Influence of Acacia Mearnsii Fodder on Rumen Digestion and Mitigation of Greenhouse Gas Production
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Animals
2.3. Forage Samples and Treatments
2.4. Ruminal Degradation Kinetic
2.5. Gas, CH4, CO2 Production and In Vitro Digestibility
2.6. Rumen pH
2.7. Chemical Analysis
2.8. Experimental Design and Statistical Analysis
3. Results
3.1. Rumen Degradation and Digestibility of DM and OM
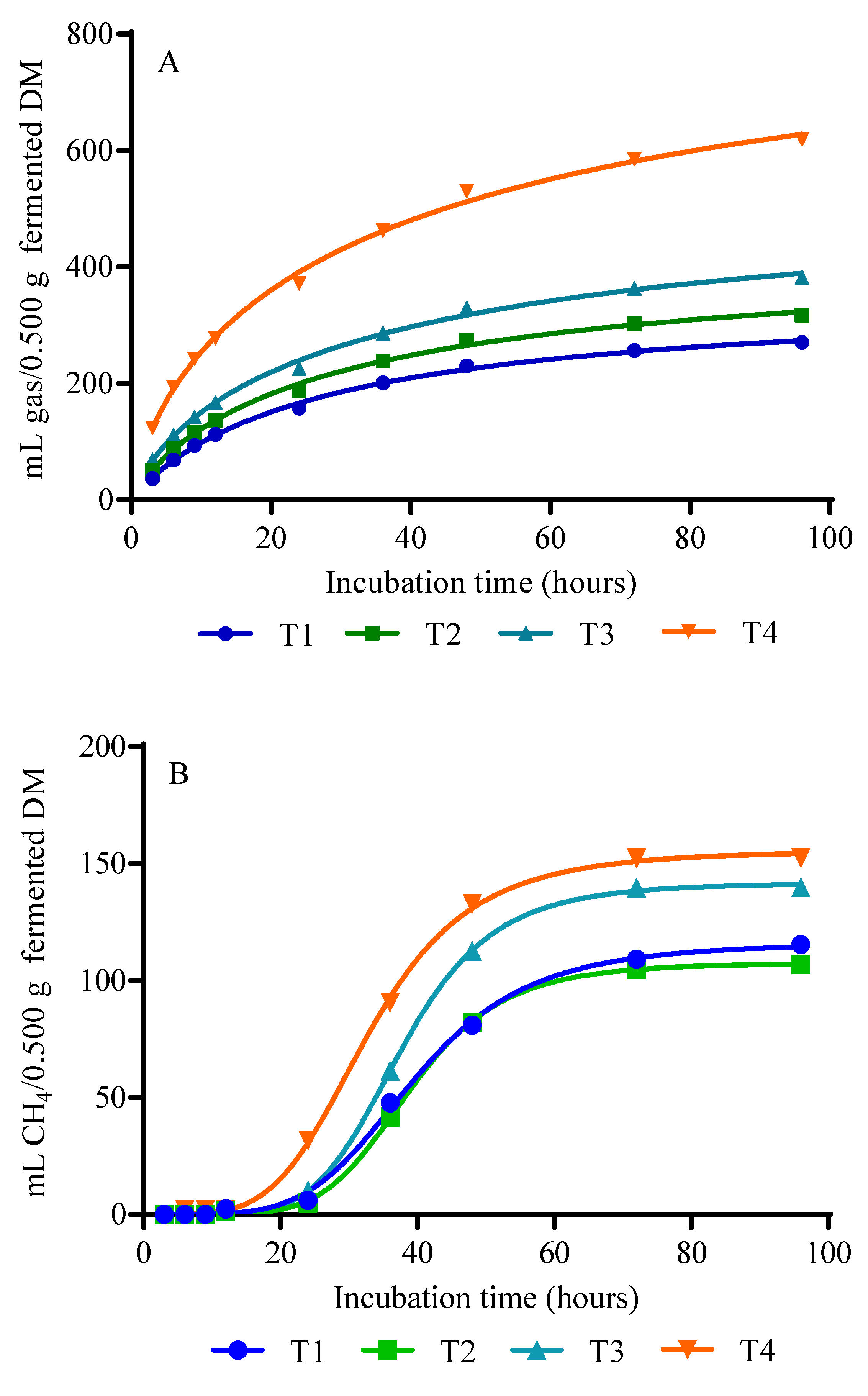
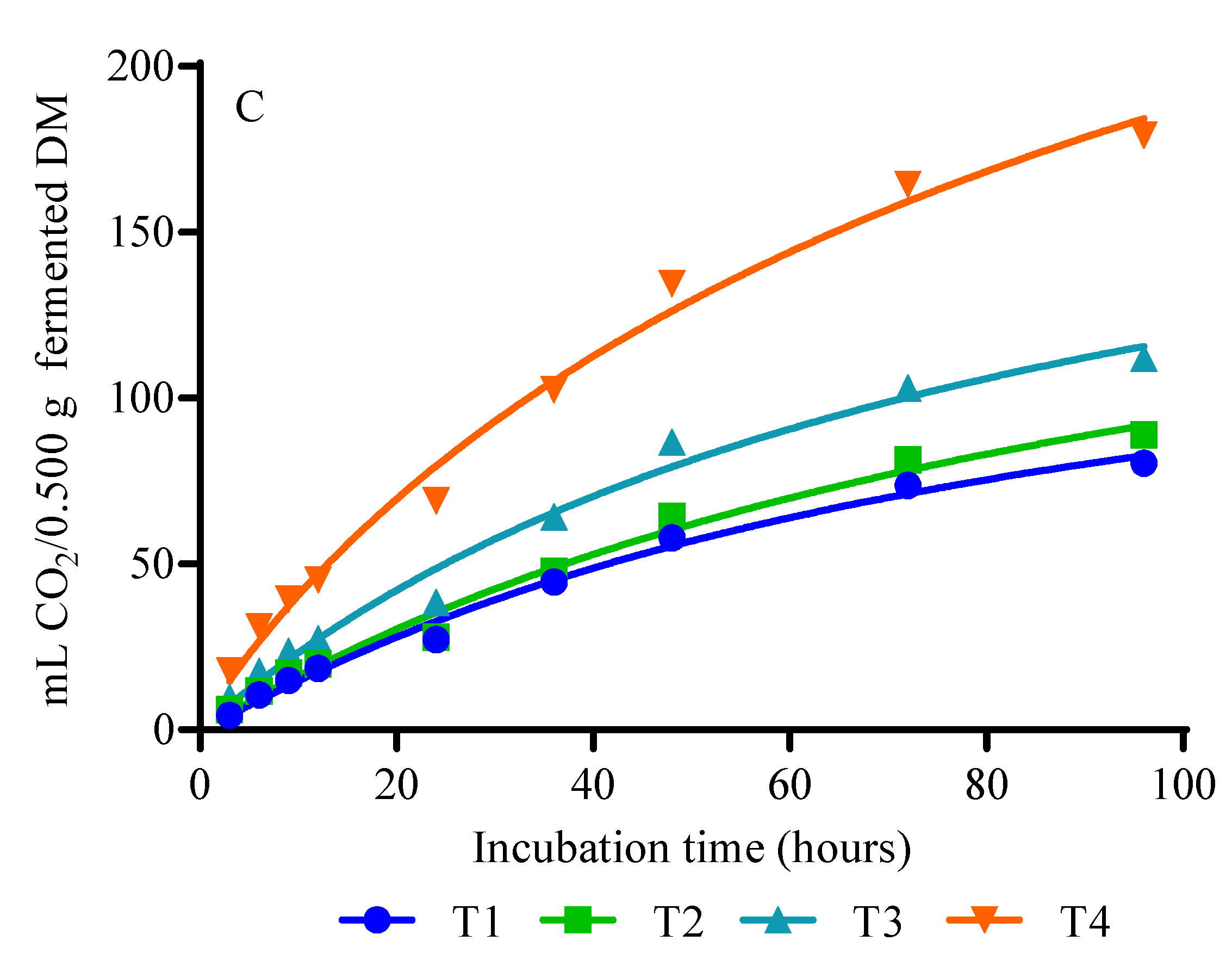
3.2. Gas, CH4, and CO2 Production
4. Discussion
4.1. Rumen Degradation and Digestibility of DM and OM
4.2. Gas, CH4, and CO2 Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nocek, J.E.; Russell, J. Protein and energy as an integrated system. Relationship of ruminal protein and carbohydrate availability to microbial synthesis and milk production. J. Dairy Sci. 1988, 71, 2070–2107. [Google Scholar] [CrossRef]
