Simple Summary
Tibial dyschondroplasia (TD) is a metabolic disorder that impairs bony and cartilage processes. It is common in broilers due to the consumption of thiram, especially in the industrial and agriculture zones. During the condition, cartilage does not only seem to develop ossification during its occurrence but also causes lameness, mortality, and moral convictions in commercial poultry. Moreover, it has been characterized as an economically significant condition since it causes carcass damage due to the involvement of different biological pathways that lead to a particular change in the chondrocytes. These entire cellular pathways are interconnected through various cellular inputs, including anti-apoptotic, pro-apoptotic, and executioner caspases that modulate the other essential chondrogenic proteins (collagen and aggrecan), extracellular metalloproteinases, and NLRP3 base inflammasome.
Abstract
Tibial dyschondroplasia debilities apoptotic and inflammasomal conditions that can further destroy chondrocytes. Inflammasomes are specialized protein complexes that process pro-inflammatory cytokines, e.g., interleukin-1β (IL-1β) and IL-18. Moreover, there is mounting evidence that many of the signaling molecules that govern programmed cell death also affect inflammasome activation in a cell-intrinsic way. During the last decade, apoptotic functions have been described for signaling molecules involving inflammatory responses and cell death pathways. Considering these exceptional developments in the knowledge of processes, this review gives a glimpse of the significance of these two pathways and their connected proteins in tibial dyschondroplasia. The current review deeply elaborates on the elevated level of signaling mediators of mitochondrial-mediated apoptosis and the inflammasome. Although investigating these pathways’ mechanisms has made significant progress, this review identifies areas where more study is especially required. It might lead to developing innovative therapeutics for tibial dyschondroplasia and other associated bone disorders, e.g., osteoporosis and osteoarthritis, where apoptosis and inflammasome are the significant pathways.
1. Introduction
Cellular and molecular pathways regulate bone formation and growth. Any variation from the normal process may result in bone abnormalities, which pose a significant economic challenge to the poultry business [1]. The control of bone formation and growth seems complicated, with several layers of interaction between the regulatory factors [2]. Chondrocytes’ formation and differentiation occur on the growth plate in a particular region. Pre-hypertrophic chondrocytes form a columnar layer once proliferating chondrocytes stop replicating. These phases are determined by the cellular phenotype and extracellular matrix metalloproteinase (ECM) proteins. Columnar cells, pre-hypertrophic cells, and hypertrophic cells express distinct transcription factors and ECM proteins as they progress through the embryonic stages [3]. As a result, interactions between these essential processes in the growth plate become necessary for appropriate long bone development [4].
Tibial dyschondroplasia is among the remarkably prevailing skeletal abnormalities affecting young poultry birds [5]. The prevalence of this tibiotarsal bone condition has increased by 30% in the flock at broiler farms. Due to the majority of its symptoms being sub-clinical [6], it is often difficult to adequately detect the prevalence of TD at these farms; as a result, farmers frequently find it easy to let their guard down. In fact, broilers with TD experience leg weakness, limited motion, and even walking difficulties. Broilers are more likely to sustain fractures during the feeding process, which negatively impacts the welfare of the birds and reduces production, which further causes significant financial loss for the poultry industry. Various researchers worldwide have constantly focused on the etiology and prevention of TD [7,8]. In most cases, nutritional, ecological, and genetic factors have been implicated in its etiology [9]. For instance, soybean meal in feeding has been associated with TD pervasiveness, along with further concerns, including ergocalciferol insufficiency, hyperthyroidism (overactive thyroid), and abnormal levels of biological parameters such as interleukin-1β and nitric oxide [10]. Moreover, according to some studies, copper deficiency, fusarochromanone, excessive dietary levels of cysteine and homocysteine, metabolic acidosis [11], vitamin D deficiency [12], disbalance of calcium and phosphorus [13], and thiram contamination [14] may also cause the condition. The condition of TD has been linked to aberrant ossification and prolongation of tibial growth plates (GP) as a result of reduced chondrocyte propagation and differentiation [15]. An ideal cartilage matrix has enough blood supply and mineralization; however, this is not always the case for TD [16]. During TD conditions, chondrocytes are premature and more prominent than usual because of pre-hypertrophic enlargement with avascular ossein zones in cartilage [17].
Pesticides are widely used in agriculture to eradicate or control many agricultural bugs, herbicides, and diseases that may harm crops and animals. On the other side, pesticides have become a hazard due to their toxicity. Living organisms may be exposed precisely or periphrastically over the food chain, air, soil, and water [18]. Thiram (Tetramethyl thiuram disulfide) is a dithiocarbamate pesticide and fungicide commonly used in horticulture to treat grains for seed protection and preservation [19]. It has a lipophilic character that can effortlessly combine with cell membranes to induce cytotoxicity, bone formation problems, cartilage damage, and immunological downturns. It may also cause membrane disruption, bone biosynthetic pathway inactivation, and angiogenesis inhibition [20]. So, it is highly associated with the induction of TD, with symptoms that resemble commonly occurring tibial dyschondroplasia. Additionally, earlier research has shown that TH (thiram) may cause TD in chickens at the dose rate of 50 mg/kg [5,21]. Moreover, it has been frequently mobilized to imitate TD in numerous research trials [22,23,24,25]. Our prior studies indicate that thiram induces apoptosis in chondrocytes raising the number of apoptotic chondrocytes inside the osteogenesis area [26,27,28]. Besides, thiram inhibits angiogenesis within the GP, reducing chondrocytes function and osteogenesis [29]. Among thiram’s most visible and damaging effects is a bone cartilage disorder in broilers when fed thiram-containing diets [24,29]. During this disorder, non-mineralized avascular cartilage accumulates in the growth plates of the proximal tibia, resulting in lameness [30].
2. Prospective Tibial Cartilage Development
The growth and development of long bones in poultry are accomplished by chondrocytes and a matrix composed of highly organized growth plates [31]. These cells in the growth plate can be subdivided into five phases for endochondral ossification (EO) [32]. OE is a term used to describe the process of bone formation. It is a process that includes the continuous replacement of growing cartilage to generate a thick bony structure [33]. The chondrocytes multiply, grow, and die during the ossification process, while the extracellular matrix that builds during this process is subsequently invaded by the vascular system and various bone cells, forming bone over cartilage matrix remnants [34]. The process occurs in three unique locations: the physis, the epiphysis, and the cuboidal. During bone development, chondrocytes may be subdivided into layers or zones in which the hypertrophic zone is the most important for the formation of endochondral ossification [35,36]. Chondrocytes are formed in this area by final differentiation of the proliferating zone farthest from the epiphyseal plate. When these cells stop proliferating, they enlarge, profoundly impacting the development process [37,38]. Once the bone has developed to both ends, the hypertrophic zone forms at the bottom of the growth plate, between the preceding propagating cell layers and the epiphyseal bone (Figure 1) [35]. Here, the chondrocytes are surrounded by an extracellular matrix, which eventually mineralizes in the zone of preliminary calcification. After the invasion of the chondrocyte columns by the metaphyseal vascular system, bone grows on the remaining columns of hardened cartilage. The combination of hardened cartilage and undeveloped bone is then progressively reshaped to become mature bone [39].
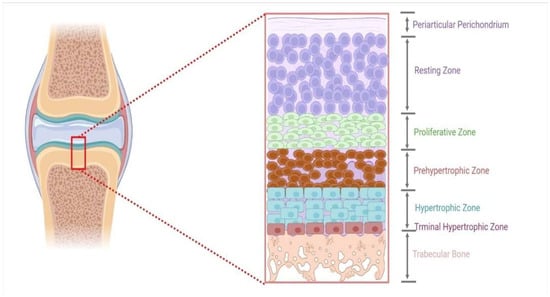
Figure 1.
An illustration of a growth plate’s different zones.
Each growth plate is a multilayer sandwich structure organized into four distinct zones: reserve, proliferating, transformational, and degeneration zones [40]. Immature cells in the growth plate are included in the resting zone because they are located near the epiphysis. This zone comprises tiny, homogeneous, compactly placed chondrocytes that appear alone or in couples and are positioned inside the reserve zone (also called resting or germinal area). This zone is further distinguished by a low proliferation rate, proteoglycan, and collagen type II production [41,42]. The proliferating zone is the next layer down from the reserve area. Chondrocytes are flattened and well-separated into columns in this area. During mitosis, cells only divide at the bottom of a column. Collagen production in the true germinal layer has risen significantly in type II and type XI [43]. The transformation zone, which lies beneath the proliferative zone, is divided into a top and bottom hypertrophic zone and a degenerative zone. Chondrocytes at this stage are distinguished by their lack of cellular proliferation and decreased DNA synthesis [15,43].
