Echocardiographic Features of the Ductus Arteriosus and the Foramen Ovale in a Hospital-Based Population of Neonatal Foals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Echocardiography and Flow Doppler Examination
2.3. Data Analysis
3. Results
3.1. Study Population
3.2. Cardiac Auscultation
3.3. Echocardiographic Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marr, C.M. The Equine Neonatal Cardiovascular System in Health and Disease. Vet. Clin. N. Am. Equine Pract. 2015, 31, 545–565. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.; Palmer, L.; Marr, C. Two-dimensional and M-mode echocardiographic findings in healthy Thoroughbred foals. Aust. Vet. J. 2010, 88, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Scansen, B.A. Equine Congenital Heart Disease. Vet. Clin. N. Am. Equine Pract. 2019, 35, 103–117. [Google Scholar] [CrossRef]
- Macdonald, A.A.; Fowden, A.L.; Silver, M.; Ousey, J.; Rossdale, P.D. The foramen ovale of the foetal and neonatal foal. Equine Vet. J. 1988, 20, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Redpath, A.; Marr, C.M.; Bullard, C.; Hallowell, G.D. Real-time three-dimensional (3D) echocardiographic characterisation of an atrial septal defect in a horse. Vet. Med. Sci. 2020, 6, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Praud, J.-P.; Miura, Y.; Frasch, M.G. Animal Models for the Study of Neonatal Disease. In Animal Models for the Study of Human Disease; Academic Press: Cambridge, MA, USA, 2017; pp. 806–808. [Google Scholar]
