Comparison of Sources and Methods for the Isolation of Equine Adipose Tissue-Derived Stromal/Stem Cells and Preliminary Results on Their Reaction to Incubation with 5-Azacytidine
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation of Equine ASCs
2.2. Antibody Staining and Flow Cytometry
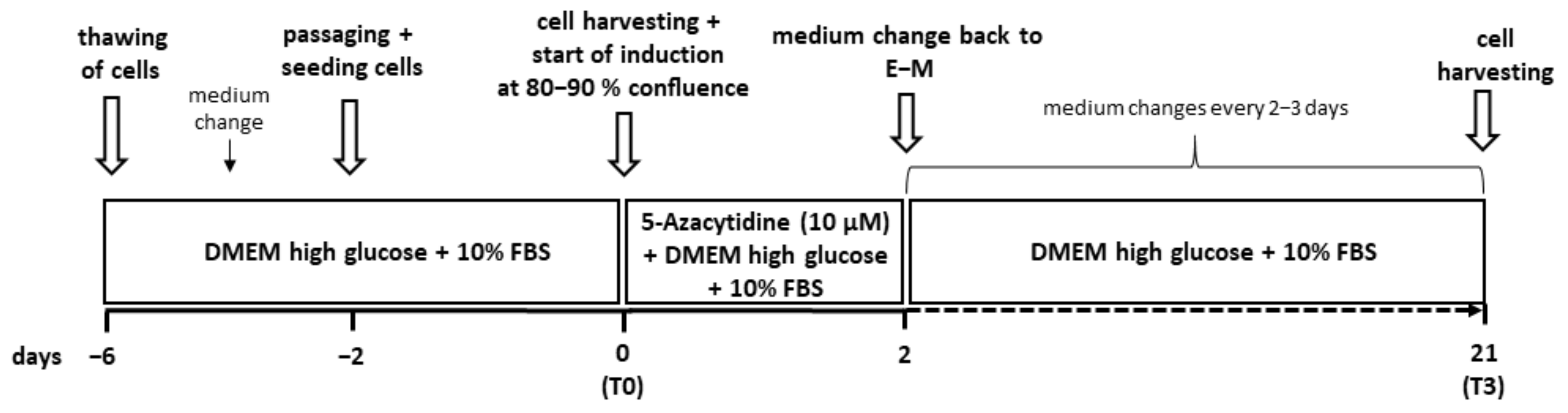
2.3. Cardiomyogenic Induction
2.4. Real-Time Quantitative PCR
2.5. Statistical Analyses
3. Results
3.1. Isolation and Cultivation of Equine ASCs
3.2. Flow Cytometry
3.3. Cardiomyogenic Differentiation
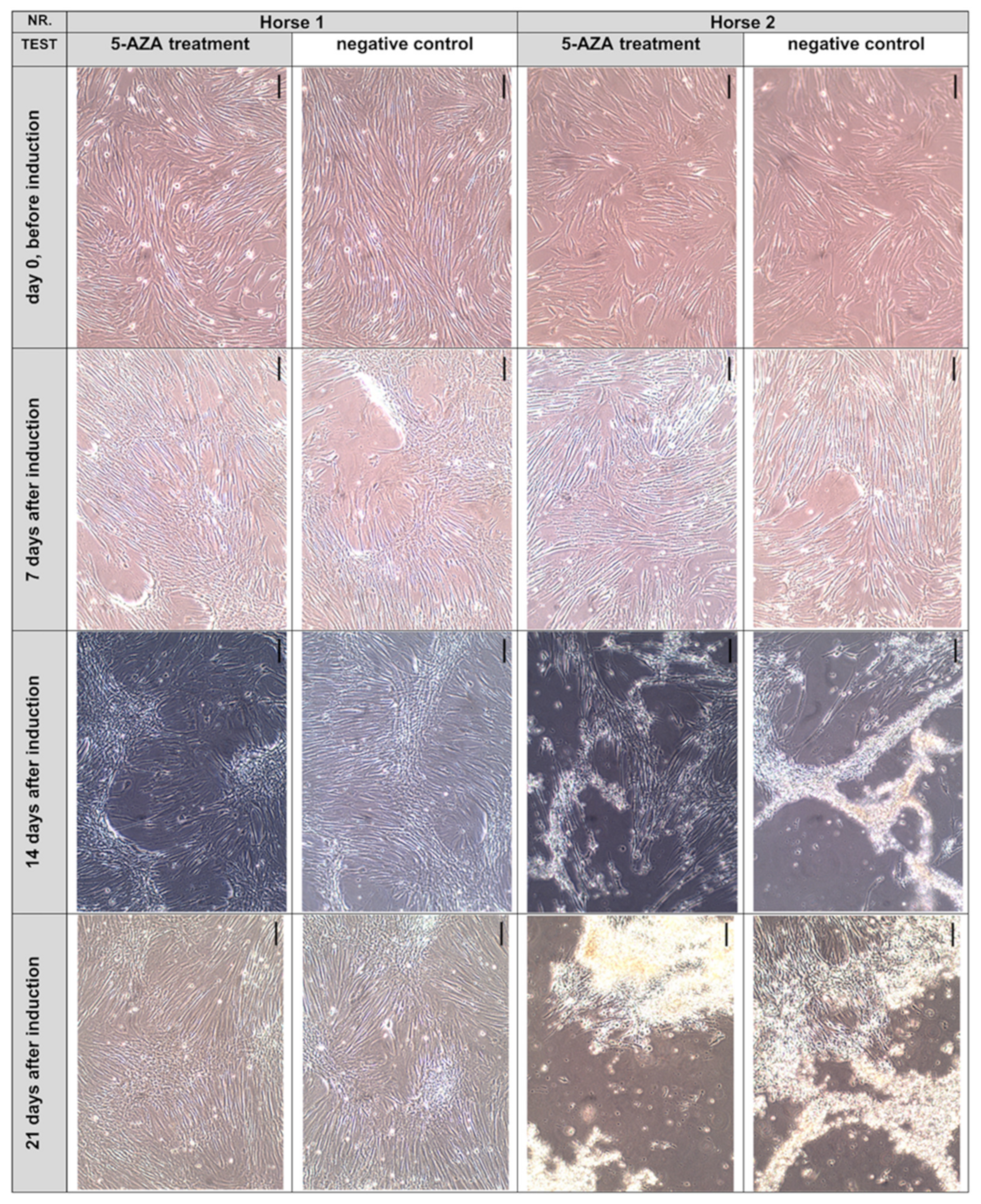
3.3.1. Cell Morphology
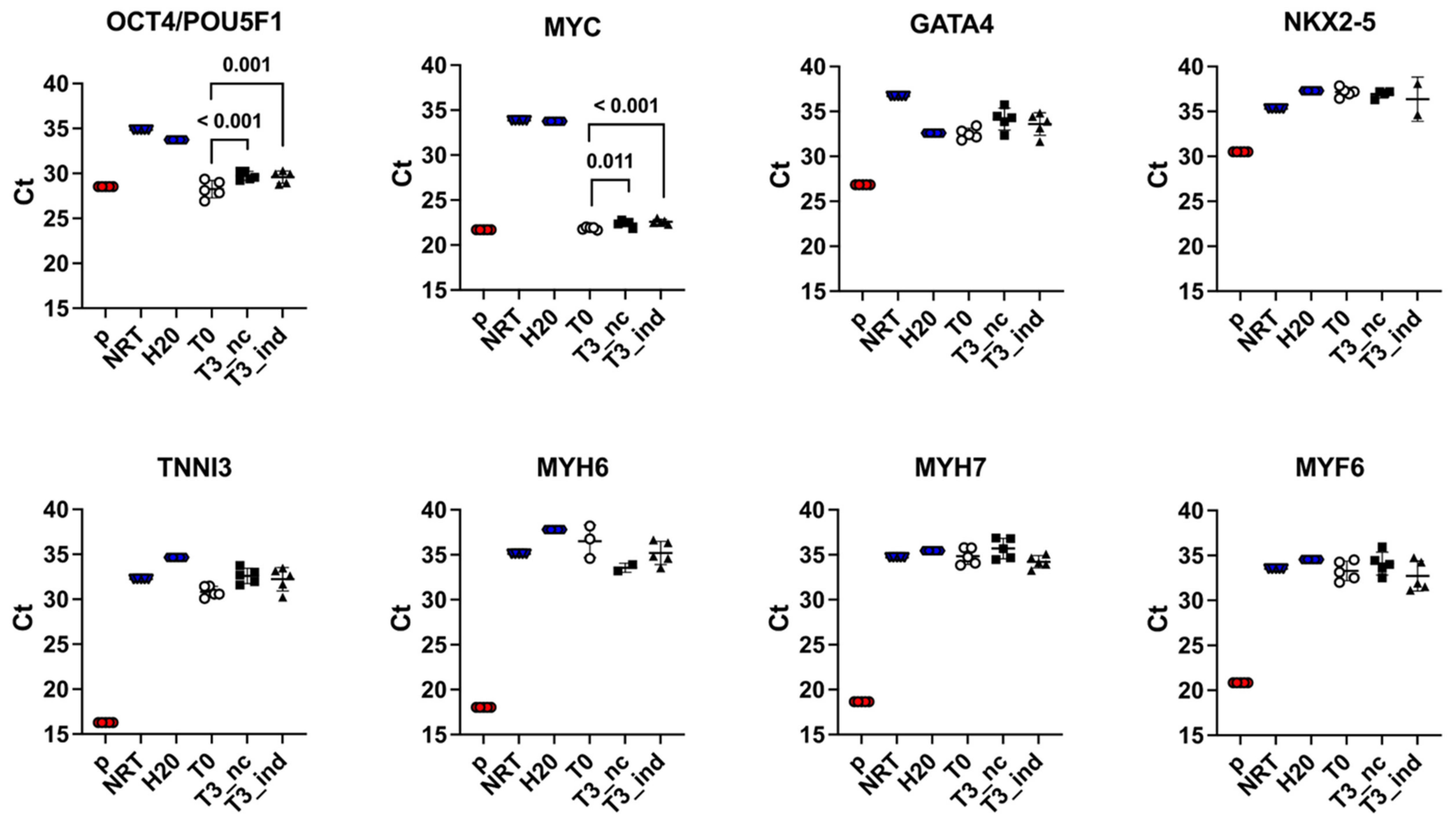
3.3.2. Reverse-Transcription Real-Time Quantitative PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamlin, R.L. QRS in pigs versus in dogs. J. Pharmacol. Toxicol. Methods 2010, 62, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, S.W. Measurement of the QT interval: Lessons from thirty-two animal species for the correction of the QT interval by heart rate. Int. J. Clin. Cardiol. 2018, 5, 127. [Google Scholar] [CrossRef]
- Trachsel, D.S.; Tejada, M.A.; Groesfjeld Christensen, V.; Pedersen, P.J.; Kanters, J.K.; Buhl, R.; Calloe, K.; Klaerke, D.A. Effects of trimethoprim-sulfadiazine and detomidine on the function of equine Kv 11.1 channels in a two-electrode voltage-clamp (TEVC) oocyte model. J. Vet. Pharmacol. Ther. 2018, 41, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Calloe, K.; Rognant, S.; Friis, S.; Shaughnessy, C.; Klaerke, D.A.; Trachsel, D.S. Compounds commonly used in equine medicine inhibits the voltage-gated potassium channel Kv11.1. Res. Vet. Sci. 2019, 123, 239–246. [Google Scholar] [CrossRef]
- Pedersen, P.J.; Thomsen, K.B.; Flak, J.B.; Tejada, M.A.; Hauser, F.; Trachsel, D.; Buhl, R.; Kalbfleisch, T.; DePriest, M.S.; MacLeod, J.N.; et al. Molecular cloning and functional expression of the K+ channel KV7.1 and the regulatory subunit KCNE1 from equine myocardium. Res. Vet. Sci. 2017, 113, 79–86. [Google Scholar] [CrossRef]