3. Growth Plate Associated Tibial Dyschondroplasia in Poultry
During TD condition, the chondrocytes in the growth plate region are unorganized, having fewer blood vessels with lesions in proliferative and hypertrophic zones [29]. These lesions include avascular, noncalcified tissue and soft cartilage. Histologically, hypertrophic zone enlarges and combines with avascular cartilage zones [17]. Thiram is highly associated with the induction of TD, with symptoms resembling tibial dyschondroplasia (Figure 2). Additionally, earlier research has shown that TH (Thiram) may cause TD in chickens. Moreover, it has been frequently mobilized to imitate TD in numerous research trials [14,22,44]. Our prior studies indicate that thiram induces apoptosis in chondrocytes, raising the number of apoptotic chondrocytes inside the osteogenesis area [27,45]. Besides, thiram inhibits angiogenesis within the GP, reducing chondrocytes function and osteogenesis [23,29].
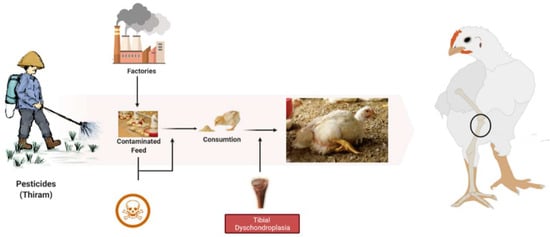
Figure 2.
The root cause of tibial dyschondroplasia in broilers.
4. Role of Different Proteins in the Pathogenesis of TD
Research on disease pathogenesis and treatment involves comparing a diseased condition to a healthy one. The discovery of differentially regulated proteins in a disease seems to be a particular focus of inquiry. Those proteins might be a promising biomarker in pharmacological and clinical research. This kind of data might be helpful after combining with other biological data to establish a disease’s target picture. Too far, a plethora of research has demonstrated the essential proteins encode in combat against TD. Following are some significant proteins involved in tibial dyschondroplasia.
4.1. Role of Chondrogenic Marker Proteins
The damaged articular cartilage has a deficient ability to mend itself from causing any impairment [46]. Collagen II and aggrecan are two of the bones’ most crucial extracellular matrix components [47]. Any defect in collagen (type II) results either in approximate or quantitative alteration, depending on the mutant site. As a result, the clinical symptoms of collagen II abnormalities range from neonatal mortality to minor skeletal dysplasia [48]. Similarly, aggrecan is also a significant proteoglycan in the articular cartilage, expressed by chondrocytes. This protein is essential in chondro-skeletal morphogenesis during development [49]. Furthermore, due to its glycosaminoglycan concentration, aggrecan plays an integral part in producing the cartilage’s persistent negative charge, resulting in its water-attracting qualities [50].
In this way, these macromolecules are helpful in the process of “decellularized extracellular matrix” (dECM), which is directly connected with the maintenance of the native environment for promoting cell proliferation and differentiation [51] (Figure 3). Hence, reducing these macromolecules may affect normal and pathological bone development [52].
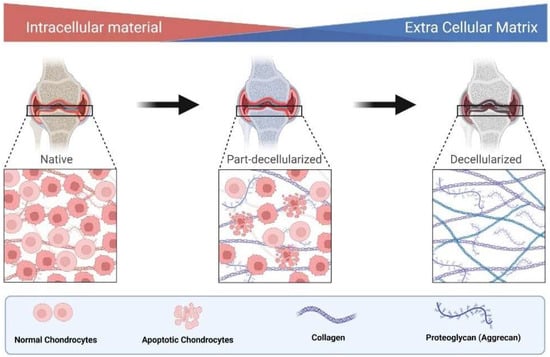
Figure 3.
Role of collagen and aggrecan mediated ECM in decellularization.
4.2. Role of CD147 (EMMPRIN/Basigin) Protein
The Cluster of differentiation 147 (CD147) protein decrypted by the BSG gene is an immunoglobulin superfamily member interacting with the cell membrane. It has a type I integral membrane binding site with 269 amino acids that features two ig domains at the N-terminal [53]. It is also known as an extracellular matrix metalloproteinase inducer (EMMPRIN) or basigin [54], located on the exterior of apoptotic cells. It may stimulate the transcription or activation of matrix metalloproteinase (MMP) in neighboring mesenchymal and malignant cells, hence promoting tumor invasion [55]. Recent investigations have shown that CD147 is expressed in malignant cells and various other cells, including fibroblast and keratinocytes [56]. Additionally, it is vital to replenish the extracellular matrix components required for continuous bone formation [57]. Furthermore, it is involved in cellular reflexes, metabolism, inflammation, distant metastasis, metalloproteinase production, apoptosis, angiogenesis, proliferation, and differentiation [58,59]. According to Asgari et al., CD147 has a compelling part in cell migration and survival/apoptosis. It can control p53, Bax, and Bcl-2 independently [60].
4.3. Role of Angiogenesis Proteins
Angiogenesis is the process through which new capillaries develop from pre-existing vessels under different growth factors [61]. Almost all cells survive due to growth factors and interaction with the extracellular matrix. The depletion of growth factors, including VEGF, bFGF, and angiopoietin-1, may cause uncontrolled apoptosis. So, their activation has been shown to promote survival by blocking apoptosis [62]. The action of VEGF is transduced by two tyrosine kinase receptors, flt-1, and flk-1. Cell multiplication and survival have been linked to flk-1, whereas chemotaxis and vascular permeability have been linked to flt-1 [62]. The protein kinase B (or Akt) and MAPK are parts of the flk-1-activated signaling pathway [62,63]. Moreover, VEGF has been demonstrated to enhance cell survival by activating the PI3K/Akt pathway [61,64]. Surprisingly, VEGF’s survival function relies on VEGF binding to VEGFR2 (KDR/flk-1) [64]. Hence, VEGFR2 and PI3K/Akt signal transduction pathways are critical in VEGF-induced survival enhancement. Furthermore, Akt-dependent stimulation of endothelial nitric oxide synthase (NOS) results in increased endothelial NO production, which is one of the downstream effector mechanisms, mediating the antiapoptotic VEGF action [65,66]. Alternatively, the PI3K/Akt pathway promotes survivin transcription and can suppress the p38 mitogen-activated protein kinase (MAPK) [67,68]. The VEGF induces MAPK/ERK pathway stimulation and suppresses the stress activated protein kinase/c-Jun amino-terminal kinase pathway, which is implicated in the antiapoptotic action mediated by VEGF. Interestingly, activation of the PI3K/Akt pathway mediates the survival impact of VEGF on cells and the migrating effect of VEGF via Akt-dependent phosphorylation and eNOS activation [69] (Figure 4).
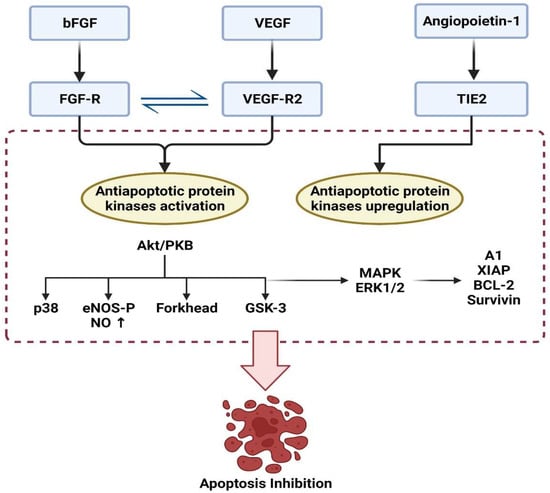
Figure 4.
Regulation of apoptosis under the effect of angiogenic factors.
4.4. Role of Apoptotic Proteins
Apoptosis participates in the development, regeneration, and integrity of multicellular organisms. It is critical to multiply, eliminate and maintain homeostatic physiological functions during the transformation, development, and tissue renewal processes [70]. This natural cell death process is genetically predetermined and involves the destruction of cells in response to specific signals under different mediators (Table 1) [71]. However, if this common cell death mechanism fails, the effects might be disastrous. This entire mechanism is interconnected to multiple conserved anticlines. It terminates for stimulating and destroying cellular inputs [72] in different growth plate zones under the effect of pesticides, e.g., thiram. Typically, programmed cellular senescence is regulated by a range of intra and extracellular signals guided by the cell’s surroundings and internal signaling [73]. It has been shown that some proteins hold both pro and anti-apoptotic functions in the cell, which plays a pivotal part in the governance of apoptosis [74]. The apoptosis inducer, mediator, and executioner genes are regularly transduced into those cells to compensate for the absence of the endogenous homologue [75]. Moreover, several factors contribute to the effectiveness of molecular-targeted specific medications; understanding these factors offers insight into an effective treatment plan for designing molecular-targeted medicines [70].

Table 1.
The various mediators of apoptosis and their functions [76].
There are two primary apoptotic mechanisms: the intrinsic approach, which involves early mitochondrial disruption caused by cellular stress or cytotoxic assaults, and the extrinsic system, activated by death receptor stimulation [77].