- Lara, P.E.; Canché, M.C.; Magaña, H.; Aguilar, E.; Sanginés, J.R. Producción de gas in vitro y cinética de degradación de harina de forraje de morera (Morus alba) mezclada con maíz. Rev. Cubana Cienc. Agríc. 2009, 43, 273–279. [Google Scholar]
- Rojas García, A.R.; Hernández Ayona, A.; Sánchez-Santillán, P.; Alaniz Gutierrez, L.; Torres Salado, N.; Herrera Pérez, J.; Escobar España, J.C. Cinética de fermentación y degradación in vitro de tres leguminosas rastreras nativas del municipio de Cuajinicuilapa, Guerrero. Rev. Investig. Vet. Perú 2018, 29, 1229–1236. [Google Scholar] [CrossRef]
- Mosisa, A.; Nurfeta, A.; Bezabih, M.; Tolera, A.; Mengistu, S.; Yigrem, S.; Hassen, A. Assessment of botanical composition, biomass yield, nutritional quality and methane production of forages in selected grasslands, southern highlands of Ethiopia. Sci. Afr. 2021, 12, e00726. [Google Scholar] [CrossRef]
- Gerber, P.J.; Hristov, A.N.; Henderson, B.; Makkar, H.; Oh, J.; Lee, C.; Meinen, R.; Montes, F.; Ott, T.; Firkins, J.; et al. Technical options for the mitigation of direct methane and nitrous oxide emissions from livestock: A review. Animals 2013, 7, 220–234. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.L.; Dijkstra, J.; Kebreab, E.; Bannink, A.; Odongo, N.E.; McBride, B.W.; France, J. Aspects of rumen microbiology central to mechanistic modelling of methane production in cattle. J. Agric. Sci. 2008, 146, 213–233. [Google Scholar] [CrossRef]
- Moumen, A.; Azizi, G.; Chekroun, K.B.; Baghour, M. The effects of livestock methane emission on the global warming: A review. Int. J. Glob. Warm. 2016, 9, 229–253. [Google Scholar] [CrossRef]
- Smith, P.; Reay, D.; Smith, J. Agricultural methane emissions and the potential formitigation. Philos. Trans. R. Soc. A 2021, 379, 20200451. [Google Scholar] [CrossRef]
- Eugène, M.; Klumpp, K.; Sauvant, D. Methane mitigating options with forages fed to ruminants. Grass Forage Sci. 2021, 76, 196–204. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Global Livestock Environmental Assessment Model (GLEAM); FAO: Rome, Italy, 2021; Available online: https://www.fao.org/gleam/en (accessed on 27 June 2022).
