Nociception-Induced Changes in Electroencephalographic Activity and FOS Protein Expression in Piglets Undergoing Castration under Isoflurane Anaesthesia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocol
2.3. Statistical Analysis
2.3.1. EEG
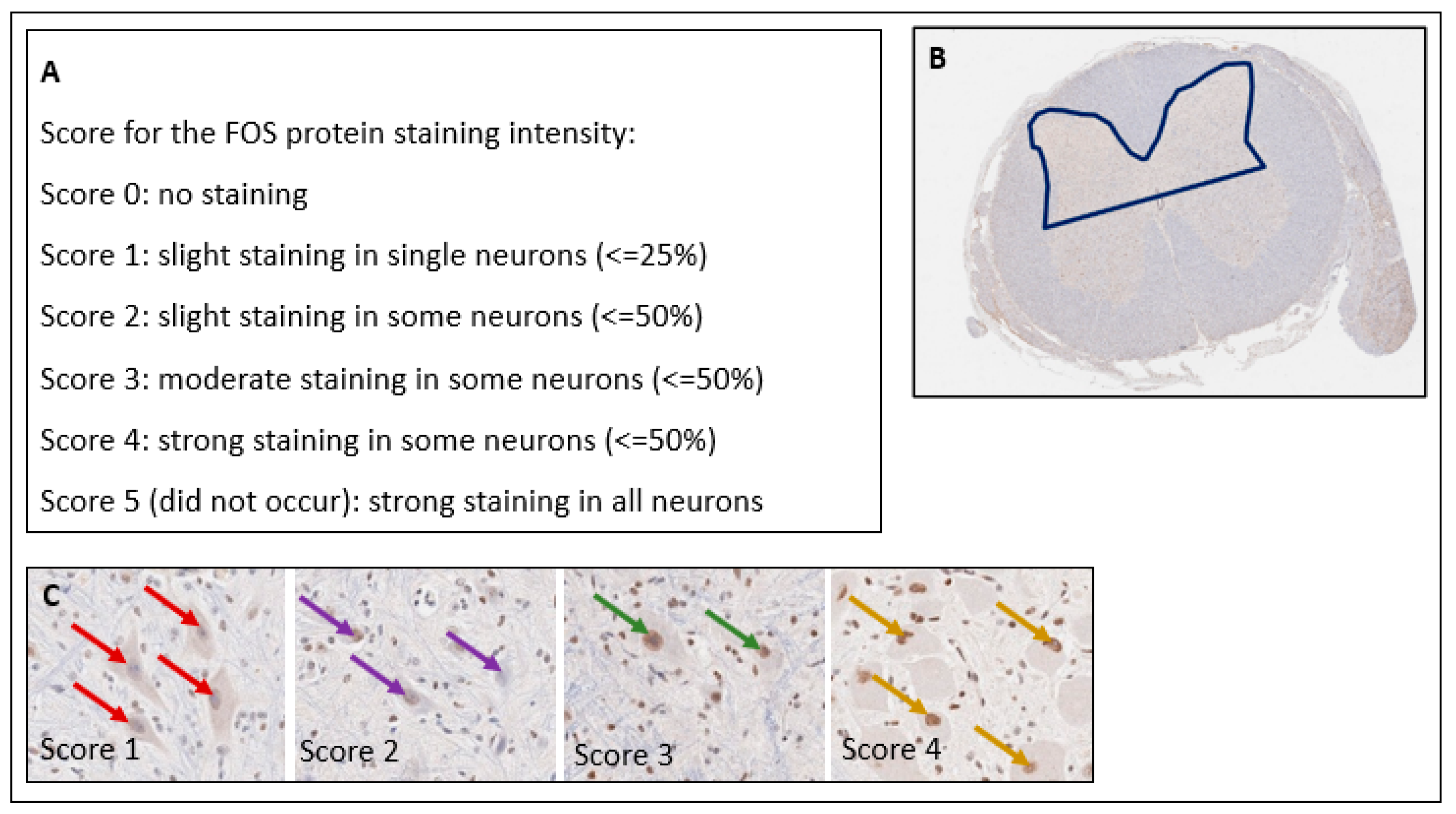
2.3.2. FOS Protein
3. Results
3.1. Demographics
3.2. Interdigital Pinch
3.3. Injections
3.4. Skin Incision
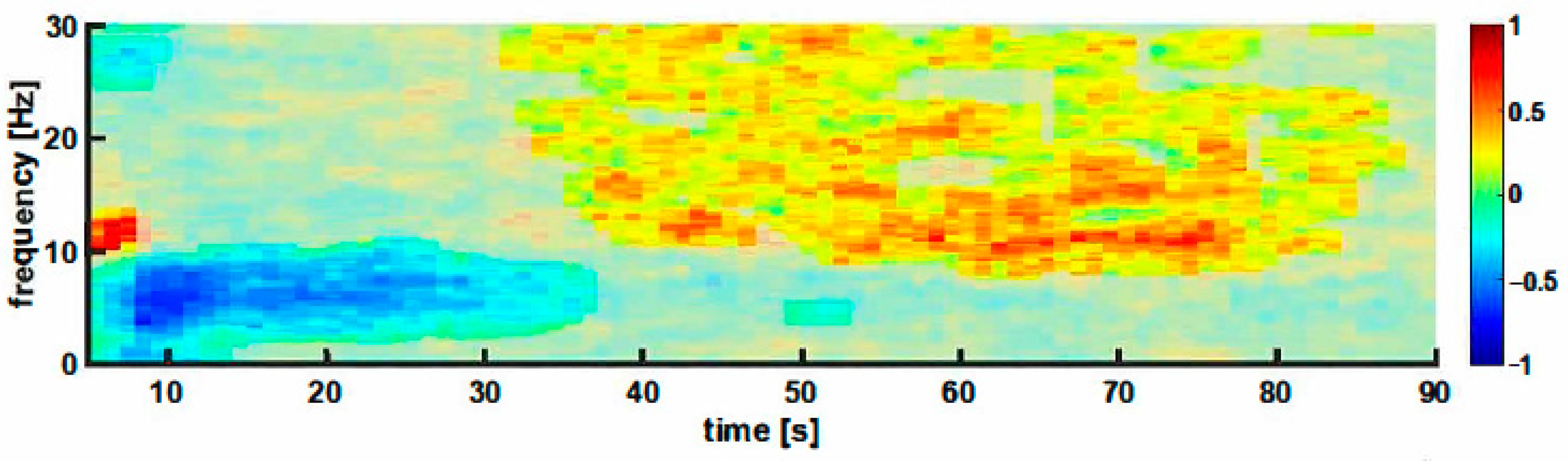
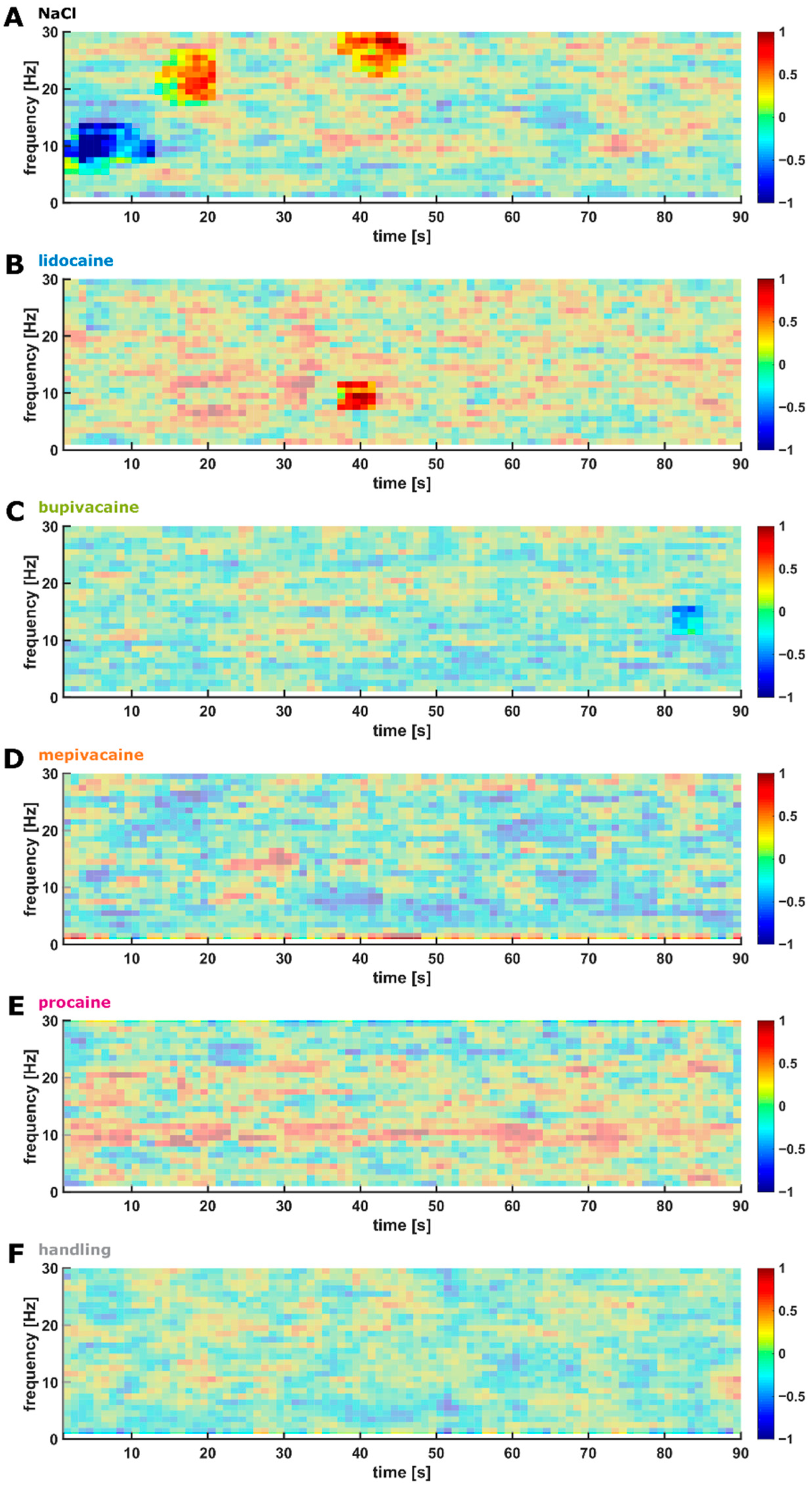
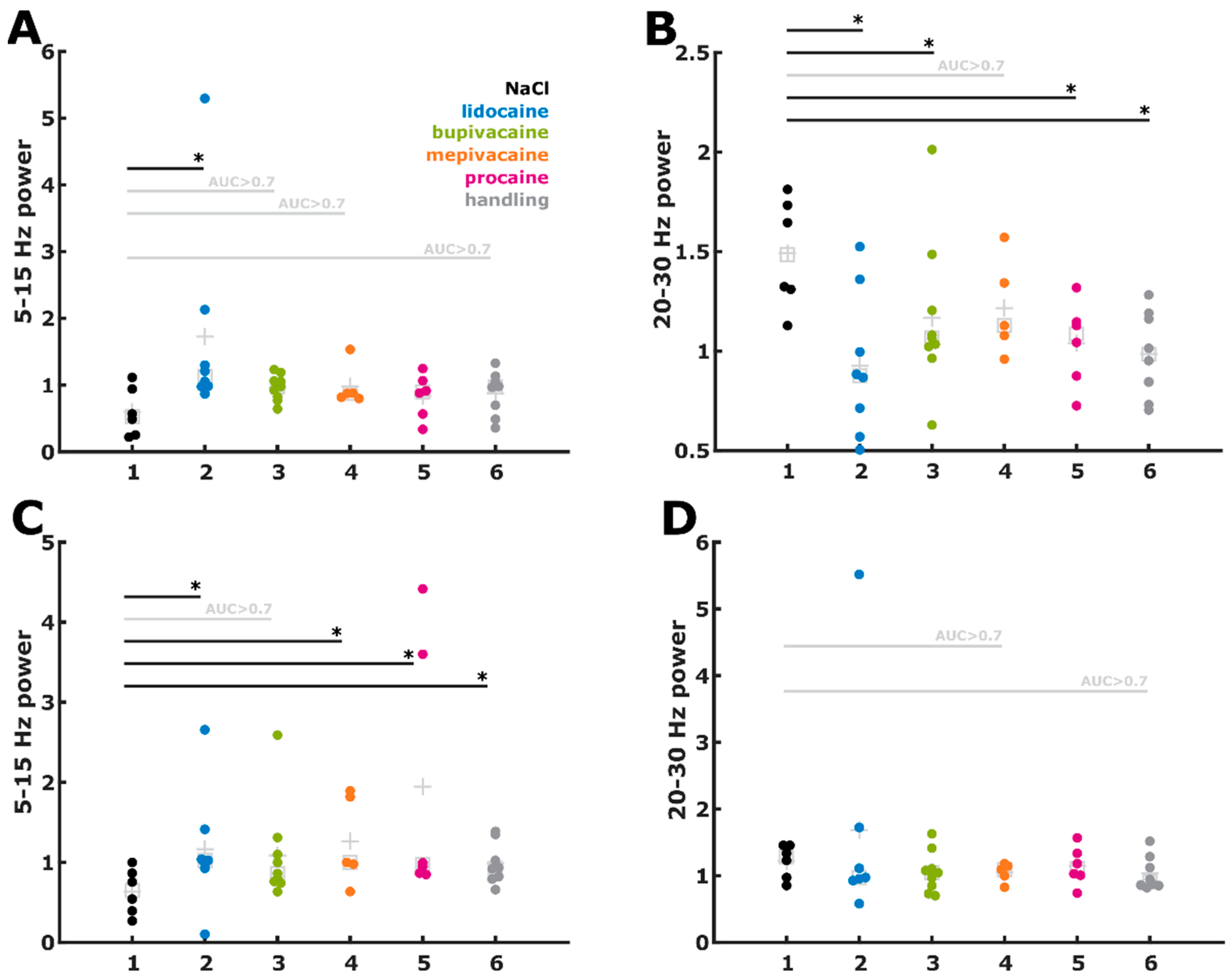
3.5. Cutting of the Spermatic Cord
3.6. Evaluation of the Early and Late Responses
3.7. FOS Protein
4. Discussion
4.1. EEG Reaction
4.2. FOS Protein
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansson, M.; Lundeheim, N.; Nyman, G.; Johansson, G. Effect of local anaesthesia and/or analgesia on pain responses induced by piglet castration. Acta Vet. Scand. 2011, 53, 34. [Google Scholar] [CrossRef]
- Kluivers, M.; Hopster, H.; Spoolder, H.A.M. Castration under Anaesthesia and/or Analgesia in Commercial Pig Production; Animal Sciences Group: Lelystad, The Netherlands, 2007. [Google Scholar]
- Saller, A.M.; Werner, J.; Reiser, J.; Senf, S.; Deffner, P.; Abendschön, N.; Weiß, C.; Fischer, J.; Schörwerth, A.; Miller, R.; et al. Local anesthesia in piglets undergoing castration-A comparative study to investigate the analgesic effects of four local anesthetics on the basis of acute physiological responses and limb movements. PLoS ONE 2020, 15, e0236742. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.; Arlinghaus, R.; Cooke, S.; Diggles, D.B.K.; Sawynok, W.; Stevens, D.; Wynne, C.D.L. Can fish really feel pain? Fish Fish. 2013, 15, 97–133. [Google Scholar] [CrossRef]
- Mathews, K.; Kronen, P.W.; Lascelles, D.; Nolan, A.; Robertson, S.; Steagall, P.V.; Wright, B.; Yamashita, K. Guidelines for recognition, assessment and treatment of pain: WSAVA Global Pain Council members and co-authors of this document. J. Small Anim. Pract. 2014, 55, E10–E68. [Google Scholar] [CrossRef] [PubMed]
