LAMP Coupled CRISPR-Cas12a Module for Rapid, Sensitive and Visual Detection of Porcine Circovirus 2
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Clinical Samples
2.2. Nucleic Acid Preparation
2.3. LAMP Primers Design and crRNA Preparation
2.4. Preparation of huLbCas12a
2.5. Determination of Target Cleavage Activity of the huLbCas12a Protein
2.6. Optimization and Evaluation of the LAMP-CRISPR Detection Method
2.7. LAMP-CRISPR Fluorescence Assay
2.8. Efficiency Assessment of the LAMP-CRISPR Detection System for Clinical Samples Detection
2.9. Statistic Analysis
3. Results
3.1. Expression and Purification of the huLbCas12a Protein in E. coli
3.2. Target Cleavage Activity of huLbCas12a Protein
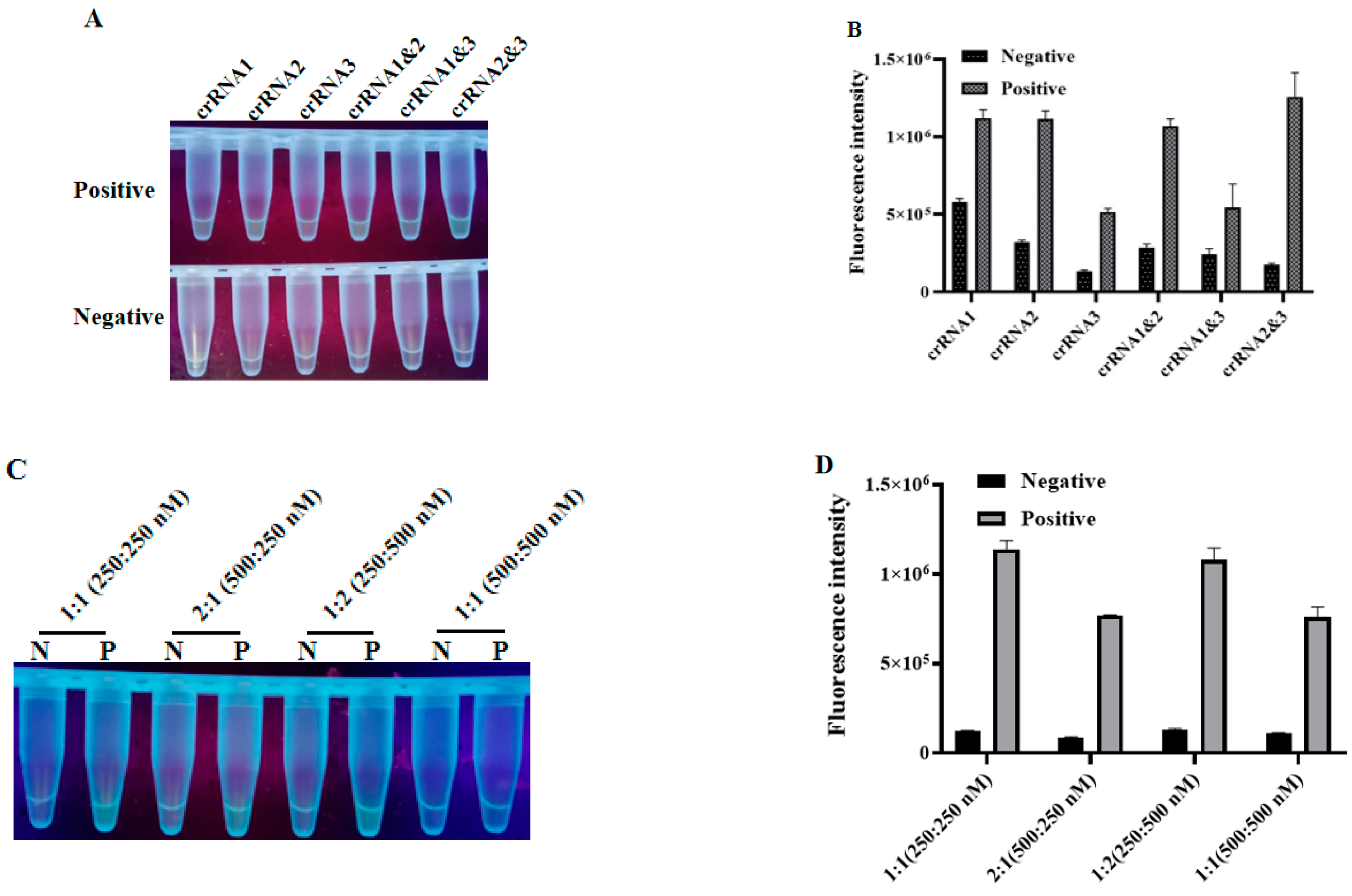
3.3. Optimization of the LAMP-CRISPR Detection
3.4. Sensitivity and Specificity Test of LAMP-CRISPR Detection
3.5. Comparation of LAMP-CRISPR and Taqman-Based qPCR in Detecting Clinical Samples
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allan, G.M.; McNeilly, F.; Kennedy, S.; Daft, B.; Clarke, E.G.; Ellis, J.A.; Haines, D.M.; Meehan, B.M.; Adair, B.M. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J. Vet. Diagn. Investig. 1998, 10, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Kekarainen, T.; Cortey, M. The natural history of porcine circovirus type 2: From an inoffensive virus to a devastating swine disease? Vet. Microbiol. 2013, 165, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.J. Porcine circovirus type 2 (PCV2): Pathogenesis and interaction with the immune system. Annu. Rev. Anim. Biosci. 2013, 1, 43–64. [Google Scholar] [CrossRef]
- Han, L.; Yuan, G.F.; Chen, S.J.; Dai, F.; Hou, L.S.; Fan, J.H.; Zuo, Y.Z. Porcine circovirus type 2 (PCV2) infection in Hebei Province from 2016 to 2019: A retrospective study. Arch. Virol. 2021, 166, 2159–2171. [Google Scholar] [CrossRef]
- Savic, B.; Milicevic, V.; Jakic-Dimic, D.; Bojkovski, J.; Prodanovic, R.; Kureljusic, B.; Potkonjak, A.; Savic, B. Genetic characterization and phylogenetic analysis of porcine circovirus type 2 (PCV2) in Serbia. Arch. Virol. 2012, 157, 21–28. [Google Scholar] [CrossRef]
- Franzo, G.; Cortey, M.; Segalés, J.; Hughes, J.; Drigo, M. Phylodynamic analysis of porcine circovirus type 2 reveals global waves of emerging genotypes and the circulation of recombinant forms. Mol. Phylogenet. Evol. 2016, 100, 269–280. [Google Scholar] [CrossRef]