- Carmona, J.C.; Bolívar, D.M.; Giraldo, L.A. El gas metano en la producción ganadera y alternativas para medir sus emisiones y aminorar su impacto a nivel ambiental y productivo. Rev. Colomb. Cienc. Pecu 2005, 18, 49–63. [Google Scholar]
- Bharanidharan, R.; Arokiyaraj, S.; Baik, M.; Ibidhi, R.; Lee, S.J.; Lee, Y.; Kim, K.H. In Vitro Screening of East Asian Plant Extracts for Potential Use in Reducing Ruminal Methane Production. Animals 2021, 11, 1020. [Google Scholar] [CrossRef] [PubMed]
- Barros-Rodríguez, M.; Oña-Rodríguez, J.; Mera-Andrade, R.; Artieda-Rojas, J.; Curay-Quispe, S.; Avilés-Esquivel, D.; Guishca-Cunuhay, C. Degradación ruminal de dietas a base de biomasa pos-cosecha de Amaranthus cruentus: Efecto sobre los protozoos del rumen y producción de gas in vitro. Rev. Investig. Vet. Perú 2017, 28, 812–821. [Google Scholar] [CrossRef][Green Version]
- Aragadvay-Yungán, R.G.; Barros-Rodríguez, M.; Ortiz, L.; Carro, M.D.; Navarro Marcos, C.; Elghandour, M.M.M.Y.; Salem, A.Z.M. Mitigation of ruminal methane production with enhancing the fermentation by supplementation of different tropical forage legumes. Environ. Sci. Pollut. Res. 2021, 29, 3438–3445. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Bodas, R.; Prieto, N.; García-González, R.; Andrés, S.; Giráldez, F.J.; López, S. Manipulation of rumen fermentation and methane production with plant secondary metabolites. Anim. Feed Sci. Techol. 2012, 176, 78–93. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P. Methane mitigation from ruminants using tannins and saponins. Trop. Anim. Health Prod. 2012, 44, 729–739. [Google Scholar] [CrossRef]
- Hoste, H.; Martinez-Ortiz-De-Montellano, C.; Manolaraki, F.; Brunet, S.; Ojeda-Robertos, N.; Fourquaux, I.; Sandoval-Castro, C.A. Direct and indirect effects of bioactive tannin-rich tropical and temperate legumes against nematode infections. Vet. Parasitol. 2012, 186, 18–27. [Google Scholar] [CrossRef]
- Engström, M.T.; Karonen, M.; Ahern, J.R.; Baert, N.; Payré, B.; Hoste, H.; Salminen, J.P. Chemical Structures of Plant Hydrolyzable Tannins Reveal Their in vitro Activity against Egg Hatching and Motility of Haemonchus contortus Nematodes. J. Agric. Food Chem. 2016, 64, 840–851. [Google Scholar] [CrossRef]
- Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production-Progress and challenges. Anim. Feed Sci. Techol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Ku-Vera, J.C.; Jiménez-Ocampo, R.; Valencia-Salazar, S.S.; Montoya-Flores, M.D.; Molina-Botero, I.C.; Arango, J.; Solorio Sánchez, F.J. Role of secondary plant metabolites on enteric methane mitigation in ruminants. Front. Vet. Sci. 2020, 7, 584. [Google Scholar] [CrossRef] [PubMed]
- Rubanza, C.D.K.; Shem, M.N.; Otsyina, R.; Bakengesa, S.S.; Ichinohe, T.; Fujihara, T. Polyphenolics and tannins effect on in vitro digestibility of selected Acacia species leaves. Anim. Feed Sci. Technol. 2005, 119, 129–142. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Beauchemin, K.A. Potential of molecular weight and structure of tannins to reduce methane emissions from ruminants: A review. Animals 2019, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Velasco, B.; Mella, M.R. Vegetable tannins, ruminal microbiota and ruminant metabolism interaction. Trop. Subtrop. Agroecosyst. 2022, 25, 1. [Google Scholar] [CrossRef]