- Pedersen, P.J.; Thomsen, K.B.; Olander, E.R.; Hauser, F.; Tejada Mde, L.; Poulsen, K.L.; Grubb, S.; Buhl, R.; Calloe, K.; Klaerke, D.A. Molecular cloning and functional expression of the equine K+ channel KV11.1 (Ether à Go-Go-Related/KCNH2 gene) and the regulatory subunit KCNE2 from equine myocardium. PLoS ONE 2015, 10, e0138320. [Google Scholar] [CrossRef]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in pluripotent stem cells: History, mechanisms, technologies, and applications. Stem Cell Rev. Rep. 2020, 16, 3–32. [Google Scholar] [CrossRef]
- Kornicka, K.; Geburek, F.; Rocken, M.; Marycz, K. Stem cells in equine veterinary practice-current trends, risks, and perspectives. J. Clin. Med. 2019, 8, 675. [Google Scholar] [CrossRef]
- Gattegno-Ho, D.; Argyle, S.A.; Argyle, D.J. Stem cells and veterinary medicine: Tools to understand diseases and enable tissue regeneration and drug discovery. Vet. J. 2012, 191, 19–27. [Google Scholar] [CrossRef]
- Przadka, P.; Buczak, K.; Frejlich, E.; Gasior, L.; Suliga, K.; Kielbowicz, Z. The Role of mesenchymal stem cells (MSCs) in veterinary medicine and their use in musculoskeletal disorders. Biomolecules 2021, 11, 1141. [Google Scholar] [CrossRef]
- Arnhold, S.; Wenisch, S. Adipose tissue derived mesenchymal stem cells for musculoskeletal repair in veterinary medicine. Am. J. Stem. Cells 2015, 4, 1–12. [Google Scholar]
- Khazaei, S.; Keshavarz, G.; Bozorgi, A.; Nazari, H.; Khazaei, M. Adipose tissue-derived stem cells: A comparative review on isolation, culture, and differentiation methods. Cell Tissue Bank. 2021, 23, 1–16. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef]
- Shen, J.F.; Sugawara, A.; Yamashita, J.; Ogura, H.; Sato, S. Dedifferentiated fat cells: An alternative source of adult multipotent cells from the adipose tissues. Int. J. Oral Sci. 2011, 3, 117–124. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kano, K.; Kondo, D.; Fukuda, N.; Iribe, Y.; Tanaka, N.; Matsubara, Y.; Sakuma, T.; Satomi, A.; Otaki, M.; et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J. Cell. Physiol. 2008, 215, 210–222. [Google Scholar] [CrossRef]
- Jumabay, M.; Bostrom, K.I. Dedifferentiated fat cells: A cell source for regenerative medicine. World J. Stem. Cells 2015, 7, 1202–1214. [Google Scholar] [CrossRef]
- Sandhu, M.A.; Jurek, S.; Trappe, S.; Kolisek, M.; Sponder, G.; Aschenbach, J.R. Influence of bovine serum lipids and fetal bovine serum on the expression of cell surface markers in cultured bovine preadipocytes. Cells Tissues Organs 2017, 204, 13–24. [Google Scholar] [CrossRef]
