; Decuypere, E. Performance and physiological parameters of broiler chickens subjected to fasting on the neonatal period. Poult. Sci. 2003, 82, 1250–1256. [Google Scholar] [CrossRef]
- Wen, C.; Wu, P.; Chen, Y.; Wang, T.; Zhou, Y. Methionine improves the performance and breast muscle growth of broilers with lower hatching weight by altering the expression of genes associated with the insulin-like growth factor-I signalling pathway. Br. J. Nutr. 2014, 111, 201–206. [Google Scholar] [CrossRef] [PubMed]
| Name of the Genee | Sequence | References |
|---|---|---|
| myoD | F-GATGGCATGATGGAGTACAG R-AGCTTCAGCTGGAGGGAGTA | [13] |
| myoG | F-GGCTTTGGAGGGAAGGACT R-CAGAGTGCTGCGTTTCAGAG | [13] |
| MRF4 | F-AGGCTCTGAAAAGAGGACTG R-AGGCTGCTGGAAGCCGACGAC | [13] |
| GADPH | F-GAGGGTAGTGAAGGCTGCTG R-CCACAACACGGTTGCTGTAT | [13] |
| I Experiment | II Experiment | III Experiment | Mean for All Experiments | |||||
|---|---|---|---|---|---|---|---|---|
| ST | HC | ST | HC | ST | HC | ST | HC | |
| Body weight (BW) | ||||||||
| D1 | 42.27 ± 4.17 a | 46.63 ± 3.28 b | 43.58 ± 3.99 a | 49.37 ± 5.32 b | 46.25 ± 5.85 b | 43.08 ± 4.90 a | 44.04 a | 46.47 b |
| D7 | 160.83 ± 28.30 a | 198.34 ± 30.15 b | 159.63 ± 16.31 a | 182.90 ± 20.00 b | 182.59 ± 17.76 a | 203.07 ± 30.59 b | 167.23 a | 194.73 b |
| D21 | 959.53 ± 91.17 | 912.32 ± 165.39 | 618.77 ± 99.78 a | 748.33 ± 102.46 b | 979.94 ± 74.52 | 968.80 ± 103.46 | 852.74 | 876.88 |
| D35 | 1855.93 ± 262.19 a | 2062.73 ± 224.93 b | 1505.48 ± 259.42 a | 1674.87 ± 279.37 b | 2245.9 ± 250.92 | 2174.13 ± 306.06 | 1873.19 | 1970.58 |
| Breast-muscle index (BMI) | ||||||||
| D1 | 1.35 ± 0.20 a | 1.50 ± 0.24 b | 1.43 ± 0.28 a | 1.67 ± 0.40 b | 1.54 ± 0.46 | 1.40 ± 0.44 | 1.45 | 1.51 |
| D7 | 11.47 ± 1.18 a | 12.55 ± 1.02 b | 8.92 ± 0.88 a | 9.58 ± 1.14 b | 9.40 ± 1.22 a | 11.15 ± 1.27 b | 9.95 a | 10.90 b |
| D21 | 15.91 ± 1.48 | 16.13 ± 1.84 | 14.76 ± 1.74 a | 15.76 ± 1.95 b | 16.94 ± 1.28 | 17.98 ± 2.11 | 15.98 a | 16.71 b |
| D35 | 19.24 ± 3.31 | 19.81 ± 2.19 | 17.49 ± 5.33 | 17.41 ± 1.78 | 20.55 ± 1.83 | 19.53 ± 2.27 | 19.28 | 19.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaweł, A.; Madej, J.P.; Kozak, B.; Bobrek, K. Early Post-Hatch Nutrition Influences Performance and Muscle Growth in Broiler Chickens. Animals 2022, 12, 3281. https://doi.org/10.3390/ani12233281
Gaweł A, Madej JP, Kozak B, Bobrek K. Early Post-Hatch Nutrition Influences Performance and Muscle Growth in Broiler Chickens. Animals. 2022; 12(23):3281. https://doi.org/10.3390/ani12233281
Chicago/Turabian StyleGaweł, Andrzej, Jan Paweł Madej, Bartosz Kozak, and Kamila Bobrek. 2022. "Early Post-Hatch Nutrition Influences Performance and Muscle Growth in Broiler Chickens" Animals 12, no. 23: 3281. https://doi.org/10.3390/ani12233281
APA StyleGaweł, A., Madej, J. P., Kozak, B., & Bobrek, K. (2022). Early Post-Hatch Nutrition Influences Performance and Muscle Growth in Broiler Chickens. Animals, 12(23), 3281. https://doi.org/10.3390/ani12233281