- García, P.S.; Kreuzer, M.; Hight, D.; Sleigh, J.W. Effects of noxious stimulation on the electroencephalogram during general anaesthesia: A narrative review and approach to analgesic titration. Br. J. Anaesth. 2021, 126, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Murrell, J.C.; Johnson, C.B. Neurophysiological techniques to assess pain in animals. J. Vet. Pharmacol. Ther. 2006, 29, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Otto, K.A. Effects of averaging data series on the electroencephalographic response to noxious visceral stimulation in isoflurane-anaesthetized dogs. Res. Vet. Sci. 2007, 83, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Otto, K.A.; Mally, P. Noxious stimulation during orthopaedic surgery results in EEG ‘arousal’ or ‘paradoxical arousal’ reaction in isoflurane-anaesthetised sheep. Res. Vet. Sci. 2003, 75, 103–112. [Google Scholar] [CrossRef]
- Antognini, J.F.; Carstens, E. Isoflurane blunts electroencephalographic and thalamic-reticular formation responses to noxious stimulation in goats. Anesthesiology 1999, 91, 1770–1779. [Google Scholar] [CrossRef]
- Johnson, C.B.; Sylvester, S.P.; Stafford, K.J.; Mitchinson, S.L.; Ward, R.N.; Mellor, D.J. Effects of age on the electroencephalographic response to castration in lambs anaesthetized with halothane in oxygen from birth to 6 weeks old. Vet. Anaesth. Analg. 2009, 36, 273–279. [Google Scholar] [CrossRef]
- Murrell, J.C.; Mitchinson, S.L.; Waters, D.; Johnson, C.B. Comparative effect of thermal, mechanical, and electrical noxious stimuli on the electroencephalogram of the rat. Br. J. Anaesth. 2007, 98, 366–371. [Google Scholar] [CrossRef]
- Orth, M.; Barter, L.; Dominguez, C.; Atherley, R.; Carstens, E.; Antognini, J. Halothane and propofol differentially affect electroencephalographic responses to noxious stimulation. Br. J. Anaesth. 2005, 95, 477–484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murrell, J.C.; Johnson, C.B.; White, K.L.; Taylor, P.M.; Haberham, Z.L.; Waterman-Pearson, A.E. Changes in the EEG during castration in horses and ponies anaesthetized with halothane. Vet. Anaesth. Analg. 2003, 30, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Haga, H.A.; Dolvik, N.I. Electroencephalographic and cardiovascular variables as nociceptive indicators in isoflurane-anaesthetized horses. Vet. Anaesth. Analg. 