- Franzo, G.; Segalés, J. Porcine Circovirus 2 Genotypes, Immunity and Vaccines: Multiple Genotypes but One Single Serotype. Pathogens 2020, 9, 1049. [Google Scholar] [CrossRef]
- Franzo, G.; Segalés, J.; Giovanni, F.; Joaquim, S. Porcine circovirus 2 (PCV-2) genotype update and proposal of a new genotyping methodology. PLoS ONE 2018, 13, e0208585. [Google Scholar] [CrossRef]
- Wang, Y.; Noll, L.; Lu, N.; Porter, E.; Stoy, C.; Zheng, W.; Liu, X.; Peddireddi, L.; Niederwerder, M.; Bai, J. Genetic diversity and prevalence of porcine circovirus type 3 (PCV3) and type 2 (PCV2) in the Midwest of the USA during 2016–2018. Transbound. Emerg. Dis. 2020, 67, 1284–1294. [Google Scholar] [CrossRef]
- Guo, Z.; Ruan, H.; Qiao, S.; Deng, R.; Zhang, G. Co-infection status of porcine circoviruses (PCV2 and PCV3) and porcine epidemic diarrhea virus (PEDV) in pigs with watery diarrhea in Henan province, central China. Microb. Pathog. 2020, 142, 104047. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, J.; Qi, J.; Hao, F.; Xu, L.; Guo, K. Genetic Diversity and Prevalence of Porcine Circovirus Type 2 in China During 2000–2019. Front. Vet. Sci. 2021, 8, 788172. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Q.; Guo, H.C.; Sun, D.H.; Yin, S.H.; Shang, Y.J.; Cai, X.P.; Liu, X.T. Development and validation of an ELISA using a protein encoded by ORF2 antigenic domain of porcine circovirus type 2. Virol. J. 2010, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, U.; Sen, A.; Sharma, I. Development of cost-effective quantitative PCR method for parallel detection of porcine circovirus2 and porcine parvovirus in perspective of North-eastern India. Trop. Anim. Health Prod. 2021, 53, 177. [Google Scholar] [CrossRef] [PubMed]
- Brunborg, I.M.; Moldal, T.; Jonassen, C.M. Quantitation of porcine circovirus type 2 isolated from serum/plasma and tissue samples of healthy pigs and pigs with postweaning multisystemic wasting syndrome using a TaqMan-based real-time PCR. J. Virol. Methods 2004, 122, 171–178. [Google Scholar] [CrossRef]
- Kim, J.; Chae, C. Double in situ hybridization for simultaneous detection and differentiation of porcine circovirus 1 and 2 in pigs with postweaning multisystemic wasting syndrome. Vet. J. 2002, 164, 247–253. [Google Scholar] [CrossRef]