- Scott, E.A.; Kneller, S.K.; Witherspoon, D.M. Closure of ductus arteriosus determined by cardiac catheterization and angiography in newborn foals. Am. J. Vet. Res. 1975, 36, 1021–1023. [Google Scholar]
- Machida, N.; Yasuda, J.; Too, K.; Kudo, N. A morphological study on the obliteration processes of the ductus arteriosus in the horse. Equine Vet. J. 1988, 20, 249–254. [Google Scholar] [CrossRef]
- Bonagura, J.D. Overview of Equine Cardiac Disease. Vet. Clin. N. Am. Equine Pract. 2019, 35, 1–22. [Google Scholar] [CrossRef]
- Reef, V.B. Cardiovascular Disease in the Equine Neonate. Vet. Clin. N. Am. Equine Pract. 1985, 1, 117–130. [Google Scholar] [CrossRef]
- Marr, C.M.; Reimer, J.M. Chapter 8—The cardiovascular system. In Equine Man; Springer Publishing: New York, NY, USA, 2006; pp. 455–483. [Google Scholar]
- Livesey, L.; Marr, C.M.; Boswood, A.; Freeman, S.; Bowen, I.; Corley, K. Auscultatory and two-dimensional, M-Mode, spectral and colour flow Doppler echocardiographic findings in pony foals from birth to seven weeks of age. J. Vet. Intern. Med. 1998, 12, 255. [Google Scholar]