- Saminathan, M.; Sieo, C.C.; Abdullah, N.; Wong, C.M.V.L.; Ho, Y.W. Effects of condensed tannin fractions of different molecular weights from a Leucaena leucocephala hybrid on in vitro methane production and rumen fermentation. J. Sci. Food Agric. 2015, 95, 2742–2749. [Google Scholar] [CrossRef]
- Lamy, E.; Rawel, H.; Schweigert, F.J.; Capela e Silva, F.; Ferreira, A.; Costa, A.R.; Antunes, C.; Almeida, A.; Varela, A.; Sales-Baptista, E. The effect of tannins on Mediterranean ruminant ingestive behavior: The role of the oral cavity. Molecules 2011, 16, 2766–2784. [Google Scholar] [CrossRef]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: A review. Anim. Feed Sci. Techol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Reed, J.D. Nutritional toxicology of tannins and related polyphenols in forage legumes. J. Anim. Sci. 1995, 73, 1516–1528. [Google Scholar] [CrossRef]
- Silanikove, N.; Perevolotsky, A.; Provenza, F.D. Use of tannin-binding chemicals to assay for tannins and their negative postingestive effects in ruminants. Anim. Feed Sci. Techol. 2001, 91, 69–81. [Google Scholar] [CrossRef]
- Robins, C.; Brooker, J.D. The effects of Acacia aneura feeding on abomasal and intestinal structure and function in sheep. Anim. Feed Sci. Techol. 2005, 121, 205–215. [Google Scholar] [CrossRef]
- Henke, A.; Dickhoefer, U.; Westreicher-Kristen, E.; Knappstein, K.; Molkentin, J.; Hasler, M.; Susenbeth, A. Effect of dietary Quebracho tannin extract on feed intake, digestibility, excretion of urinary purine derivatives and milk production in dairy cows. Arch. Anim. Nutr. 2017, 71, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Makkar, H.P.; Nagal, K.B.; Sharma, S.K.; Wadhwa, D.R.; Singh, B. Oak (Quercus incana) leaf poisoning in cattle. Vet. Hum. Toxicol. 1992, 34, 161–164. [Google Scholar]
- Lamy, E.; Rodrigues, L.; Guerreiro, O.; Soldado, D.; Francisco, A.; Lima, M.; Capela e Silva, F.; Lopes, O.; Santos-Silva, J.; Jerónimo, E. Changes in salivary protein composition of lambs supplemented with aerial parts and condensed tannins: Extract from Cistus ladanifer L.—A preliminary study. Agroforest. Syst. 2020, 94, 1501–1509. [Google Scholar] [CrossRef]
- Ahmed, E.; Fukuma, N.; Hanada, M.; Nishida, T. The Efficacy of Plant-Based Bioactives Supplementation to Different Proportion of Concentrate Diets on Methane Production and Rumen Fermentation Characteristics In Vitro. Animals 2021, 11, 1029. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, G.M.; Benetel, G.; Carriero, M.M.; Sousa, R.L.M.; Muir, J.P.; Macedo, R.O.; Bueno, I.C.S. Tannin-rich forage as a methane mitigation strategy for cattle and the implications for rumen microbiota. Anim. Prod. Sci. 2021, 61, 26. [Google Scholar] [CrossRef]
- Honan, M.; Feng, X.; Tricarico, J.M.; Kebreab, E. Feed additives as a strategic approach to reduce enteric methane production in cattle: Modes of action, effectiveness and safety. Anim. Prod. Sci. 2021, in press. [Google Scholar] [CrossRef]