- Schwarz, C.; Leicht, U.; Rothe, C.; Drosse, I.; Luibl, V.; Rocken, M.; Schieker, M. Effects of different media on proliferation and differentiation capacity of canine, equine and porcine adipose derived stem cells. Res. Vet. Sci. 2012, 93, 457–462. [Google Scholar] [CrossRef]
- Vidal, M.A.; Kilroy, G.E.; Lopez, M.J.; Johnson, J.R.; Moore, R.M.; Gimble, J.M. Characterization of equine adipose tissue-derived stromal cells: Adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet. Surg. 2007, 36, 613–622. [Google Scholar] [CrossRef]
- Hillmann, A.; Ahrberg, A.B.; Brehm, W.; Heller, S.; Josten, C.; Paebst, F.; Burk, J. Comparative characterization of human and equine mesenchymal stromal cells: A basis for translational studies in the equine model. Cell Transplant. 2016, 25, 109–124. [Google Scholar] [CrossRef]
- Ranera, B.; Lyahyai, J.; Romero, A.; Vázquez, F.J.; Remacha, A.R.; Bernal, M.L.; Zaragoza, P.; Rodellar, C.; Martín-Burriel, I. Immunophenotype and gene expression profiles of cell surface markers of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Vet. Immunol. Immunopathol. 2011, 144, 147–154. [Google Scholar] [CrossRef]
- Burk, J.; Ribitsch, I.; Gittel, C.; Juelke, H.; Kasper, C.; Staszyk, C.; Brehm, W. Growth and differentiation characteristics of equine mesenchymal stromal cells derived from different sources. Vet. J. 2013, 195, 98–106. [Google Scholar] [CrossRef]
- Barberini, D.J.; Freitas, N.P.; Magnoni, M.S.; Maia, L.; Listoni, A.J.; Heckler, M.C.; Sudano, M.J.; Golim, M.A.; da Cruz Landim-Alvarenga, F.; Amorim, R.M. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: Immunophenotypic characterization and differentiation potential. Stem Cell Res. Ther. 2014, 5, 25. [Google Scholar] [CrossRef]
- Gittel, C.; Brehm, W.; Burk, J.; Juelke, H.; Staszyk, C.; Ribitsch, I. Isolation of equine multipotent mesenchymal stromal cells by enzymatic tissue digestion or explant technique: Comparison of cellular properties. BMC Vet. Res. 2013, 9, 221. [Google Scholar] [CrossRef][Green Version]
- Kono, S.; Kazama, T.; Kano, K.; Harada, K.; Uechi, M.; Matsumoto, T. Phenotypic and functional properties of feline dedifferentiated fat cells and adipose-derived stem cells. Vet. J. 2014, 199, 88–96. [Google Scholar] [CrossRef]