4.4.1. Intrinsic/Mitochondrial-Mediated Proteins
The intrinsic mechanism mainly relates to mitochondrial-mediated apoptotic pathways, activated by numerous extra and intracellular stressors, including oxidative damage, irradiation, and cytotoxic medication [78]. Besides being controlled or triggered by extraneous stimulants, apoptosis may also be governed by stimuli such as cellular infliction and oxidative strain [79]. The attributes of the Bcl family (Bax and Bcl-2) are known as pro-apoptotic or anti-apoptotic proteins and are found on the mitochondrial membrane. They are critical mediators of the intrinsic apoptotic process [80], resulting in cytochrome c (Cyto C) release within the cytoplasm upon disrupting the mitochondrial membrane. The clemency of Cyto C within the cytoplasm results in forming a network including APAF1 and pro-caspase 9, which is referred to as an apoptosome (Figure 5). This combination cleaves and activates executioners, such as Caspase-3 and Caspase-7, ensuring cell death at the end of the process [81]. Thus, intrinsic mitochondrial dysfunction leads to decreased inner mitochondrial function, increased superoxide ion synthesis, mitochondrial malfunction, and the affluence of MCG (matrix calcium glutathione) [82].
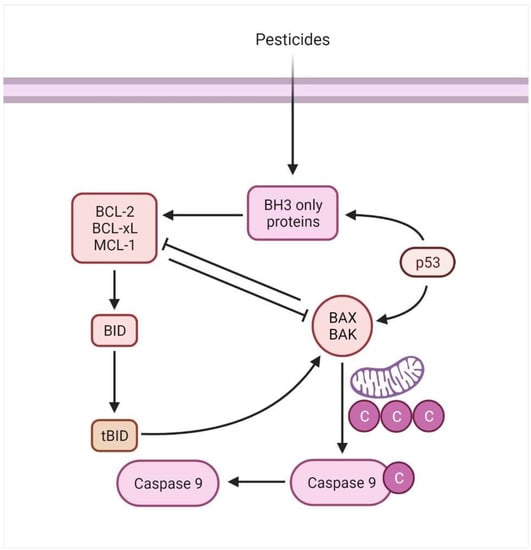
Figure 5.
The mitochondrial mediated/intrinsic apoptosis is associated with the activation of BH3 proteins.
4.4.2. Bcl-2 Family Proteins
The Bcl-2 proteins family firmly guards against intrinsic or mitochondrial cell death. This family group is unruffled of similar constructional proteins along with antagonistic activities [83]. Initiators, effectors, and anti-apoptotic proteins are all members of the Bcl-2 family. Usually, pro-apoptotic proteins and stimulants counteract apoptotic-promoting activities directly with the anti-apoptotic proteins. When these pro and anti-apoptotic proteins are balanced, a cell’s survival or death is determined [4]. The balance among these proteins is tangled with programmed cell death through the involvement of mitochondria [84]. This is why it’ is essential to keep an eye out for abnormalities that might cause an imbalance in mitochondrial biogenesis, resulting in the destruction of inner membrane potentiality and an upturn in superoxide ions generation [70].
All Bcl-2 proteins have a unique series of homology in sustained areas, called BH (Bcl-2 homology) motifs, that determine shape and activity [85]. All candidates of the anti-apoptotic category and a subgroup of the representative pro-apoptotic type are a multi-domain group of proteins having a series similarity within three to four BH domains. Such a subgroup of pro-apoptotic members termed BH3 exclusive proteins demising domain since mandatory multi-domain Bcl-2 family proteins [86].
4.4.3. Anti-Apoptotic Bcl-2 Proteins
The resistance mechanism against apoptosis is recognized as one of the defining characteristics of cells [87]. Many studies have proved that the increase of anti-apoptotic Bcl-2 proteins plays a central role in B cell lymphomagenesis [88]. Another possibility is that the expression level of these proteins is a sign of how reliant the cell is on the protein to keep itself stable [89]. Apoptosis is inhibited initially by the competence of anti-apoptotic members to pickle and detain the pro-apoptotic proteins (e.g., Bax and Bak), preventing mitochondrial membrane damage [90]. To prevent apoptosis from releasing Cyto C from the membrane, these Bcl-2 family proteins reside on the outer mitochondrial subunit [83].
The dysregulation of anti-apoptotic proteins occurs during malignancies that further promote tumor formation [91]. The equilibrium of such proteins is disrupted when Bcl-2 (or related proteins) are dysregulated, or BH3-only proteins or effector proteins are depleted [92] (Figure 6). The numerous genetic pathways behind these anomalies are discussed elsewhere [93]. However, it is critical to recognize that the elevated levels of pro-survival protein members may be an epigenetically mediated adaptive response to cellular stress. In summary, cells with increased Bcl-2 expression function as a safeguard against apoptosis in cellular stressors (Figure 5). As Letai describes, these cells are “primed for death” and should thus be very sensitive to the lack of Bcl-2′s protective role [92].
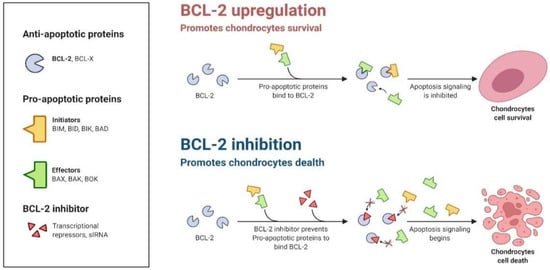
Figure 6.
The imbalance or inhibition of anti-apoptotic proteins (e.g., Bcl-2) may initiate apoptosis.
4.4.4. Pro-Apoptotic Bcl-2 Proteins
The anti-apoptotic proteins contribute to cell continuity by controlling or keeping the levels of pro-apoptotic proteins [94]. In contrast, the pro-apoptotic proteins are members of the Bcl-2 family with several BH domains. These members are structurally related and have pro-survival relatives. During an apoptotic event, Bax and Bak homodimerize and oligomerize in the exterior mitochondrial layer, resulting in the discharge of Cyto C within the cytoplasm [84]. Numerous research has been conducted to determine the prognostic value of pro-apoptotic proteins. For instance, the mRNA intensity of Bax and Bcl-2 was evaluated in colorectal cancer patients. According to the results, Bax substantially induced tumor cell apoptosis and regulated Bcl-2 to prevent apoptosis [95].
The mitochondrial cell death pathway is interceded by multi-domain proteins such as Bax and Bak, with anti-apoptotic proteins. Various genetic and biochemical studies act as upstream regulating entities that resist the intrinsic death-inducing behavior at mitochondrial membranes [96]. Their interaction with mitochondrial membranes has been extensively investigated, revealing that these proteins regulate mitochondrial outer membrane permeability (MOP) for apoptotic proteins’ discharge, e.g., cytochrome [97,98]. Various conditions have been found to activate their action, showing that these pro-apoptotic proteins may remain dormant until triggered. For example, Bax is located in the cytosol instead of interacting membrane organelles before any cell death signal [99]. The presence of Bax in its inactive soluble form shows that the C-terminal membrane-anchoring motif is snuggled into the same niche that most likely engages BH3 peptides [100]. Bax changes its shape in response to still-unknown signals, forming oligomers, revealing its C-terminal membrane-anchoring motif, and entering into mitochondrial membranes [96,101]. Additional proteins (e.g., Bak and Bok) seem to be present in membranes on a constitutive basis. Even if Bax or Bak are embedded in the outer mitochondrial membrane, oligomerization appears to be required to discharge cytochrome c and other apoptotic proteins [96]. Additionally, anti-apoptotic proteins of the Bcl-2 family may create pathological conditions conducive to apoptosis by their capacity to attach and trigger Bax, Bak, and Bok.
4.4.5. Activation of the Executioner Caspase-3 and Caspase-7
The execution phase is the last stage in the induction of apoptosis. It is distinguished by vacuolization, chromatin condensation, genomic instability, and blebbing of the cell membrane [82]. It is initiated by a series of events, with the executioner (e.g., caspases-3 and -7) acting as the culmination point of the process [102]. It is thought that the cleavage and activation of executioners cause significant intracellular proteolysis and cellular functioning impairment [103,104]. Additionally, activation and aggregation of these two caspases have already been distinguished as biochemical apoptotic hallmarks [105]. Because of their almost identical activity toward specific synthetic peptide substrates, the primary executioners are widely believed to have functionally similar roles inside the cell death mechanism [106]. As a result, the expression of these executor caspases is critical for providing a reliable biomarker of disease progression. For instance, a study that evaluated Caspase-3 expression in healthy and cancerous prostates concluded that the absence of Caspase-3 in cancerous affected cells may destroy apoptotic components [107] (Figure 7). A study on MCF7 cells discovered that the overexpression of Caspase-3 increases chemosensitivity to develop drug counteraction [108]. Moreover, it is an efficient marker for detecting gastric cancer differentiation, development, invasion, and dissemination through modulation of infiltrating lymphocyte apoptosis [109]. In contrast, Caspase-7 activates the spontaneously anti-parallel complex formation of two precursor variants. Few research studies have investigated the role of this caspase type in apoptosis regulatory oversight and its relationship to clinicopathological characteristics. For example, its reduced protein expression level was found to be a strong predictor in all breast tumors [110]. The findings further indicated that Caspase-7 is abnormally produced and contributes to cellular adhesion and division, making it a potential target for the treatment of different disorders [111].