- de Souza Congio, G.F.; Bannink, A.; Mogollón, O.L.M.; Jaurena, G.; Gonda, H.; Gere, J.I.; Hristov, A.N. Enteric methane mitigation strategies for ruminant livestock systems in the Latin America and Caribbean region: A meta-analysis. J. Clean. Prod. 2021, 312, 127693. [Google Scholar] [CrossRef]
- Carulla, J.E.; Kreuzer, M.; Machmüller, A.; Hess, H.D. Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Aust. J. Agric. Res. 2005, 56, 961–970. [Google Scholar] [CrossRef]
- Correia, R.; Quintela, J.C.; Duarte, M.P.; Gonçalves, M. Insights for the valorization of biomass from portuguese invasive Acacia spp. in a biorefinery perspective. Forests 2020, 11, 1342. [Google Scholar] [CrossRef]
- Hassanpour, S.; Baghbani Mehmandar, F. Anthelmintic effects of Acacia mearnsii (wattle tannin) in small ruminants; a review. J. Comp. Clin. Path Res. 2012, 1, 1–8. [Google Scholar]
- Avila, A.S.; Zambom, M.A.; Faccenda, A.; Fischer, M.L.; Anschau, F.A.; Venturini, T.; Faciola, A.P. Effects of black wattle (Acacia mearnsii) condensed tannins on intake, protozoa population, ruminal fermentation, and nutrient digestibility in Jersey steers. Animals 2020, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Maamouri, O.; Atti, N.; Kraiem, K.; Mahouachi, M. Effects of concentrate and Acacia cyanophylla foliage supplementation on nitrogen balance and milk production of grazing ewes. Livest. Sci. 2011, 139, 264–270. [Google Scholar] [CrossRef]
- Ørskov, E.R.; Hovell, D.; Mould, F. The use of the nylon bag technique for the evaluation of feedstuffs. Trop. Anim. Prod. 1980, 5, 195–213. [Google Scholar]
- Ørskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; Mcallan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminants feeds. Anim. Feed Sci. Techol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Rodríguez-Ocampo, I.; Parra-Garcia, A.; Salem, A.Z.M.; Greiner, R.; Marquez-Molina, O.; Barros-Rodriguez, M.; Barbabosa-Pilego, A. Biogas production from prickly pear cactus containing diets supplemented with Moringa oleifera leaf extract for a cleaner environmental livestock production. J. Clean. Prod. 2018, 185, 547–553. [Google Scholar] [CrossRef]
- Groot, J.C.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.; Lantinga, E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim. Feed Sci. Techol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Price, M.L.; Van Scoyoc, S.; Butler, L.G. A critical evaluation of the vanillin reaction assay for tannin in sorghum grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw-Hill: Boston, MA, USA, 1997. [Google Scholar]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Kozloski, G.V.; Härter, C.J.; Hentz, F.; de Ávila, S.C.; Orlandi, T.; Stefanello, C.M. Intake, digestibility and nutrients supply to wethers fed ryegrass and intraruminally infused with levels of Acacia mearnsii tannin extract. Small Rumin. Res. 2012, 106, 125–130. [Google Scholar] [CrossRef]
- de Oliveira, S.G.; Berchielli, T.T.; dos Santos Pedreira, M.; Primavesi, O.; Frighetto, R.; Lima, M.A. Effect of tannin levels in sorghum silage and concentrate supplementation on apparent digestibility and methane emission in beef cattle. Anim. Feed Sci. Techol. 2007, 135, 236–248. [Google Scholar] [CrossRef]