- Priya, N.; Sarcar, S.; Majumdar, A.S.; SundarRaj, S. Explant culture: A simple, reproducible, efficient and economic technique for isolation of mesenchymal stromal cells from human adipose tissue and lipoaspirate. J. Tissue Eng. Regen. Med. 2014, 8, 706–716. [Google Scholar] [CrossRef]
- Lee, D.H.; Joo, S.D.; Han, S.B.; Im, J.; Lee, S.H.; Sonn, C.H.; Lee, K.M. Isolation and expansion of synovial CD34−CD44+CD90+ mesenchymal stem cells: Comparison of an enzymatic method and a direct explant technique. Connect. Tissue Res. 2011, 52, 226–234. [Google Scholar] [CrossRef]
- Dominici, M.L.B.K.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Paebst, F.; Piehler, D.; Brehm, W.; Heller, S.; Schroeck, C.; Tarnok, A.; Burk, J. Comparative immunophenotyping of equine multipotent mesenchymal stromal cells: An approach toward a standardized definition. Cytom. Part A 2014, 85, 678–687. [Google Scholar] [CrossRef]
- Braun, J.; Hack, A.; Weis-Klemm, M.; Conrad, S.; Treml, S.; Kohler, K.; Walliser, U.; Skutella, T.; Aicher, W.K. Evaluation of the osteogenic and chondrogenic differentiation capacities of equine adipose tissue-derived mesenchymal stem cells. Am. J. Vet. Res. 2010, 71, 1228–1236. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Wystrychowski, W.; Patlolla, B.; Zhuge, Y.; Neofytou, E.; Robbins, R.C.; Beygui, R.E. Multipotency and cardiomyogenic potential of human adipose-derived stem cells from epicardium, pericardium, and omentum. Stem. Cell Res. Ther. 2016, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.H.; Daibert, A.P.; Monteiro, B.S.; Okano, B.S.; Carvalho, J.L.; Cunha, D.N.; Favarato, L.S.; Pereira, V.G.; Augusto, L.E.; Del Carlo, R.J. Differentiation of adipose tissue-derived mesenchymal stem cells into cardiomyocytes. Arq. Bras. Cardiol. 2013, 100, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Rangappa, S.; Fen, C.; Lee, E.H.; Bongso, A.; Sim, E.K. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann. Thorac. Surg. 2003, 75, 775–779. [Google Scholar] [CrossRef]
- Yang, J.; Song, T.; Wu, P.; Chen, Y.; Fan, X.; Chen, H.; Zhang, J.; Huang, C. Differentiation potential of human mesenchymal stem cells derived from adipose tissue and bone marrow to sinus node-like cells. Mol. Med. Rep. 2012, 5, 108–113. [Google Scholar] [CrossRef]