2005, 32, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Diesch, T.J.; Mellor, D.J.; Johnson, C.B.; Lentle, R.G. Electroencephalographic responses to tail clamping in anaesthetized rat pups. Lab. Anim. 2009, 43, 224–231. [Google Scholar] [CrossRef]
- Haga, H.A.; Ranheim, B. Castration of piglets: The analgesic effects of intratesticular and intrafunicular lidocaine injection. Vet. Anaesth. Analg. 2005, 32, 1–9. [Google Scholar] [CrossRef]
- Waldmann, V.; Otto, K.H.; Bollwahn, W. Piglet castration-pain sensation and pain elimination. Dtsch. Tierarztl. Wochenschr. 1994, 101, 105–109. [Google Scholar]
- Kells, N.J.; Beausoleil, N.J.; Chambers, J.P.; Sutherland, M.A.; Morrison, R.S.; Johnson, C.B. Electroencephalographic responses of anaesthetized pigs (Sus scrofa) to tail docking using clippers or cautery iron performed at 2 or 20 days of age. Vet. Anaesth. Analg. 2017, 44, 1156–1165. [Google Scholar] [CrossRef]
- Kells, N.J.; Beausoleil, N.J.; Sutherland, M.A.; Morrison, R.M.; Johnson, C.B. Electroencephalographic assessment of oral meloxicam, topical anaesthetic cream and cautery iron for mitigating acute pain in pigs (Sus scrofa) undergoing tail docking. Vet. Anaesth. Analg. 2017, 44, 1166–1174. [Google Scholar] [CrossRef]
- Johnson, C.; Sutherland, M.; Beausoleil, N.; Kells, N. Validation of EEG Measures for Pain Assessment in Piglets Aged 0 to 10 Days. pork.org. NPB# C-13–188; Massey Univesity: Palmerston North, New Zealand, 2015. [Google Scholar]
- Haga, H.A.; Tevik, A.; Moerch, H. Electroencephalographic and cardiovascular indicators of nociception during isoflurane anaesthesia in pigs. Vet. Anaesth. Analg. 2001, 28, 126–131. [Google Scholar] [CrossRef]
- Munglani, R.; Hunt, S.P. Molecular biology of pain. Br. J. Anaesth. 1995, 75, 186–192. [Google Scholar] [CrossRef][Green Version]
- Hunt, S.P.; Pini, A.; Evan, G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 1987, 328, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A. Using c-fos as a neural marker of pain. Brain Res. Bull. 1998, 45, 1–8. [Google Scholar] [CrossRef]