- Liu, Y.B.; Zhang, L.; Xue, Q.H.; Ning, Y.B.; Zhang, Z.G. Development of a loop-mediated isothermal amplification assay for porcine circovirus type 2. Virol. Sin. 2011, 26, 214–220. [Google Scholar] [CrossRef]
- Racine, S.; Kheyar, A.; Gagnon, C.A.; Charbonneau, B.; Dea, S. Eucaryotic expression of the nucleocapsid protein gene of porcine circovirus type 2 and use of the protein in an indirect immunofluorescence assay for serological diagnosis of postweaning multisystemic wasting syndrome in pigs. Clin. Diagn. Lab. Immunol. 2004, 11, 736–741. [Google Scholar] [CrossRef]
- Szczotka, A.; Stadejek, T.; Pejsak, Z. A comparison of immunohistochemistry and in situ hybridization for the detection of porcine circovirus type 2 in pigs. Pol. J. Vet. Sci. 2011, 14, 565–571. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wang, J.; Liu, G. CRISPR/Cas Systems towards Next-Generation Biosensing. Trends Biotechnol. 2019, 37, 730–743. [Google Scholar] [CrossRef]
- Myhrvold, C.; Freije, C.A.; Gootenberg, J.S.; Abudayyeh, O.O.; Metsky, H.C.; Durbin, A.F.; Kellner, M.J.; Tan, A.L.; Paul, L.M.; Parham, L.A.; et al. Field-deployable viral diagnostics using CRISPR-Cas13. Science 2018, 360, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Patchsung, M.; Jantarug, K.; Pattama, A.; Aphicho, K.; Suraritdechachai, S.; Meesawat, P.; Sappakhaw, K.; Leelahakorn, N.; Ruenkam, T.; Wongsatit, T.; et al. Clinical validation of a Cas13-based assay for the detection of SARS-CoV-2 RNA. Nat. Biomed. Eng. 2020, 4, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef]
- Ding, X.; Yin, K.; Li, Z.; Lalla, R.V.; Ballesteros, E.; Sfeir, M.M.; Liu, C. Ultrasensitive and visual detection of SARS-CoV-2 using all-in-one dual CRISPR-Cas12a assay. Nat. Commun. 2020, 11, 4711. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Cheng, Q.X.; Wang, J.M.; Li, X.Y.; Zhang, Z.L.; Gao, S.; Cao, R.B.; Zhao, G.P.; Wang, J. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Tan, L.; Lei, L.; Zhan, Y.; Duan, D.Y.; Wang, A.B. Establishment and application of LAMP visual detection method for porcine circovirus type 2. Chin. Vet. Sci. 2022, 6, 679–684. (In Chinese) [Google Scholar]
- Xiao, C.T.; Halbur, P.G.; Opriessnig, T. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J. Gen. Virol. 2015, 96 Pt 7, 1830–1841. [Google Scholar] [CrossRef]