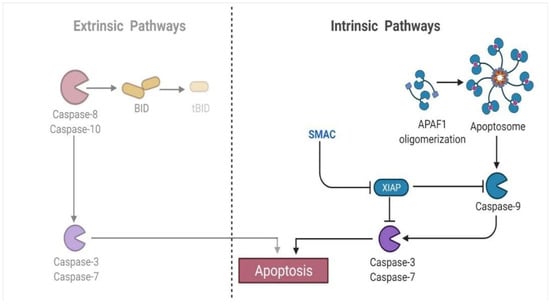
Figure 7.
The executioner caspase proteins (e.g., Caspase-3, Caspase-7) trigger apoptosis in both extrinsic and intrinsic pathways.
4.5. Role of Inflammasome Proteins
An inflammation is some kind of a defensive immunological response triggered by an innate immune system in feedback to damaging stimuli [112]. Stimulation of the inflammasome complex is triggered when chronic inflammation persists, resulting in the release of pro-inflammatory cytokines (for example, interleukin-1beta) over time. To reduce inflammation and induce cell death, this protein complex has been shown to have a substantial role in carcinogenesis and cancer progression [113]. Hence, it is a crucial modulator of the innate immune system [114].
An NLRP3 base inflammasome seems to be a critical part of the innate immunity that various stimuli may trigger. It incorporates NLRP3, an apoptosis-associated speck-like protein with a CARD (ASC), and pro-Caspase-1 [115] (Figure 8). Nevertheless, Caspase-1 is notorious for generating pyroptosis and was first discovered to activate apoptosis under beta cell lymphoma 2 (Bcl-2) inhibition [116]. Moreover, it is involved in apoptosis in several clinical situations [117]. It has recently been discovered that NLRP3 inflammasomes are formed due to intrinsic apoptosis [118,119]. The Caspase-3 and Caspase-7 break down the membrane protein pannexin1 during apoptosis. This helps free pannexin1 channel activity from self-inhibition caused by its cytoplasmic C-terminal tail. After cleaving these executioners, pannexin 1 sends K+ out of the cell to activate the NLRP3 inflammasome, which in turn produces IL-1β [120]. So, the NLRP3 base inflammasome has much importance in bone-related disorders. According to research by Kin et al., patients experiencing arthritis had increased expression of the NLRP3 inflammasome, which was associated with elevated pro-inflammatory mediators [121] (Figure 7). Another study by Pan et al. found that nucleosides, including nucleoside analogues, may stimulate host immune responses in mice with type II collagen-induced arthritis by interacting with TREM receptors on the skin and NLRP3 inflammasomes [122]. As a result, combining these pro-inflammatory cytokines may result in phenotypic alterations in cells throughout the ossification process [115,123].
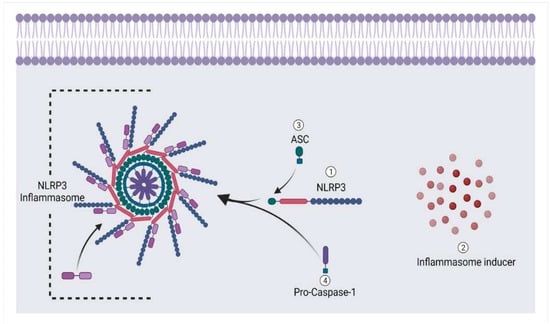
Figure 8.
Formation of NLRP3 base inflammasome under the influence of cell damage.
5. Prevention and Treatment against Apoptotic Events of TD
According to our previous studies, chlorogenic acid (CGA) in the feed may lower the prevalence of tibial dyschondroplasia as it targets specific mediators related to apoptotic events [12,26,27,45,124]. CGA is the most abundant phenolic acid in nature, being synthesized when quinic and caffeic acids are esterified. It occurs naturally in various fruits, herbs, and vegetables, including kiwi fruit, coffee beans, tobacco leaves, and honeysuckle [125]. It has been seen in pharmacological trials to have significant antioxidant, anti-inflammatory, antiviral, anticancer, cardioprotective, anti-apoptotic, and free radical scavenging properties [27,45,126,127]. Zhang et al. discovered that CGA might stimulate osteoblast growth and speed the S phase transition process. Additionally, it may promote Bcl-2 expression and limit Bax activation during apoptosis, ultimately decreasing osteoblast apoptosis [128]. It has been shown in recent work by Kulyar et al. that CGA has therapeutic benefits for TD chickens by modulating a variety of pathways associated with apoptosis and inflammasome [27,45]. Furthermore, targeting micro RNAs is a better therapy for overcoming such disorders. It is well known that miRNAs control mRNA expression via binding to their 3′-UTRs. These microRNAs (miRNAs) convoluted in various skeletal buildup aspects [129,130,131]. Such miRNAs attach to complemental bases in 3′ untranslated part of particular target mRNAs, preventing the production of specific proteins [132]. The major biological actions such as cell proliferation, apoptosis, cell differentiation, and metabolism are influenced by miRNAs. As a result, miRNA expression alterations may significantly impact normal and abnormal cells [133]. The miR-460a is an essential micro RNA involved in many structural and metabolic cellular processes [134]. Moreover, it is correlated with inflammatory genes, including IL-1β, in broiler chickens [134,135]. Some other options can be used from the treatment perspective (Table 2).

Table 2.
Alternative treatment options for controlling apoptotic events in tibial dyschondroplasia.
Recent research has focused on the idea that, in contrast to pro-apoptotic, the anti-apoptotic approach in tibial dyschondroplasia may occasionally be advantageous as it reduces the inflammatory response [45]. In fact, an earlier regulation of apoptosis may be beneficial for chondrocytes’ survival. Moreover, local and international industries adopt a proper nutritional strategy for preventing tibial dyschondroplasia (e.g., a proper ratio of calcium, phosphorus, and vitamin D [147,148]) and vaccination for other bone disorders, e.g., viral and bacterial arthritis, chondronecrosis, osteomyelitis, etc. [149,150].
These findings provide fresh knowledge to researchers. Even though several significant research studies have contributed to a deeper understanding of the treatment and prevention of tibial dyschondroplasia, the knowledge is still inadequate for such a critical issue. As a result, the discovery of effective and very sound therapy is urgently required. Moreover, future research on the mechanism of protein-to-protein interaction with the latest scientific findings may lay the foundation for associated bone disorders, e.g., osteoarthritis and osteoporosis.
6. Conclusions
Tibial dyschondroplasia (TD) has been the most severe tibiotarsus disease, causing tibial epiphysis in fast-growing chickens. It causes unusual apoptosis in the tibial growth plate (GP), reducing chondrocyte activity and compromising osteogenesis. According to different pathologic findings and molecular mechanisms, apoptosis and inflammasome are critical. Hence, a deep insight into these pathways is necessary to know the most effective therapeutic approach.
Author Contributions
M.F.-e.-A.K.; Conceptualization, data curation, writing—original draft. W.Y., Q.M., Y.D. and Y.Z.; methodology and formal analysis. J.G., K.L. and H.P.; visualization, and data curation. S.N., M.S., K.M., M.I., M.A., Z.A.B. and M.W.; formal analysis, writing—review & editing J.L. and D.Q.; project administration, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the National Natural Science Foundation of China (Grant No. 31873031; Grant No. 32172929). The figures were designed by using BioRender.com (accessed on 4 August 2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Bcl-2, B-cell lymphoma 2; Bcl-xL, Bcl-2-associated protein xL; Bax, Bcl-2-associated protein x; Bak, Bcl-2-associated protein k; c-FLIP, FLICE-like inhibitory protein; NF-κB, nuclear factor-κB; IκB, inhibitory-κB; IAPs, surviving of apoptosis proteins; XIAP, X-linked inhibitor of apoptosis protein; JAK, Janus kinase; STAT, signal transducers and activators of transcription; MAPK, mitogen-activated protein kinase; PKR, protein kinase R; CDK, cyclin-dependent kinase, TRAIL, tumor necrosis factor-related apoptosis inducing ligand; FasL, Fas ligand; DISC, death-inducing signaling complex; c-FLIP, FLICE-like inhibitory protein; MOMP, mitochondrial outer membrane permeability; FADD, Fas-associated death domain; TRADD, TNFR1-associated death domain; TWEAK, TNF-like WEAK inducer of apoptosis; NGF, nerve growth factor; Bcl-2, B-cell lymphoma 2; BH3, Bcl-homology-3; tBid, truncated Bid; Bax, Bcl-2-associated protein x; Bak, Bcl-2-associated protein k; Apaf-1, apoptotsis activating factor-1—Activates procaspase 9; AIF, apoptosis inducing factor; Endo G, endonuclease G; IAPs, surviving of apoptosis proteins; Smac/DIABLO, second mitochondrial-derived activator of caspases—Director inhibitor of apoptosis-binding protein with Low pI; TRAF2, TNF receptor associated factor 2.