- Coggeshall, R.E. Fos, nociception and the dorsal horn. Prog. Neurobiol. 2005, 77, 299–352. [Google Scholar] [CrossRef] [PubMed]
- Lykkegaard, K.; Lauritzen, B.; Tessem, L.; Weikop, P.; Svendsen, O. Local anaesthetics attenuates spinal nociception and HPA-axis activation during experimental laparotomy in pigs. Res. Vet. Sci. 2005, 79, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, O. Castration of piglets under carbon dioxide (CO2) anaesthesia. J. Vet. Pharmacol. Ther. 2006, 29, 54–55. [Google Scholar] [CrossRef]
- Nyborg, P.Y.; Sørig, A.; Lykkegaard, K.; Svendsen, O. Nociception after castration of juvenile pigs determined by quantitative estimation of c-Fos expressing neurons in the spinal cord dorsal horn. Dansk Veterinærtidsskrift 2000, 83, 16–17. [Google Scholar]
- Ruggiero, D.A.; Sica, A.L.; Anwar, M.; Frasier, I.; Gootman, N.; Gootman, P.M. Induction of c-fos gene expression by spinal cord transection in Sus scrofa. Brain Res. 1997, 759, 301–305. [Google Scholar] [CrossRef]
- Werner, J.; Saller, A.; Reiser, J.; Senf, S.; Deffner, P.; Abendschön, N.; Fischer, J.; Grott, A.; Miller, R.; Zablotski, Y.; et al. Evaluation of Two Injection Techniques in Combination with the Local Anesthetics Lidocaine and Mepivacaine for Piglets Undergoing Surgical Castration. Animals 2022, 12, 1028. [Google Scholar] [CrossRef]
- Tierschutzgesetz. Tierschutzgesetz in der Fassung der Bekanntmachung vom 18. Mai 2006 (BGBl. I S. 1206, 1313), das zuletzt durch Artikel 105 des Gesetzes vom 10. August 2021 (BGBl. I S. 3436) geändert worden ist. 2021. Available online: https://www.gesetze-im-internet.de/tierschg/BJNR012770972.html (accessed on 31 August 2022).
- Sassenhagen, J.; Draschkow, D. Cluster-based permutation tests of MEG/EEG data do not establish significance of effect latency or location. Psychophysiology 2019, 56, e13335. [Google Scholar] [CrossRef]
- Akeju, O.; Westover, M.B.; Pavone, K.J.; Sampson, A.L.; Hartnack, K.E.; Brown, E.N.; Purdon, P.L. Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology 2014, 121, 990–998. [Google Scholar] [CrossRef]