- Marr, C.M. Cardiac murmurs: Congenital heart disease. In Cardiology of the Horse; Elsevier: Amsterdam, The Netherlands, 2010; pp. 193–205. [Google Scholar]
- Vernemmen, I.; Vera, L.; Van Steenkiste, G.; Van Loon, G.; Decloedt, A. Reference values for 2-dimensional and M-mode echocardiography in Friesian and Warmblood horses. J. Vet. Intern. Med. 2020, 34, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- Keen, J.; Marr, C.M. Equine echocardiography: Abbreviations and terminology recommended by Equine Veterinary Journal. Equine Vet. J. 2017, 49, 8–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandecasteele, T.; Cornillie, P.; Van Steenkiste, G.; Vandevelde, K.; Gielen, I.; Vanderperren, K.; Van Loon, G. Echocardiographic identification of atrial-related structures and vessels in horses validated by computed tomography of casted hearts. Equine Vet. J. 2019, 51, 90–96. [Google Scholar] [CrossRef] [PubMed]
- De Lange, L.; Vera, L.; Decloedt, A.; Van Steenkiste, G.; Vernemmen, I.; van Loon, G. Prevalence and characteristics of ventricular septal defects in a non-racehorse equine population (2008–2019). J. Vet. Intern. Med. 2021, 35, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Lombard, C.W.; Evans, M.; Martin, L.; Tehrani, J. Blood pressure, electrocardiogram and echocardiogram measurements in the growing pony foal. Equine Vet. J. 1984, 16, 342–347. [Google Scholar] [CrossRef]