References
- Pines, M.; Reshef, R. Poultry bone development and bone disorders. In Sturkie’s Avian Physiology; Elsevier Inc.: New York, NY, USA, 2015; pp. 367–377. [Google Scholar]
- Gkiatas, I.; Lykissas, M.; Kostas-Agnantis, I.; Korompilias, A.; Batistatou, A.; Beris, A. Factors Affecting Bone Growth. Am. J. Orthop. 2015, 44, 61–67. [Google Scholar]
- Nishimura, R.; Hata, K.; Ikeda, F.; Ichida, F.; Shimoyama, A.; Matsubara, T.; Wada, M.; Amano, K.; Yoneda, T. Signal transduction and transcriptional regulation during mesenchymal cell differentiation. J. Bone Miner. Metab. 2008, 26, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.M.; Clark-Garvey, S.; Porcu, P.; Eischen, C.M. Targeting the Bcl-2 family in B cell lymphoma. Front. Oncol. 2019, 8, 636. [Google Scholar] [CrossRef]
- Huang, S.-C.; Rehman, M.U.; Lan, Y.-F.; Qiu, G.; Zhang, H.; Iqbal, M.K.; Luo, H.-q.; Mehmood, K.; Zhang, L.-h.; Li, J.-k. Tibial dyschondroplasia is highly associated with suppression of tibial angiogenesis through regulating the HIF-1α/VEGF/VEGFR signaling pathway in chickens. Sci. Rep. 2017, 7, 9089. [Google Scholar] [CrossRef] [PubMed]
- Groves, P.J.; Muir, W. Earlier hatching time predisposes Cobb broiler chickens to tibial dyschondroplasia. Animal 2017, 11, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Genin, O.; Hasdai, A.; Shinder, D.; Pines, M. The effect of inhibition of heat-shock proteins on thiram-induced tibial dyschondroplasia. Poult. Sci. 2012, 91, 1619–1626. [Google Scholar] [CrossRef]
- Huang, S.; Kong, A.; Cao, Q.; Tong, Z.; Wang, X. The role of blood vessels in broiler chickens with tibial dyschondroplasia. Poult. Sci. 2019, 98, 6527–6532. [Google Scholar] [CrossRef]
- Lynch, M.; Thorp, B.H.; Whitehead, C.C. Avian Tibial Dyschondroplasia as a Cause of Bone Deformity. Avian Pathol. 1992, 21, 275–285. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Mehmood, K.; Chang, Y.-F.; Tang, Z.; Li, Y. Treatment of tibial dyschondroplasia with traditional Chinese medicines:“Lesson and future directions”. Poult. Sci. 2020, 99, 6422–6433. [Google Scholar] [CrossRef]
- Orth, M.; Cook, M. Avian tibial dyschondroplasia: A morphological and biochemical review of the growth plate lesion and its causes. Vet. Pathol. 1994, 31, 403–414. [Google Scholar] [CrossRef]
- Landy, N.; Toghyani, M. Evaluation of one-alpha-hydroxy-cholecalciferol alone or in combination with cholecalciferol in CaP deficiency diets on development of tibial dyschondroplasia in broiler chickens. Anim. Nutr. 2018, 4, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, J.; Guo, Y.; Sun, Q.; Hu, X. The influence of dietary calcium and phosphorus imbalance on intestinal NaPi-Iib and calbindin mRNA expression and tibia parameters of broilers. Asian-Australas. J. Anim. Sci. 2012, 25, 552. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Zhang, H.; Jiang, X.; Mehmood, K.; Iqbal, M.; Li, A.; Zhang, J.; Wang, Y.; Waqas, M.; Shen, Y.; et al. Effect of total flavonoids of rhizoma drynariae on tibial dyschondroplasia by regulating BMP-2 and Runx2 expression in chickens. Front. Pharmacol. 2018, 9, 1251. [Google Scholar] [CrossRef] [PubMed]
- Provot, S.; Schipani, E. Fetal growth plate: A developmental model of cellular adaptation to hypoxia. Ann. N. Y. Acad. Sci. 2007, 1117, 26–39. [Google Scholar] [CrossRef]
- Pines, M.; Hasdai, A.; Monsonego-Ornan, E. Tibial dyschondroplasia–tools, new insights and future prospects. World’s Poult. Sci. J. 2005, 61, 285–297. [Google Scholar] [CrossRef]
- Leach Jr, R.; Monsonego-Ornan, E. Tibial dyschondroplasia 40 years later. Poult. Sci. 2007, 86, 2053–2058. [Google Scholar] [CrossRef]
- Osman, A.; Sherif, A.; Elhussein, A.; Mohamed, A. Sensitivity of some nitrogen fixers and the target pest Fusarium oxysporum to fungicide thiram. Interdiscip. Toxicol. 2012, 5, 25–29. [Google Scholar] [CrossRef]
- Kunkur, V.; Hunje, R.; Biradarpatil, N.K.; Vyakarnahal, B.S. Effect of seed coating with polymer, fungicide and insecticide on seed quality in cotton during storage. Karnataka J. Agric. Sci. 2010, 20, 137–139. [Google Scholar]
- Beckmann, R.; Houben, A.; Tohidnezhad, M.; Kweider, N.; Fragoulis, A.; Wruck, C.J.; Brandenburg, L.O.; Hermanns-Sachweh, B.; Goldring, M.B.; Pufe, T. Mechanical forces induce changes in VEGF and VEGFR-1/sFlt-1 expression in human chondrocytes. Int. J. Mol. Sci. 2014, 15, 15456–15474. [Google Scholar] [CrossRef]
- Zhang, H.; Mehmood, K.; Li, K.; Rehman, M.U.; Jiang, X.; Huang, S.; Wang, L.; Zhang, L.; Tong, X.; Nabi, F. Icariin ameliorate thiram-induced tibial dyschondroplasia via regulation of WNT4 and VEGF expression in broiler chickens. Front. Pharmacol. 2018, 9, 123. [Google Scholar] [CrossRef]
- Waqas, M.; Qamar, H.; Zhang, J.; Yao, W.; Li, A.; Wang, Y.; Iqbal, M.; Mehmood, K.; Jiang, X.; Li, J. Puerarin enhance vascular proliferation and halt apoptosis in thiram-induced avian tibial dyschondroplasia by regulating HIF-1α, TIMP-3 and BCL-2 expressions. Ecotoxicol. Environ. Saf. 2020, 190, 110126. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, K.; Zhang, H.; Li, K.; Wang, L.; Rehman, M.U.; Nabi, F.; Iqbal, M.K.; Luo, H.; Shahzad, M.; Li, J. Effect of tetramethylpyrazine on tibial dyschondroplasia incidence, tibial angiogenesis, performance and characteristics via HIF-1α/VEGF signaling pathway in chickens. Sci. Rep. 2018, 8, 2495. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Nabi, F.; Rehman, M.; Mehmood, K.; Huang, S.; Zhang, H.; Zhang, L.; Iqbal, M.; Li, J. FK228 recovers thiram-induced tibial dyschondroplasia in chicken via hypoxia inducible factor-1alpha. J. Biol. Regul. Homeost. Agents 2018, 32, 89–95. [Google Scholar] [PubMed]
- Shahzad, M.; Liu, J.; Gao, J.; Wang, Z.; Zhang, D.; Nabi, F.; Li, K.; Li, J. Differential Expression of Extracellular Matrix Metalloproteinase Inducer (EMMPRIN/CD147) in Avian Tibial Dyschondroplasia. Avian Pathol. 2015, 44, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Kulyar, M.F.-E.-A.; Yao, W.; Ding, Y.; Du, H.; Li, K.; Zhang, L.; Li, A.; Huachun, P.; Waqas, M.; Mehmood, K. Cluster of differentiation 147 (CD147) expression is linked with thiram induced chondrocyte’s apoptosis via Bcl-2/Bax/Caspase-3 signalling in tibial growth plate under chlorogenic acid repercussion. Ecotoxicol. Environ. Saf. 2021, 213, 112059. [Google Scholar] [CrossRef] [PubMed]
- Kulyar, M.F.-E.-A.; Yao, W.; Ding, Y.; Du, H.; Mo, Q.; Pan, H.; Shahzad, M.; Mehmood, K.; Iqbal, M.; Akhtar, M. Chlorogenic acid suppresses mitochondrial apoptotic effectors Bax/Bak to counteract Nod-like receptor pyrin domain 3 (NLRP3) inflammasome in thiram exposed chondrocytes. Phytomedicine 2022, 95, 153865. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, S.; Tong, X.; Zhang, L.; Jiang, X.; Zhang, H.; Mehmood, K.; Li, J. Chlorogenic acid alleviates thiram-induced tibial dyschondroplasia by modulating caspases, BECN1 expression and ECM degradation. Int. J. Mol. Sci. 2019, 20, 3160. [Google Scholar] [CrossRef]
- Mehmood, K.; Zhang, H.; Yao, W.; Jiang, X.; Waqas, M.; Li, A.; Wang, Y.; Lei, L.; Zhang, L.; Qamar, H. Protective effect of Astragaloside IV to inhibit thiram-induced tibial dyschondroplasia. Environ. Sci. Pollut. Res. 2019, 26, 16210–16219. [Google Scholar] [CrossRef]
- Niu, S.; Li, X.; Jahejo, A.; Zhang, N.; Yang, S.; Jia, Y.; Zhang, Y.; Tian, Z.; Li, Z.; Ning, G. Glutathione-S-transferase A3 protein suppresses thiram-induced tibial dyschondroplasia by regulating prostaglandin-related genes expression. Res. Vet. Sci. 2021, 135, 343–348. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, Z.; Jin, D.; Cai, C.; Jia, C.; Liu, W.; Wang, T.; Li, S.; Zhang, H.; Huang, B. mTORC1 regulates PTHrP to coordinate chondrocyte growth, proliferation and differentiation. Nat. Commun. 2016, 7, 11151. [Google Scholar] [CrossRef]