- Kreuzer, M.; Stern, M.A.; Hight, D.; Berger, S.; Schneider, G.; Sleigh, J.W.; Garcia, P.S. Spectral and entropic features are altered by age in the electroencephalogram in patients under sevoflurane anesthesia. Anesthesiology 2020, 135, 1003–1016. [Google Scholar] [CrossRef]
- Hentschke, H.; Stüttgen, M.C. Computation of measures of effect size for neuroscience data sets. Eur. J. Neurosci. 2011, 34, 1887–1894. [Google Scholar] [CrossRef]
- Haga, H.A.; Tevik, A.; Moerch, H. Motor responses to stimulation during isoflurane anaesthesia in pigs. Vet. Anaesth. Analg. 2002, 29, 69–75. [Google Scholar] [CrossRef]
- Satas, S.; Haaland, K.; Thoresen, M.; Steen, P.A. MAC for halothane and isoflurane during normothermia and hypothermia in the newborn piglet. Acta Anaesthesiol. Scand. 1996, 40, 452–456. [Google Scholar] [CrossRef]
- Arras, M.; Autenried, P.; Rettich, A.; Spaeni, D.; Rülicke, T. Optimization of intraperitoneal injection anesthesia in mice: Drugs, dosages, adverse effects, and anesthesia depth. Comp. Med. 2001, 51, 443–456. [Google Scholar]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar]
- Rintisch, U.; Baars, J.; Lahrmann, K.H. Evaluation of perioperative analgesia by nociceptive flexor reflex in pigs under ketamine-azaperone-general anaesthesia. Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 96–102. [Google Scholar]
- Rampil, I.J.; Weiskopf, R.B.; Brown, J.G.; Eger, E.I., 2nd; Johnson, B.H.; Holmes, M.A.; Donegan, J.H. I653 and isoflurane produce similar dose-related changes in the electroencephalogram of pigs. Anesthesiology 1988, 69, 298–302. [Google Scholar] [CrossRef]
- Niedermeyer, E.; Sherman, D.L.; Geocadin, R.J.; Hansen, H.C.; Hanley, D.F. The burst-suppression electroencephalogram. Clin. Electroencephalogr. 1999, 30, 99–105. [Google Scholar] [CrossRef]
- Haga, H.A.; Ranheim, B.; Spadavecchia, C. Effects of isoflurane upon minimum alveolar concentration and cerebral cortex depression in pigs and goats: An interspecies comparison. Vet. J. 2011, 187, 217–220. [Google Scholar] [CrossRef]
- Orth, M.; Bravo, E.; Barter, L.; Carstens, E.; Antognini, J.F. The differential effects of halothane and isoflurane on electroencephalographic responses to electrical microstimulation of the reticular formation. Anesth Analg. 2006, 102, 1709–1714. [Google Scholar] [CrossRef]