- Rossdale, P.D. Clinical studies on the newborn thoroughbred foal II. Heart rate, auscultation and electrocardiogram. Br. Vet. J. 1967, 123, 521–532. [Google Scholar] [CrossRef]
- Schwarzwald, C.C. Sequential segmental analysis—A systematic approach to the diagnosis of congenital cardiac defects. Equine Vet. Educ. 2008, 20, 305–309. [Google Scholar] [CrossRef]
- Schwarzwald, C.C. Disorders of the Cardiovascular System. In Equine Internal Medicine, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 387–541. [Google Scholar]
- Reef, V.B. Evaluation of ventricular septal defects in horses using two-dimensional and Doppler echocardiography. Equine Vet. J. 1995, 27 (Suppl. 19), 86–95. [Google Scholar] [CrossRef]
- Miller, M.W.; Gordon, S.; Saunders, A.; Arsenault, W.G.; Meurs, K.; Lehmkuhl, L.B.; Bonagura, J.; Fox, P.R. Angiographic classification of patent ductus arteriosus morphology in the dog. J. Vet. Cardiol. 2006, 8, 109–114. [Google Scholar] [CrossRef]
- Cottrill, C.M.; O’Connor, W.N.; Cudd, T.; Rantanen, N.W. Persistence of foetal circulatory pathways in a newborn foal. Equine Vet. J. 1987, 19, 252–255. [Google Scholar] [CrossRef]