- Mizuhashi, K.; Ono, W.; Matsushita, Y.; Sakagami, N.; Takahashi, A.; Saunders, T.L.; Nagasawa, T.; Kronenberg, H.M.; Ono, N. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature 2018, 563, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J. Pathogenesis of osteochondrosis. In Diagnosis and Management of Lameness in the Horse; Elsevier: Amsterdam, The Netherlands, 2003; pp. 534–543. [Google Scholar]
- Mackie, E.; Ahmed, Y.; Tatarczuch, L.; Chen, K.-S.; Mirams, M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Rivas, R.; Shapiro, F. Structural stages in the development of the long bones and epiphyses: A study in the New Zealand white rabbit. JBJS 2002, 84, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, F.; Flynn, E. Structural differences in epiphyseal and physeal hypertrophic chondrocytes. BoneKEy Rep. 2015, 4, 663. [Google Scholar] [CrossRef][Green Version]
- Breur, G.; VanEnkevort, B.; Farnum, C.; Wilsman, N. Linear relationship between the volume of hypertrophic chondrocytes and the rate of longitudinal bone growth in growth plates. J. Orthop. Res. 1991, 9, 348–359. [Google Scholar] [CrossRef]
- Abad, V.; Meyers, J.L.; Weise, M.; Gafni, R.I.; Barnes, K.M.; Nilsson, O.; Bacher, J.D.; Baron, J. The role of the resting zone in growth plate chondrogenesis. Endocrinology 2002, 143, 1851–1857. [Google Scholar] [CrossRef]
- Ono, N.; Kronenberg, H.M. Developmental Biology of Musculoskeletal Tissues for Tissue Engineers. Dev. Biol. Musculoskelet. Tissue Eng. Princ. Appl. 2018, 1–24. [Google Scholar] [CrossRef]
- Burdan, F.; Szumiło, J.; Korobowicz, A.; Farooquee, R.; Patel, S.; Patel, A.; Dave, A.; Szumiło, M.; Solecki, M.; Klepacz, R. Morphology and physiology of the epiphyseal growth plate. Folia Histochem. Et Cytobiol. 2009, 47, 5–16. [Google Scholar] [CrossRef]
- Melrose, J.; Smith, S.; Smith, M.; Little, C. The use of Histochoice™® for histological examination of articular and growth plate cartilages, intervertebral disc and meniscus. Biotech. Histochem. 2008, 83, 47–53. [Google Scholar] [CrossRef]
- Eames, B.F.; De La Fuente, L.; Helms, J.A. Molecular ontogeny of the skeleton. Birth Defects Res. Part C Embryo Today: Rev. 2003, 69, 93–101. [Google Scholar] [CrossRef]
- Ballock, R.T.; O’Keefe, R.J. The biology of the growth plate. JBJS 2003, 85, 715–726. [Google Scholar] [CrossRef]
- Qamar, H.; Waqas, M.; Li, A.; Iqbal, M.; Mehmood, K.; Li, J. Plastrum Testudinis Extract Mitigates Thiram Toxicity in Broilers via Regulating PI3K/AKT Signaling. Biomolecules 2019, 9, 784. [Google Scholar] [CrossRef] [PubMed]
- Kulyar, M.F.-A.; Mo, Q.; Yao, W.; Ding, Y.; Yan, Z.; Du, H.; Pan, H.; Li, K.; Gao, J.; Shahzad, M.; et al. Chlorogenic Acid Suppresses MiR-460a in the Regulation of Bcl-2, Causing Interleukin-1β Reduction in Thiram Exposed Chondrocytes via Caspase-3/Caspase-7 Pathway. Phytomedicine 2022, 104, 154296. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Mercer, S.; Eckert, G.J.; Trippel, S.B. Regulation of articular chondrocyte aggrecan and collagen gene expression by multiple growth factor gene transfer. J. Orthop. Res. 2012, 30, 1026–1031. [Google Scholar] [CrossRef]
- Frazer, A.; Bunning, R.A.; Thavarajah, M.; Seid, J.M.; Russell, R.G.G. Studies on type II collagen and aggrecan production in human articular chondrocytes in vitro and effects of transforming growth factor-β and interleukin-1β. Osteoarthr. Cartil. 1994, 2, 235–245. [Google Scholar] [CrossRef]
- Nishimura, G.; Haga, N.; Kitoh, H.; Tanaka, Y.; Sonoda, T.; Kitamura, M.; Shirahama, S.; Itoh, T.; Nakashima, E.; Ohashi, H. The phenotypic spectrum of COL2A1 mutations. Hum. Mutat. 2005, 26, 36–43. [Google Scholar] [CrossRef]
- Kiani, C.; Chen, L.; Wu, Y.J.; Yee, A.J.; Yang, B.B. Structure and function of aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef]
- Kim, Y.S.; Majid, M.; Melchiorri, A.J.; Mikos, A.G. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 2019, 4, 83–95. [Google Scholar] [CrossRef]
- Tselepis, C.; Hoyland, J.A.; Barber, R.E.; Thorp, B.H.; Kwan, A.P. Expression and distribution of cartilage matrix macromolecules in avian tibial dyschondroplasia. Avian Pathol. 1996, 25, 305–324. [Google Scholar] [CrossRef]
- Yurchenko, V.; Constant, S.; Bukrinsky, M. Dealing with the family: CD147 interactions with cyclophilins. Immunology 2006, 117, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Guindolet, D.; Gabison, E.E. Role of CD147 (EMMPRIN/Basigin) in tissue remodeling. Anat. Rec. 2020, 303, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hu, C.; Xiao, Z.; Luo, C.; Liu, Z. Downregulation of CyclophilinA/CD147 Axis Induces Cell Apoptosis and Inhibits Glioma Aggressiveness. BioMed Res. Int. 2020, 2020, 7035847. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jin, A.; Huang, W.; Tsang, L.L.; Cai, Z.; Zhou, X.; Chen, H.; Chan, H.C. Up-regulation of Bcl-2 by CD147 through ERK activation results in abnormal cell survival in human endometriosis. J. Clin. Endocrinol. Metab. 2015, 100, E955–E963. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Liu, C.; Xu, W.; AzhaTi, W.; Li, C.; Wang, Z. Up-regulated expression of CD147 gene in malignant bone tumor and the possible induction mechanism during osteoclast formation. Braz. J. Med. Biol. Res. 2018, 51, e6948. [Google Scholar] [CrossRef]
- Yang, D.; Liu, R.; Liu, L.; Liao, H.; Wang, C.; Cao, Z. Involvement of CD 147 in alveolar bone remodeling and soft tissue degradation in experimental periodontitis. J. Periodontal Res. 2017, 52, 704–712. [Google Scholar] [CrossRef]
- Yin, H.; Shao, Y.; Chen, X. The effects of CD147 on the cell proliferation, apoptosis, invasion, and angiogenesis in glioma. Neurol. Sci. 2017, 38, 129–136. [Google Scholar] [CrossRef]
- Asgari, R.; Mansouri, K.; Bakhtiari, M.; Vaisi-Raygani, A. CD147 as an apoptosis regulator in spermatogenesis: Deciphering its association with matrix metalloproteinases’ pathway. Mol. Biol. Rep. 2019, 46, 1099–1105. [Google Scholar] [CrossRef]
- Chavakis, E.; Dimmeler, S. Regulation of endothelial cell survival and apoptosis during angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 887–893. [Google Scholar] [CrossRef]
- Ferrari, G.; Pintucci, G.; Seghezzi, G.; Hyman, K.; Galloway, A.C.; Mignatti, P. VEGF, a prosurvival factor, acts in concert with TGF-β1 to induce endothelial cell apoptosis. Proc. Natl. Acad. Sci. USA 2006, 103, 17260–17265. [Google Scholar] [CrossRef]
- Lamalice, L.; Houle, F.; Jourdan, G.; Huot, J. Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene 2004, 23, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.-P.; McMurtrey, A.; Kowalski, J.; Yan, M.; Keyt, B.A.; Dixit, V.; Ferrara, N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway: Requirement for Flk-1/KDR activation. J. Biol. Chem. 1998, 273, 30336–30343. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.r.; Haendeler, J.; Aicher, A.; Rössig, L.; Vasa, M.; Zeiher, A.M.; Dimmeler, S. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: Important role of nitric oxide. Circ. Res. 2001, 89, 709–715. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Fulton, D.; Mahboubi, K.; Kalb, R.G.; O’Connor, D.S.; Li, F.; Altieri, D.C.; Sessa, W.C. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/surviving pathway. J. Biol. Chem. 2000, 275, 9102–9105. [Google Scholar] [CrossRef]