- Rampil, I.J.; Laster, M.J. No correlation between quantitative electroencephalographic measurements and movement response to noxious stimuli during isoflurane anesthesia in rats. Anesthesiology 1992, 77, 920–925. [Google Scholar] [CrossRef]
- Taylor, A.A.; Weary, D.M. Vocal responses of piglets to castration: Identifying procedural sources of pain. Appl. Anim. Behav. Sci. 2000, 70, 17–26. [Google Scholar] [CrossRef]
- Hight, D.F.; Gaskell, A.L.; Kreuzer, M.; Voss, L.J.; García, P.S.; Sleigh, J.W. Transient electroencephalographic alpha power loss during maintenance of general anaesthesia. Br. J. Anaesth. 2019, 122, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Hagihira, S.; Takashina, M.; Mori, T.; Ueyama, H.; Mashimo, T. Electroencephalographic bicoherence is sensitive to noxious stimuli during isoflurane or sevoflurane anesthesia. J. Am. Soc. Anesthesiol. 2004, 100, 818–825. [Google Scholar] [CrossRef]
- Kochs, E.; Bischoff, P.; Pichlmeier, U.; Schulte, A.E.J. Surgical stimulation induces changes in brain electrical activity during isoflurane/nitrous oxide anesthesia. A topographic electroencephalographic analysis. Anesthesiology 1994, 80, 1026–1034. [Google Scholar] [CrossRef]
- Rundshagen, I.; Schröder, T.; Heinze, J.; Prichep, L.; John, E.R.; Kox, W.J. Topographic electroencephalography: Endotracheal intubation during anaesthesia with propofol/fentanyl. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 2005, 40, 633–639. [Google Scholar] [CrossRef]
- Ekström, P.M.; Short, C.E.; Geimer, T.R. Electroencephalography of detomidine-ketamine-halothane and detomidine-ketamine-isoflurane anesthetized horses during orthopedic surgery. A comparison. Vet. Surg. 1993, 22, 414–418. [Google Scholar] [CrossRef]
- Murrell, J.C.; Waters, D.; Johnson, C.B. Comparative effects of halothane, isoflurane, sevoflurane and desflurane on the electroencephalogram of the rat. Lab. Anim. 2008, 42, 161–170. [Google Scholar] [CrossRef]
- Rampil, I.J. A primer for EEG signal processing in anesthesia. Anesthesiology 1998, 89, 980–1002. [Google Scholar] [CrossRef]