- Vernemmen, I.; Paulussen, E.; Dauvillier, J.; Decloedt, A.; van Loon, G. Three-dimensional and catheter-based intracardiac echocardiographic characterization of the interatrial septum in 2 horses with suspicion of a patent foramen ovale. J. Vet. Intern. Med. 2022, 36, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
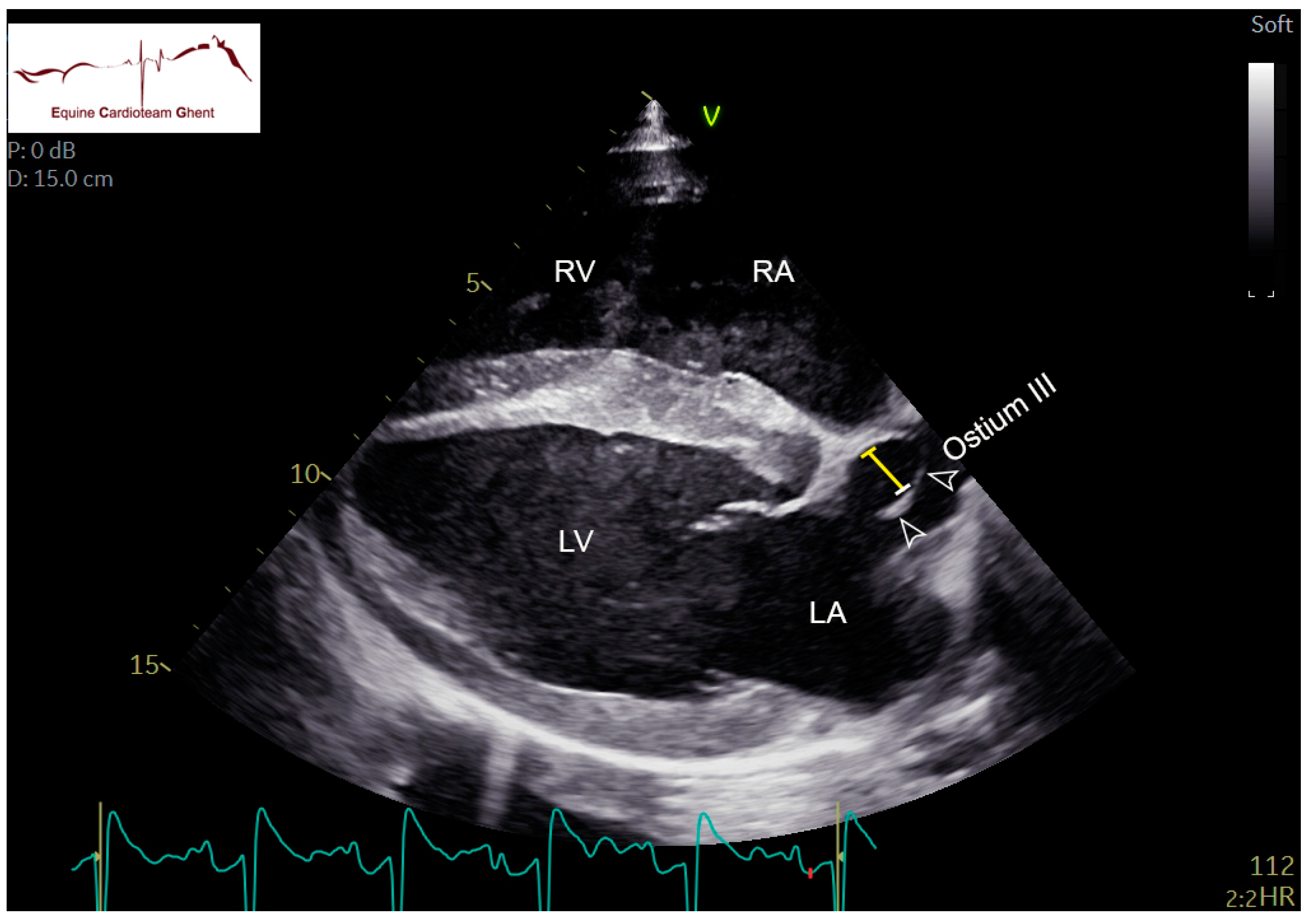
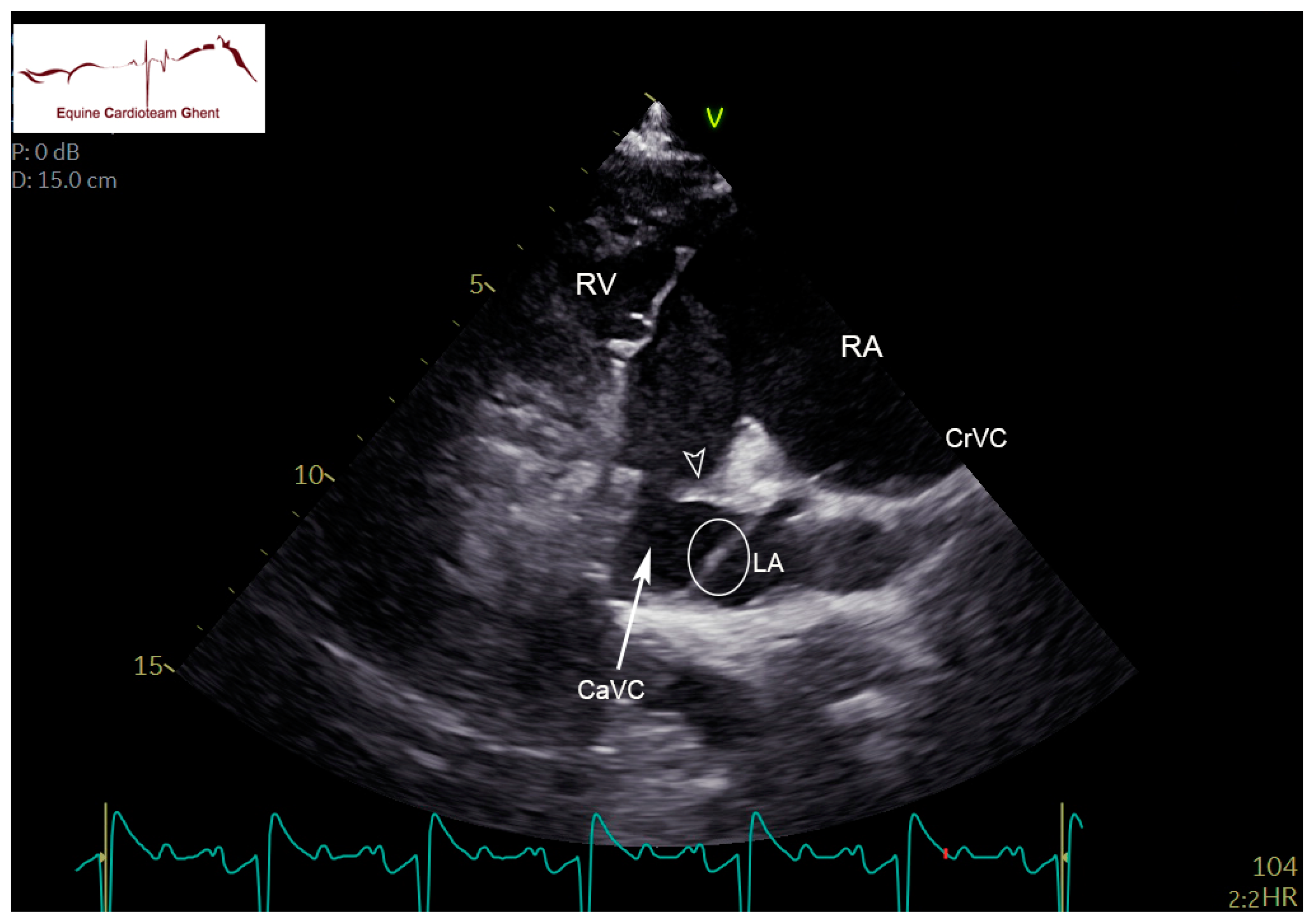
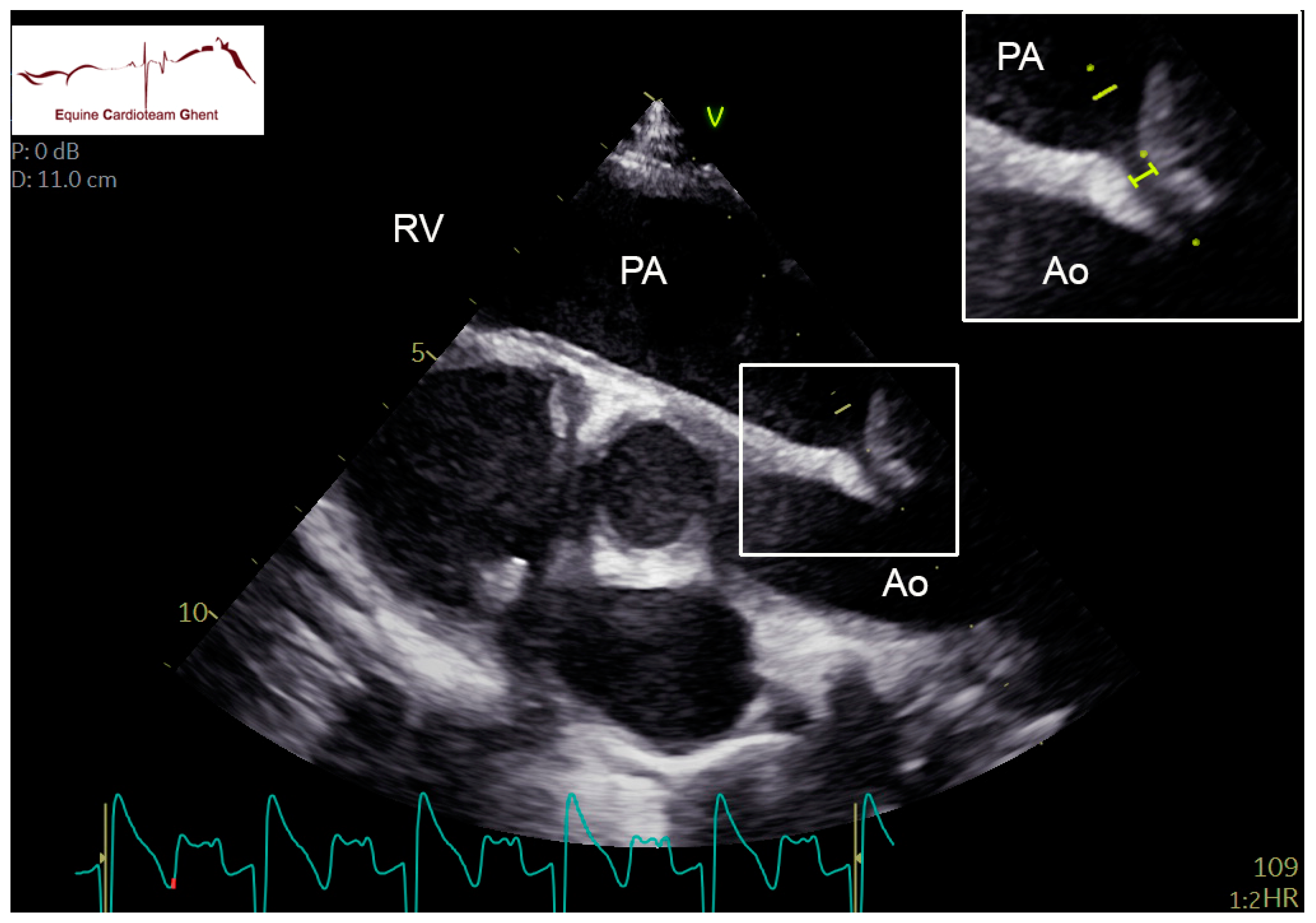
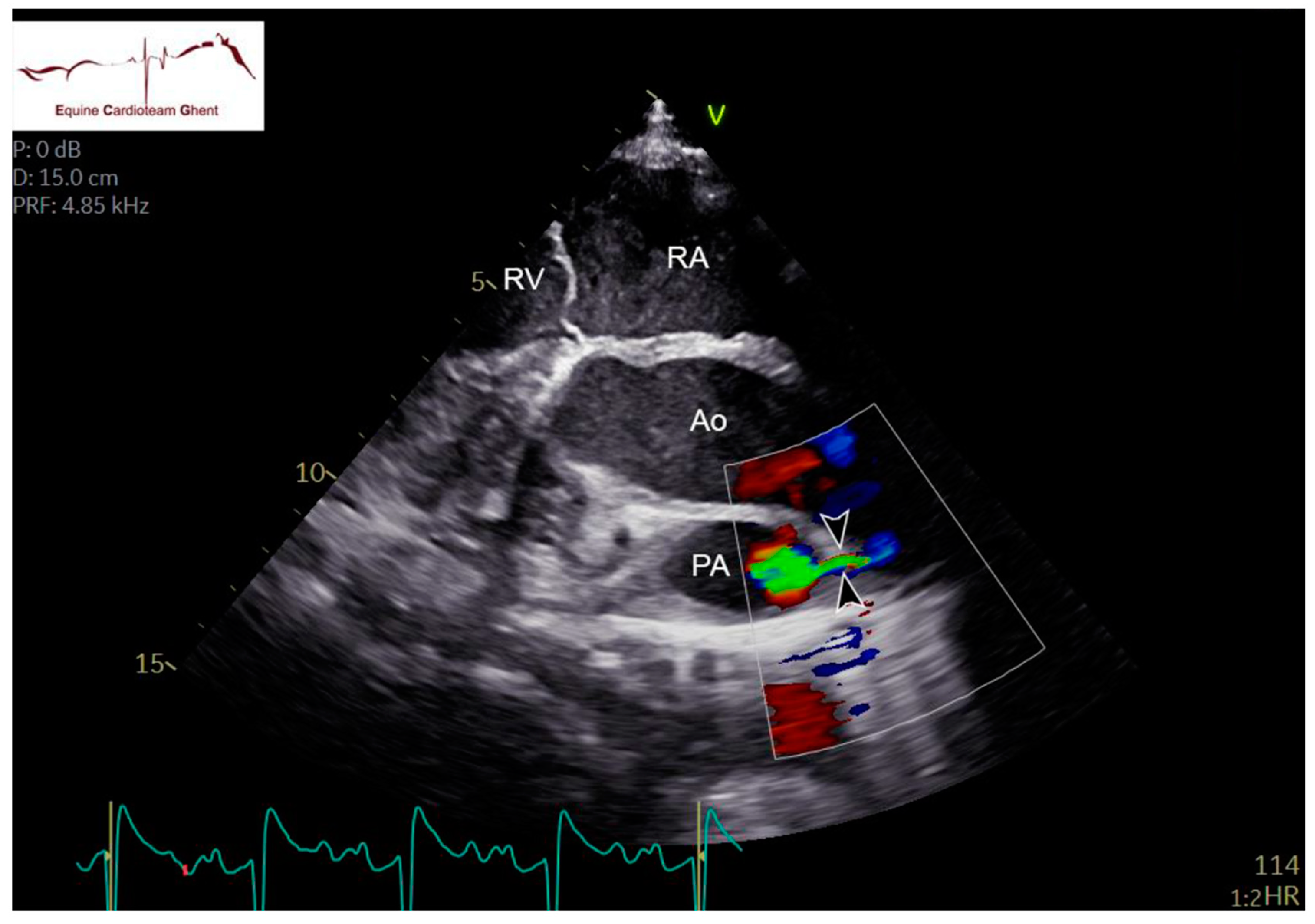
| Initial Diagnosis | Day 2 | Day 5 | Day 10 |
|---|---|---|---|
| (n = 43) | (n = 31) | (n = 9) | |
| Healthy foals | 15 | 7 | 2 |
| Premature foals (gestation length < 320 days) | 8 | 7 | 3 |
| Neonatal hypoxic encephalopathy, perinatal asphyxia syndrome, septicemia, neonatal isoerythrolysis, failure of passive transfer, foals born by cesarean section, gastrointestinal (colic, inguinal hernias, meconium impaction) and/or respiratory (ARDS) diseases | 17 | 15 | 4 |
| Orthopedic abnormalities (angular and flexural limb deformities) | 3 | 2 | 0 |
| Day 2 | Day 5 | Day 10 | ||||
|---|---|---|---|---|---|---|
| (n Open/n Total) | (n Open/n Total) | (n Open/n Total) | ||||
| Healthy | Diseased | Healthy | Diseased | Healthy | Diseased | |
| Turbulent flow through DA (Color Doppler) | 13/15 | 27/28 | 6/7 | 13/24 | 0/2 | 2/7 |
| Systolic flow through DA | 13/13 | 27/27 | 6/6 | 13/13 | - | 2/2 |
| (CW Doppler) | ||||||
| Diastolic flow through DA | 5/13 | 9/27 | 2/6 | 4/13 | - | 0/2 |