- Kissling, L.; Monfort, A.; Swarts, D.C.; Wutz, A.; Jinek, M. Preparation and electroporation of Cas12a/Cpf1-guide RNA complexes for introducing large gene deletions in mouse embryonic stem cells. Methods Enzymol. 2019, 616, 241–263. [Google Scholar]
- Chang, Y.; Deng, Y.; Li, T.; Wang, J.; Wang, T.; Tan, F.; Li, X.; Tian, K. Visual detection of porcine reproductive and respiratory syndrome virus using CRISPR-Cas13a. Transbound. Emerg. Dis. 2020, 67, 564–571. [Google Scholar] [CrossRef]
- Fozouni, P.; Son, S.; Díaz de León Derby, M.; Knott, G.J.; Gray, C.N.; D’Ambrosio, M.V.; Zhao, C.; Switz, N.A.; Kumar, G.R.; Stephens, S.I.; et al. Amplification-free detection of SARS-CoV-2 with CRISPR-Cas13a and mobile phone microscopy. Cell 2021, 184, 323–333.e9. [Google Scholar] [CrossRef] [PubMed]




| Simple Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Ct value | - | 33.82 | 23.54 | 31.55 | 32.84 | 32.13 | - | - | 25.59 | 35.44 |
| Simple number | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Ct value | 35.28 | 31.77 | 28.44 | 34.38 | 35.80 | 28.39 | 20.15 | - | - | 25.81 |
| Simple number | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| Ct value | 25.35 | - | - | - | 17.14 | 31.19 | 35.02 | - | - | 32.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, L.; Liao, F.; Tan, L.; Duan, D.; Zhan, Y.; Wang, N.; Wang, Y.; Peng, X.; Wang, K.; Huang, X.; et al. LAMP Coupled CRISPR-Cas12a Module for Rapid, Sensitive and Visual Detection of Porcine Circovirus 2. Animals 2022, 12, 2413. https://doi.org/10.3390/ani12182413
Lei L, Liao F, Tan L, Duan D, Zhan Y, Wang N, Wang Y, Peng X, Wang K, Huang X, et al. LAMP Coupled CRISPR-Cas12a Module for Rapid, Sensitive and Visual Detection of Porcine Circovirus 2. Animals. 2022; 12(18):2413. https://doi.org/10.3390/ani12182413
Chicago/Turabian StyleLei, Lei, Fan Liao, Lei Tan, Deyong Duan, Yang Zhan, Naidong Wang, Yuge Wang, Xiaoye Peng, Kaixin Wang, Xiaojiu Huang, and et al. 2022. "LAMP Coupled CRISPR-Cas12a Module for Rapid, Sensitive and Visual Detection of Porcine Circovirus 2" Animals 12, no. 18: 2413. https://doi.org/10.3390/ani12182413
APA StyleLei, L., Liao, F., Tan, L., Duan, D., Zhan, Y., Wang, N., Wang, Y., Peng, X., Wang, K., Huang, X., Yang, Y., & Wang, A. (2022). LAMP Coupled CRISPR-Cas12a Module for Rapid, Sensitive and Visual Detection of Porcine Circovirus 2. Animals, 12(18), 2413. https://doi.org/10.3390/ani12182413