- Akeju, O.; Pavone, K.J.; Westover, M.B.; Vazquez, R.; Prerau, M.J.; Harrell, P.G.; Hartnack, K.E.; Rhee, J.; Sampson, A.L.; Habeeb, K.; et al. A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis. Anesthesiology 2014, 121, 978–989. [Google Scholar] [CrossRef]
- Johnson, C.B.; Taylor, P.M. Comparison of the effects of halothane, isoflurane and methoxyflurane on the electroencephalogram of the horse. Br. J. Anaesth. 1998, 81, 748–753. [Google Scholar] [CrossRef]
- Newson, J.J.; Thiagarajan, T.C. EEG Frequency Bands in Psychiatric Disorders: A Review of Resting State Studies. Front. Hum. Neurosci. 2018, 12, 521. [Google Scholar] [CrossRef]
- Reiner, G.; Heinricy, L.; Brenig, B.; Geldermann, H.; Dzapo, V. Cloning, structural organization, and chromosomal assignment of the porcine c-fos proto-oncogene, FOS. Cytogenet. Genome Res. 2000, 89, 59–61. [Google Scholar] [CrossRef]
- Leah, J.D.; Sandkuhler, J.; Herdegen, T.; Murashov, A.; Zimmermann, M. Potentiated expression of FOS protein in the rat spinal cord following bilateral noxious cutaneous stimulation. Neuroscience 1992, 48, 525–532. [Google Scholar] [CrossRef]
- Sienkiewicz, W. Sources of the porcine testis innervation. Andrologia 2010, 42, 395–403. [Google Scholar] [CrossRef]
- Nickel, R.; Schummer, A.; Seiferle, E. Nervensystem, sinnesorgane, endokrine drüsen. In Lehrbuch der Anatomie der Haustiere; Parey Verlag: Stuttgart, Germany, 1992; p. 273. [Google Scholar]
- Kaleczyc, J.; Scheuermann, D.W.; Pidsudko, Z.; Majewski, M.; Lakomy, M.; Timmermans, J.P. Distribution, immunohistochemical characteristics and nerve pathways of primary sensory neurons supplying the porcine vas deferens. Cell Tissue Res. 2002, 310, 9–17. [Google Scholar] [CrossRef]


| Experimental Group | Noxious Stimulus | |||
|---|---|---|---|---|
| Interdigital Pinch | Injection | Incision | Cutting of the Spermatic Cord | |
| NaCl (n = 8 animals) | n = 44 datasets (pooled data) | n = 7 datasets | n = 6 datasets | n = 6 datasets |
| Lidocaine (n = 9 animals) | n = 7 datasets | n = 8 datasets | n = 7 datasets | |