| (CW Doppler) | ||||||
| Day 2 (n = 43) | Day 5 (n = 31) | Day 10 (n = 9) | |
|---|---|---|---|
| Mean ± sd | Mean ± sd | Mean ± sd | |
| HR (beats per minute) | 102 ± 16 | 96 ± 18 | 93 ± 29 |
| R-4C LADdend (cm) | 3.8 ± 0.5 | 3.8 ± 0.7 | 3.7 ± 0.6 |
| R-4C LADsend (cm) | 4.2 ± 0.6 | 4.1 ± 0.7 | 4.2 ± 0.8 |
| R-4C LAAdend (cm2) | 9.5 ± 2.2 | 10 ± 3.6 | 8.7 ± 2.6 |
| R-4C LAAsend (cm2) | 16 ± 3 | 17 ± 4.2 | 13.8 ± 4.0 |
| R-4C LVAdend (cm2) | 31 ± 6.1 | 36 ± 9.2 | 38.5 ± 9.0 |
| R-4C LVAsend (cm2) | 16 ± 4.4 | 17 ± 6.6 | 19.7 ± 3.7 |
| R-LVSAXch M-modeLVIDdend (cm) | 5.1 ± 0.7 | 5.3 ± 0.7 | 5.3 ± 1.0 |
| R-LVSAXch M-modeLVIDspeak (cm) | 3.1 ± 0.6 | 3.4 ± 0.6 | 3.6 ± 0.8 |
| R-LVSAXch M-modeRVIDdend (cm) | 2.1 ± 0.6 | 2.2 ± 0.6 | 2.2 ± 1.1 |
| R-LVSAXch M-modeRVIDspeak (cm) | 1.3 ± 0.5 | 1.3 ± 0.6 | 1.2 ± 0.8 |
| R-RVOT PADdend (cm) | 2.3 ± 0.2 | 2.2 ± 0.4 | 2.2 ± 0.5 |
| R-LVOT PADspeak (cm) | 1.8 ± 0.3 | 1.9 ± 0.3 | 1.9 ± 0.2 |
| R-LVOT AoDspeak (cm) | 2.6 ± 0.3 | 2.7 ± 0.3 | 2.7 ± 0.5 |
| R-LVOT AoDdend (cm) | 2.2 ± 0.3 | 2.4 ± 0.4 | 2.3 ± 0.5 |
| R-LVOT PADspeak/AoDspeak | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.2 |
| L-PALAX PWD PA Velmax (m/s) | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.4 |
| L-PALAX PWD PA VTI (cm) | 24 ± 8.0 | 23 ± 5.0 | 22 ± 5.9 |
| L-PALAX DAD (mm) * | 2 ± 1 | 2 ± 1 | 1 |