- Sulewska, A.; Niklinska, W.; Kozlowski, M.; Minarowski, L.; Naumnik, W.; Niklinski, J.; Dabrowska, K.; Chyczewski, L. DNA methylation in states of cell physiology and pathology. Folia Histochem. CytoBiol. 2007, 45, 149–158. [Google Scholar]
- Kakkar, A.; Nandy, S.B.; Gupta, S.; Bharagava, B.; Airan, B.; Mohanty, S. Adipose tissue derived mesenchymal stem cells are better respondents to TGFβ1 for in vitro generation of cardiomyocyte-like cells. Mol. Cell Biochem. 2019, 460, 53–66. [Google Scholar] [CrossRef]
- Safwani, W.K.; Makpol, S.; Sathapan, S.; Chua, K.H. 5-Azacytidine is insufficient for cardiogenesis in human adipose-derived stem cells. J. Negat. Results Biomed. 2012, 11, 3. [Google Scholar] [CrossRef]
- Lee, W.C.; Sepulveda, J.L.; Rubin, J.P.; Marra, K.G. Cardiomyogenic differentiation potential of human adipose precursor cells. Int. J. Cardiol. 2009, 133, 399–401. [Google Scholar] [CrossRef]
- Ong, W.K.; Chakraborty, S.; Sugii, S. Adipose Tissue: Understanding the Heterogeneity of Stem Cells for Regenerative Medicine. Biomolecules 2021, 11, 918. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Reynolds, A.; Keen, J.A.; Fordham, T.; Morgan, R.A. Adipose tissue dysfunction in obese horses with equine metabolic syndrome. Equine. Vet. J. 2019, 51, 760–766. [Google Scholar] [CrossRef]
- Tan, K.; Zheng, K.; Li, D.; Lu, H.; Wang, S.; Sun, X. Impact of adipose tissue or umbilical cord derived mesenchymal stem cells on the immunogenicity of human cord blood derived endothelial progenitor cells. PLoS ONE 2017, 12, e0178624. [Google Scholar] [CrossRef]
- Russo, V.; Yu, C.; Belliveau, P.; Hamilton, A.; Flynn, L.E. Comparison of human adipose-derived stem cells isolated from subcutaneous, omental, and intrathoracic adipose tissue depots for regenerative applications. Stem Cells Transl. Med. 2014, 3, 206–217. [Google Scholar] [CrossRef]
- Bahamondes, F.; Flores, E.; Cattaneo, G.; Bruna, F.; Conget, P. Omental adipose tissue is a more suitable source of canine Mesenchymal stem cells. BMC Vet. Res. 2017, 13, 166. [Google Scholar] [CrossRef]