- Hariadi, B.T.; Santoso, B. Evaluation of tropical plants containing tannin on in vitro methanogenesis and fermentation parameters using rumen fluid. J. Sci. Food Agric. 2010, 90, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. 2012, 96, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Blümmel, M.; Makkar, H.P.S.; Becker, K. In vitro gas production: A technique revisited. J. Anim. Physiol. Anim. Nutr. 1997, 77, 24–34. [Google Scholar] [CrossRef]
- Kelln, B.M.; Penner, G.B.; Acharya, S.N.; McAllister, T.A.; Lardner, H.A. Impact of condensed tannin-containing legumes on ruminal fermentation, nutrition, and performance in ruminants: A review. Can. J. Anim. Sci. 2020, 101, 210–223. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, O. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef]
- Barros-Rodríguez, M.A.; Solorio-Sánchez, F.J.; Sandoval-Castro, C.A.; Klieve, A.; Rojas-Herrera, R.A.; Briceño-Poot, E.G.; Ku-Vera, J.C. Rumen function in vivo and in vitro in sheep fed Leucaena leucocephala. Trop. Anim. Health Prod. 2015, 47, 757–764. [Google Scholar] [CrossRef]
| Items | Treatments | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| Forage meal (A. mearnsii) | 0.00 | 20.00 | 40.00 | 60.00 |
| Forage meal (L. perenne) | 66.45 | 54.28 | 42.10 | 29.93 |
| Forage meal (M. sativa) | 33.55 | 25.72 | 17.90 | 10.07 |
| Chemical composition | ||||
| Dry matter | 88.67 | 89.37 | 90.08 | 90.80 |
| Organic matter | 90.39 | 91.21 | 92.04 | 92.86 |
| Crude protein | 19.00 | 20.10 | 20.01 | 21.21 |
| Ether extract | 3.51 | 3.35 | 3.19 | 3.03 |
| Neutral detergent fiber | 43.77 | 41.27 | 40.77 | 39.28 |
| Acid detergent fiber | 23.54 | 23.94 | 24.34 | 24.74 |
| Metabolizable energy (MJ/kg DM) | 9.21 | 9.20 | 9.20 | 9.19 |
| Condensed tannins | 0 | 3.56 | 6.03 | 7.97 |
| Treatment | SE | p-Value | Contrasts | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | L | Q | C | |||
| Degradation DM | |||||||||
| A | 45.6 a | 40.3 b | 34.7 c | 29.6 d | 1.16 | 0.0001 | 0.0001 | 0.9033 | 0.9142 |
| B | 44.1 a | 40.0 a | 37.5 a | 37.4 a | 1.85 | 0.0641 | 0.0144 | 0.2652 | 0.8983 |
| c | 0.05 a | 0.05 a | 0.04 a | 0.04 a | 0.007 | 0.3210 | 0.0777 | 0.7802 | 0.6712 |
| A + B | 89.8 a | 80.2 b | 72.1 c | 67.1 c | 2.01 | 0.0001 | 0.0001 | 0.2718 | 0.8570 |
| Effective Degradation * | |||||||||
| 0.02 | 77.0 a | 68.1 b | 58.7 c | 51.0 d | 0.94 | 0.0001 | 0.0001 | 0.5360 | 0.5790 |
| 0.05 | 67.8 a | 59.6 b | 50.6 c | 43.6 d | 1.06 | 0.0001 | 0.0001 | 0.5583 | 0.5504 |
| 0.08 | 62.9 a | 55.1 b | 46.6 c | 40.1 d | 0.96 | 0.0001 | 0.0001 | 0.5039 | 0.5245 |
| Degradation OM | |||||||||
| A | 43.2 a | 38.3 b | 32.6 c | 29.0 c | 1.19 | 0.0001 | 0.0001 | 0.5896 | 0.5824 |
| B | 46.5 a | 41.5 a | 38.7 a | 38.9 a | 2.27 | 0.0810 | 0.0195 | 0.2631 | 0.9205 |
| c | 0.06 a | 0.05 a | 0.04 a | 0.04 a | 0.01 | 0.2513 | 0.0517 | 0.8550 | 0.7429 |
| A + B | 89.7 a | 79.8 b | 71.2 b. c | 67.9 c | 2.45 | 0.0001 | 0.0001 | 0.1977 | 0.7189 |
| Effective Degradation * | |||||||||