| Bupivacaine (n = 9 animals) | n = 9 datasets | n = 9 datasets | n = 9 datasets | |
| Mepivacaine (n = 6 animals) | n = 5 datasets | n = 5 datasets | n = 5 datasets | |
| Procaine (n = 8 animals) | n = 6 datasets | n = 5 datasets | n = 5 datasets | |
| Handling (n = 9 animals) | n = 7 datasets | n = 8 datasets | n = 8 datasets | |
| Total n = 49 animals | Total n = 166 datasets | |||
| FOS protein Analysis | NaCl | Lidocaine | Bupivacaine | Mepivacaine | Procaine | Handling |
|---|---|---|---|---|---|---|
| (n = 42 animals) | n = 7 | n = 7 | n = 8 | n = 7 | n = 6 | n = 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiser, J.; Kreuzer, M.; Werner, J.; Saller, A.M.; Fischer, J.; Senf, S.; Deffner, P.; Abendschön, N.; Groll, T.; Grott, A.; et al. Nociception-Induced Changes in Electroencephalographic Activity and FOS Protein Expression in Piglets Undergoing Castration under Isoflurane Anaesthesia. Animals 2022, 12, 2309. https://doi.org/10.3390/ani12182309
Reiser J, Kreuzer M, Werner J, Saller AM, Fischer J, Senf S, Deffner P, Abendschön N, Groll T, Grott A, et al. Nociception-Induced Changes in Electroencephalographic Activity and FOS Protein Expression in Piglets Undergoing Castration under Isoflurane Anaesthesia. Animals. 2022; 12(18):2309. https://doi.org/10.3390/ani12182309
Chicago/Turabian StyleReiser, Judith, Matthias Kreuzer, Julia Werner, Anna M. Saller, Johannes Fischer, Steffanie Senf, Pauline Deffner, Nora Abendschön, Tanja Groll, Andrea Grott, and et al. 2022. "Nociception-Induced Changes in Electroencephalographic Activity and FOS Protein Expression in Piglets Undergoing Castration under Isoflurane Anaesthesia" Animals 12, no. 18: 2309. https://doi.org/10.3390/ani12182309
APA StyleReiser, J., Kreuzer, M., Werner, J., Saller, A. M., Fischer, J., Senf, S., Deffner, P., Abendschön, N., Groll, T., Grott, A., Miller, R., Bergmann, S., Erhard, M. H., Ritzmann, M., Zöls, S., Schneider, G., Steiger, K., & Baumgartner, C. (2022). Nociception-Induced Changes in Electroencephalographic Activity and FOS Protein Expression in Piglets Undergoing Castration under Isoflurane Anaesthesia. Animals, 12(18), 2309. https://doi.org/10.3390/ani12182309