| L-PALAX CWD DA Velmax (m/s) | 3.8 ± 0.6 | 3.3 ± 1.0 | 2.7 ± 1.1 |
| R-4C SP thickness (mm) | 3 ± 1 | 4 ± 1 | 4 ± 2 |
| R-4C SS thickness (mm) | 3 ± 1 | 3 ± 1 | 4 ± 2 |
| Maximal distance between SP and SS (mm) | 12 ± 5 | 11 ± 4 | 6 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lange, L.; Vernemmen, I.; van Loon, G.; Decloedt, A. Echocardiographic Features of the Ductus Arteriosus and the Foramen Ovale in a Hospital-Based Population of Neonatal Foals. Animals 2022, 12, 2242. https://doi.org/10.3390/ani12172242
De Lange L, Vernemmen I, van Loon G, Decloedt A. Echocardiographic Features of the Ductus Arteriosus and the Foramen Ovale in a Hospital-Based Population of Neonatal Foals. Animals. 2022; 12(17):2242. https://doi.org/10.3390/ani12172242
Chicago/Turabian StyleDe Lange, Lisa, Ingrid Vernemmen, Gunther van Loon, and Annelies Decloedt. 2022. "Echocardiographic Features of the Ductus Arteriosus and the Foramen Ovale in a Hospital-Based Population of Neonatal Foals" Animals 12, no. 17: 2242. https://doi.org/10.3390/ani12172242
APA StyleDe Lange, L., Vernemmen, I., van Loon, G., & Decloedt, A. (2022). Echocardiographic Features of the Ductus Arteriosus and the Foramen Ovale in a Hospital-Based Population of Neonatal Foals. Animals, 12(17), 2242. https://doi.org/10.3390/ani12172242