| 0.02 | 76.7 a | 67.6 b | 57.8 c | 50.5 d | 0.95 | 0.0001 | 0.0001 | 0.3247 | 0.4577 |
| 0.05 | 67.1 a | 58.7 b | 49.3 c | 43.0 d | 1.08 | 0.0001 | 0.0001 | 0.3634 | 0.4193 |
| 0.08 | 61.8 a | 54.0 b | 45.2 c | 39.5 d | 0.98 | 0.0001 | 0.0001 | 0.2926 | 0.3563 |
| Treatment | SE | p-Value | Contrasts | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | L | Q | C | |||
| Digestibility | |||||||||
| DM | 74.8 a | 58.1 a b | 41.5 b | 20.7 c | 4.57 | 0.0001 | 0.0001 | 0.9115 | 0.7292 |
| OM | 77.1 a | 59.5 a b | 41.5 b | 20.6 c | 4.93 | 0.0001 | 0.0001 | 0.6918 | 0.9360 |
| pH | |||||||||
| 6 h | 6.98 a | 7.00 a | 6.98 a | 6.97 a | 0.02 | 0.7078 | 0.5613 | 0.3746 | 0.6364 |
| 12 h | 6.95 a | 7.00 a | 7.00 a | 7.02 a | 0.03 | 0.3319 | 0.1009 | 0.5161 | 0.6338 |
| 24 h | 7.26 a | 7.23 a | 7.28 a | 7.29 a | 0.06 | 0.8729 | 0.5607 | 0.7940 | 0.6048 |
| Treatment | SE | p-Value | Contrasts | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | L | Q | C | |||
| Gas production | |||||||||
| GV (mL) | 360.9 c | 434.7 c | 561.3 b | 944.1 a | 20.80 | 0.0001 | 0.0001 | 0.0001 | 0.0404 |
| B | 28.9 a | 29.4 a | 35.8 a | 38.6 a | 3.29 | 0.1340 | 0.0268 | 0.7162 | 0.5274 |
| c | 1.0 a | 0.9 a b | 0.8 b c | 0.8 c | 0.02 | 0.0001 | 0.0001 | 0.8372 | 0.7898 |
| CH4 production | |||||||||
| GV (mL) | 116.7 b c | 107.7 c | 141.8 a b | 156.1 a | 7.26 | 0.0004 | 0.0001 | 0.1249 | 0.0671 |
| B | 39.9 a | 39.1 a | 37.8 a | 33.2 b | 1.07 | 0.0013 | 0.0003 | 0.0975 | 0.5768 |
| c | 4.8 a | 6.0 a | 5.7 a | 5.1 a | 0.41 | 0.1938 | 0.7180 | 0.0412 | 0.6001 |
| CO2 production | |||||||||
| GV (mL) | 170.9 b | 189.7 b | 229.7 a b | 425.4 a | 49.75 | 0.0063 | 0.0017 | 0.0905 | 0.5528 |
| B | 108.8 a | 105.9 a | 106.5 a | 132.5 a | 33.50 | 0.9315 | 0.6377 | 0.6709 | 0.8856 |
| c | 1.1 a | 1.0 a | 1.0 a | 0.9 a | 0.05 | 0.2739 | 0.0674 | 0.6085 | 0.6846 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Ortiz, L.; Andrade-Yucailla, V.; Barros-Rodríguez, M.; Lima-Orozco, R.; Macías-Rodríguez, E.; Contreras-Barros, K.; Guishca-Cunuhay, C. Influence of Acacia Mearnsii Fodder on Rumen Digestion and Mitigation of Greenhouse Gas Production. Animals 2022, 12, 2250. https://doi.org/10.3390/ani12172250
Vargas-Ortiz L, Andrade-Yucailla V, Barros-Rodríguez M, Lima-Orozco R, Macías-Rodríguez E, Contreras-Barros K, Guishca-Cunuhay C. Influence of Acacia Mearnsii Fodder on Rumen Digestion and Mitigation of Greenhouse Gas Production. Animals. 2022; 12(17):2250. https://doi.org/10.3390/ani12172250
Chicago/Turabian StyleVargas-Ortiz, Luis, Veronica Andrade-Yucailla, Marcos Barros-Rodríguez, Raciel Lima-Orozco, Edis Macías-Rodríguez, Katherine Contreras-Barros, and Carlos Guishca-Cunuhay. 2022. "Influence of Acacia Mearnsii Fodder on Rumen Digestion and Mitigation of Greenhouse Gas Production" Animals 12, no. 17: 2250. https://doi.org/10.3390/ani12172250
APA StyleVargas-Ortiz, L., Andrade-Yucailla, V., Barros-Rodríguez, M., Lima-Orozco, R., Macías-Rodríguez, E., Contreras-Barros, K., & Guishca-Cunuhay, C. (2022). Influence of Acacia Mearnsii Fodder on Rumen Digestion and Mitigation of Greenhouse Gas Production. Animals, 12(17), 2250. https://doi.org/10.3390/ani12172250