- Gratton, J.-P.; Morales-Ruiz, M.; Kureishi, Y.; Fulton, D.; Walsh, K.; Sessa, W.C. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J. Biol. Chem. 2001, 276, 30359–30365. [Google Scholar] [CrossRef]
- Dimmeler, S.; Dernbach, E.; Zeiher, A.M. Phosphorylation of the endothelial nitric oxide synthase at ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett. 2000, 477, 258–262. [Google Scholar] [CrossRef]
- Jan, R. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 2019, 9, 205. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Programmed cell death (apoptosis). In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- LeBlanc, A.C. Natural cellular inhibitors of caspases. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 215–229. [Google Scholar] [CrossRef]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef] [PubMed]
- Sayers, T.J. Targeting the extrinsic apoptosis signaling pathway for cancer therapy. Cancer Immunol. Immunother. 2011, 60, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.A.; Kirby, R. Apoptosis: A review of pro-apoptotic and anti-apoptotic pathways and dysregulation in disease. J. Vet. Emerg. Crit. Care 2008, 18, 572–585. [Google Scholar] [CrossRef]
- Inoue, S.; Browne, G.; Melino, G.; Cohen, G. Ordering of caspases in cells undergoing apoptosis by the intrinsic pathway. Cell Death Differ. 2009, 16, 1053–1061. [Google Scholar] [CrossRef]
- Ghavami, S.; Kerkhoff, C.; Los, M.; Hashemi, M.; Sorg, C.; Karami-Tehrani, F. Mechanism of apoptosis induced by S100A8/A9 in colon cancer cell lines: The role of ROS and the effect of metal ions. J. Leukoc. Biol. 2004, 76, 169–175. [Google Scholar] [CrossRef]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and quercetin: Promising flavonoids with chemopreventive potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Goel, N. Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. In Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 125, pp. 73–120. [Google Scholar]
- Moldoveanu, T.; Follis, A.V.; Kriwacki, R.W.; Green, D.R. Many players in BCL-2 family affairs. Trends Biochem. Sci. 2014, 39, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Zamarin, D.; Holmgaard, R.B.; Subudhi, S.K.; Park, J.S.; Mansour, M.; Palese, P.; Merghoub, T.; Wolchok, J.D.; Allison, J.P. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014, 6, 226ra32. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Cory, S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018, 25, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Rooswinkel, R.W.; van de Kooij, B.; de Vries, E.; Paauwe, M.; Braster, R.; Verheij, M.; Borst, J. Antiapoptotic potency of Bcl-2 proteins primarily relies on their stability, not binding selectivity. Blood J. Am. Soc. Hematol. 2014, 123, 2806–2815. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef]
- Letai, A.G. Diagnosing and exploiting cancer’s addiction to blocks in apoptosis. Nat. Rev. Cancer 2008, 8, 121–132. [Google Scholar] [CrossRef]
- Roberts, A.; Huang, D. Targeting BCL2 with BH3 mimetics: Basic science and clinical application of venetoclax in chronic lymphocytic leukemia and related B cell malignancies. Clin. Pharmacol. Ther. 2017, 101, 89–98. [Google Scholar] [CrossRef]
- Carrington, E.M.; Zhan, Y.; Brady, J.L.; Zhang, J.-G.; Sutherland, R.M.; Anstee, N.S.; Schenk, R.L.; Vikstrom, I.B.; Delconte, R.B.; Segal, D. Anti-apoptotic proteins BCL-2, MCL-1 and A1 summate collectively to maintain survival of immune cell populations both in vitro and in vivo. Cell Death Differ. 2017, 24, 878–888. [Google Scholar] [CrossRef]
- Katsumata, K.; Sumi, T.; Tomioka, H.; Aoki, T.; Koyanagi, Y. Induction of apoptosis by p53, bax, bcl-2, and p21 expressed in colorectal cancer. Int. J. Clin. Oncol. 2003, 8, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Reed, J. Proapoptotic multidomain Bcl-2/Bax-family proteins: Mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006, 13, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Martinou, J.-C.; Green, D.R. Breaking the mitochondrial barrier. Nat. Rev. Mol. Cell Biol. 2001, 2, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, N.J.; Ricci, J.-E.; Green, D.R. And all of a sudden it’s over: Mitochondrial outer-membrane permeabilization in apoptosis. Biochimie 2002, 84, 113–121. [Google Scholar] [CrossRef]
- Wolter, K.G.; Hsu, Y.-T.; Smith, C.L.; Nechushtan, A.; Xi, X.-G.; Youle, R.J. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 1997, 139, 1281–1292. [Google Scholar] [CrossRef]
- Suzuki, M.; Youle, R.J.; Tjandra, N. Structure of Bax: Coregulation of dimer formation and intracellular localization. Cell 2000, 103, 645–654. [Google Scholar] [CrossRef]
- Nechushtan, A.; Smith, C.L.; Hsu, Y.T.; Youle, R.J. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999, 18, 2330–2341. [Google Scholar] [CrossRef]
- Fuentes-Prior, P.; Salvesen, G.S. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem. J. 2004, 384, 201–232. [Google Scholar] [CrossRef]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef]
- Riedl, S.J.; Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [PubMed]
- Walsh, J.G.; Cullen, S.P.; Sheridan, C.; Lüthi, A.U.; Gerner, C.; Martin, S.J. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc. Natl. Acad. Sci. USA 2008, 105, 12815–12819. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.D.; Saunders, M.; Dische, S.; Richman, P.; Daley, F.; Bentzen, S.M. Bcl-2 expression in head and neck cancer: An enigmatic prognostic marker. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 435–441. [Google Scholar] [CrossRef]
- Friedrich, K.; Wieder, T.; Von Haefen, C.; Radetzki, S.; JaÈnicke, R.; Schulze-Osthoff, K.; DoÈrken, B.; Daniel, P.T. Overexpression of caspase-3 restores sensitivity for drug-induced apoptosis in breast cancer cell lines with acquired drug resistance. Oncogene 2001, 20, 2749–2760. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-C.; Sun, J.-M.; Wei, Z.-L.; Yang, X.-F.; Zhang, Y.-C.; Xin, Y. Expression of Fas ligand and caspase-3 contributes to formation of immune escape in gastric cancer. World J. Gastroenterol. WJG 2003, 9, 1415. [Google Scholar] [CrossRef]
- Lindner, A.U.; Lucantoni, F.; Varešlija, D.; Resler, A.; Murphy, B.M.; Gallagher, W.M.; Hill, A.D.; Young, L.S.; Prehn, J.H. Low cleaved caspase-7 levels indicate unfavourable outcome across all breast cancers. J. Mol. Med. 2018, 96, 1025–1037. [Google Scholar] [CrossRef]
- Kasakura, K.; Takahashi, K.; Itoh, T.; Hosono, A.; Nunomura, S.; Ra, C.; Momose, Y.; Itoh, K.; Nishiyama, C.; Kaminogawa, S. C/EBPα controls mast cell function. FEBS Lett. 2014, 588, 4645–4653. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Balahura, L.R.; Selaru, A.; Dinescu, S.; Costache, M. Inflammation and inflammasomes: Pros and cons in tumorigenesis. J. Immunol. Res. 2020, 2020, 2549763. [Google Scholar]
- Lamkanfi, M.; Dixit, V.M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 2012, 28, 137–161. [Google Scholar]
- Yu, C.; Zhang, C.; Kuang, Z.; Zheng, Q. The Role of NLRP3 Inflammasome Activities in Bone Diseases and Vascular Calcification. Inflammation 2021, 44, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Guégan, C.; Vila, M.; Teissman, P.; Chen, C.; Onténiente, B.; Li, M.; Friedlander, R.M.; Przedborski, S. Instrumental activation of bid by caspase-1 in a transgenic mouse model of ALS. Mol. Cell. Neurosci. 2002, 20, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.M.; Hahn, H.S.; Odley, A.; Guo, Y.; Vallejo, J.G.; Lynch, R.A.; Mann, D.L.; Bolli, R.; Dorn, G.W. Proapoptotic effects of caspase-1/interleukin-converting enzyme dominate in myocardial ischemia. Circ. Res. 2005, 96, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.W.; Demarco, B.; Heilig, R.; Shkarina, K.; Boettcher, A.; Farady, C.J.; Pelczar, P.; Broz, P. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP 3 inflammasome assembly. EMBO J. 2019, 38, e101638. [Google Scholar] [CrossRef] [PubMed]
- Vince, J.E.; De Nardo, D.; Gao, W.; Vince, A.J.; Hall, C.; McArthur, K.; Simpson, D.; Vijayaraj, S.; Lindqvist, L.M.; Bouillet, P.; et al. The mitochondrial apoptotic effectors BAX/BAK activate caspase-3 and-7 to trigger NLRP3 inflammasome and caspase-8 driven IL-1β activation. Cell Rep. 2018, 25, 2339–2353. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, H.; Chen, D.; Yu, B.; Mao, X.; Zheng, P.; Yu, J.; Luo, Y.; Luo, J.; He, J. Chlorogenic acid improves intestinal development via suppressing mucosa inflammation and cell apoptosis in weaned pigs. ACS Omega 2018, 3, 2211–2219. [Google Scholar] [CrossRef]
- Kim, S.-K.; Cho, Y.J.; Choe, J.-Y. NLRP3 inflammasomes and NLRP3 inflammasome-derived proinflammatory cytokines in peripheral blood mononuclear cells of patients with ankylosing spondylitis. Clin. Chim. Acta 2018, 486, 269–274. [Google Scholar] [CrossRef]
- Pan, G.; Zheng, R.; Yang, P.; Li, Y.; Clancy, J.P.; Liu, J.; Feng, X.; Garber, D.A.; Spearman, P.; McDonald, J.M. Nucleosides accelerate inflammatory osteolysis, acting as distinct innate immune activators. J. Bone Miner. Res. 2011, 26, 1913–1925. [Google Scholar] [CrossRef]
- Tang, C.; Chen, Y.; Huang, J.; Zhao, K.; Chen, X.; Yin, Z.; Heng, B.C.; Chen, W.; Shen, W. The roles of inflammatory mediators and immunocytes in tendinopathy. J. Orthop. Transl. 2018, 14, 23–33. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, B.; Liu, J.; Waqas, M.; Kulyar, M.F.-e.-A.; Guo, K.; Li, J. Chlorogenic acid inhibits apoptosis in thiram-induced tibial dyschondroplasia via intrinsic pathway. Environ. Sci. Pollut. Res. 2021, 28, 68288–68299. [Google Scholar] [CrossRef]
- Yang, F.; Luo, L.; Zhu, Z.-D.; Zhou, X.; Wang, Y.; Xue, J.; Zhang, J.; Cai, X.; Chen, Z.-L.; Ma, Q. Chlorogenic acid inhibits liver fibrosis by blocking the miR-21-regulated TGF-β1/Smad7 signaling pathway in vitro and in vivo. Front. Pharmacol. 2017, 8, 929. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.-C.; Lee, C.; Kim, J.-Y.; Oh, H.M.; So, H.-S.; Lee, M.S.; Rho, M.C.; Oh, J. Chlorogenic acid inhibits of osteoclast differentiation and bone resorption by down-regulation of receptor activator of nuclear factor kappa-b ligand-induced nuclear factor of activated T cells c1 expression. Biol. Pharm. Bull. 2013, 36, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, X. Mechanism of chlorogenic acid treatment on femoral head necrosis and its protection of osteoblasts. Biomed. Rep. 2016, 5, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Gennari, L.; Bianciardi, S.; Merlotti, D. MicroRNAs in bone diseases. Osteoporos. Int. 2017, 28, 1191–1213. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.-M.; Fu, B.-L.; Xu, L.-L.; Wang, B. MicroRNA-21: An Emerging Player in Bone Diseases. Front. Pharmacol. 2021, 12, 722804. [Google Scholar] [CrossRef]
- Nakasa, T.; Yoshizuka, M.; Andry Usman, M.; Elbadry Mahmoud, E.; Ochi, M. MicroRNAs and bone regeneration. Curr. Genom. 2015, 16, 441–452. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Loh, H.-Y.; Lau, Y.-Y.; Lai, K.-S.; Osman, M.A. MicroRNAs in Bone Diseases: Progress and Prospects. In Transcriptional and Post-transcriptional Regulation; IntechOpen: London, UK, 2018. [Google Scholar]
- Naraballobh, W.; Trakooljul, N.; Murani, E.; Krischek, C.; Janisch, S.; Wicke, M.; Ponsuksili, S.; Wimmers, K. miRNAs regulate acute transcriptional changes in broiler embryos in response to modification of incubation temperature. Sci. Rep. 2018, 8, 11371. [Google Scholar] [CrossRef]
- Liu, P.; Yang, F.; Zhuang, Y.; Xiao, Q.; Cao, H.; Zhang, C.; Wang, T.; Lin, H.; Guo, X.; Hu, G. Dysregulated expression of microRNAs and mRNAs in pulmonary artery remodeling in ascites syndrome in broiler chickens. Oncotarget 2017, 8, 1993. [Google Scholar] [CrossRef]
- Weng, X.; Lin, P.; Liu, F.; Chen, J.; Li, H.; Huang, L.; Zhen, C.; Xu, H.; Liu, X.; Ye, H. Achyranthes bidentata polysaccharides activate the Wnt/β-catenin signaling pathway to promote chondrocyte proliferation. Int. J. Mol. Med. 2014, 34, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, H.; Ma, T.; Lan, H.; Feng, S.; Zhu, H.; Ji, Y. Resveratrol protects murine chondrogenic ATDC5 cells against LPS-induced inflammatory injury through up-regulating MiR-146b. Cell. Physiol. Biochem. 2018, 47, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.U.; Kim, M.O. Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef]
- Xue, Y.; Li, D.; Zhang, Y.; Gao, H.; Li, H. Angelica polysaccharide moderates hypoxia-evoked apoptosis and autophagy in rat neural stem cells by downregulation of BNIP3. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.T.; Chiang, L.-C.; Lin, Y.-T.; Lin, C.-C. Antiproliferative and apoptotic effects of tetrandrine on different human hepatoma cell lines. Am. J. Chin. Med. 2006, 34, 125–135. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Liu, J.; Wang, K.; Guo, X.; Ji, B.; Wu, W.; Zhou, F. Protective effects of genistein and puerarin against chronic alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms. J. Agric. Food Chem. 2016, 64, 7291–7297. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.-Q.; Yu, L.; He, B.; Wu, S.-H.; Zhao, Q.; Xia, S.-Q.; Mei, H.-J. Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis 2015, 20, 1187–1199. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, R.; Zhu, X.-Y.; Hao, Y.-J.; Li, N.; Wang, J.; Niu, Y.; Sun, T.; Li, Y.-X.; Yu, J.-Q. Matrine attenuates focal cerebral ischemic injury by improving antioxidant activity and inhibiting apoptosis in mice. Int. J. Mol. Med. 2015, 36, 633–644. [Google Scholar] [CrossRef]
- Jian, J.; Xuan, F.; Qin, F.; Huang, R. The antioxidant, anti-inflammatory and anti-apoptotic activities of the bauhinia championii flavone are connected with protection against myocardial ischemia/reperfusion injury. Cell. Physiol. Biochem. 2016, 38, 1365–1375. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Xu, T.; Qin, S. β-Ecdysterone Suppresses Interleukin-1β-Induced Apoptosis and Inflammation in Rat Chondrocytes via Inhibition of NF-κB Signaling Pathway. Drug Dev. Res. 2014, 75, 195–201. [Google Scholar] [CrossRef]
- Ju, X.-d.; Deng, M.; Ao, Y.-f.; Yu, C.-l.; Wang, J.-q.; Yu, J.-k.; Cui, G.-q.; Hu, Y.-l. Protective effect of sinomenine on cartilage degradation and chondrocytes apoptosis. Yakugaku Zasshi 2010, 130, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Shafey, T.; McDonald, M.; Pym, R. Effects of dietary calcium, available phosphorus and vitamin D on growth rate, food utilisation, plasma and bone constituents and calcium and phosphorus retention of commercial broiler strains. Br. Poult. Sci. 1990, 31, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.; Shinder, D.; Yosefi, S.; Vax, E.; Plavnik, I. Metabolism and requirements for calcium and phosphorus in the fast-growing chicken as affected by age. Br. J. Nutr. 2003, 89, 51–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hester, P.Y. The role of environment and management on leg abnormalities in meat-type fowl. Poult. Sci. 1994, 73, 904–915. [Google Scholar] [CrossRef]
- McNamee, P.T.; Smyth, J.A. Bacterial chondronecrosis with osteomyelitis (femoral head necrosis) of broiler chickens: A review. Avian Pathol. 2000, 29, 477–495. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).