- Yaneselli, K.M.; Kuhl, C.P.; Terraciano, P.B.; de Oliveira, F.S.; Pizzato, S.B.; Pazza, K.; Magrisso, A.B.; Torman, V.; Rial, A.; Moreno, M.; et al. Comparison of the characteristics of canine adipose tissue-derived mesenchymal stem cells extracted from different sites and at different passage numbers. J. Vet. Sci. 2018, 19, 13–20. [Google Scholar] [CrossRef]
- Arnhold, S.; Elashry, M.I.; Klymiuk, M.C.; Geburek, F. Investigation of stemness and multipotency of equine adipose-derived mesenchymal stem cells (ASCs) from different fat sources in comparison with lipoma. Stem Cell Res. Ther. 2019, 10, 309. [Google Scholar] [CrossRef]
- Metcalf, G.L.; McClure, S.R.; Hostetter, J.M.; Martinez, R.F.; Wang, C. Evaluation of adipose-derived stromal vascular fraction from the lateral tailhead, inguinal region, and mesentery of horses. Can. J. Vet. 2016, 80, 294–301. [Google Scholar]
- Jurek, S.; Sandhu, M.A.; Trappe, S.; Bermudez-Pena, M.C.; Kolisek, M.; Sponder, G.; Aschenbach, J.R. Optimizing adipogenic transdifferentiation of bovine mesenchymal stem cells: A prominent role of ascorbic acid in FABP4 induction. Adipocyte 2020, 9, 35–50. [Google Scholar] [CrossRef]
- Spaas, J.H.; De Schauwer, C.; Cornillie, P.; Meyer, E.; Van Soom, A.; Van de Walle, G.R. Culture and characterisation of equine peripheral blood mesenchymal stromal cells. Vet. J. 2013, 195, 107–113. [Google Scholar] [CrossRef]
- Schröck, C.; Eydt, C.; Geburek, F.; Kaiser, L.; Päbst, F.; Burk, J.; Pfarrer, C.; Staszyk, C. Bone marrow-derived multipotent mesenchymal stromal cells from horses after euthanasia. Vet. Med. Sci. 2017, 3, 239–251. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Gayraud-Morel, B.; Chretien, F.; Flamant, P.; Gomes, D.; Zammit, P.S.; Tajbakhsh, S. A role for the myogenic determination gene Myf5 in adult regenerative myogenesis. Dev. Biol. 2007, 312, 13–28. [Google Scholar] [CrossRef]
- Paoletti, C.; Divieto, C.; Chiono, V. Impact of biomaterials on differentiation and reprogramming approaches for the generation of functional cardiomyocytes. Cells 2018, 7, 114. [Google Scholar] [CrossRef]
- McCulley, D.J.; Black, B.L. Transcription factor pathways and congenital heart disease. Curr. Top. Dev. Biol. 2012, 100, 253–277. [Google Scholar] [CrossRef]
- Jumabay, M.; Zhang, R.; Yao, Y.; Goldhaber, J.I.; Bostrom, K.I. Spontaneously beating cardiomyocytes derived from white mature adipocytes. Cardiovasc. Res. 2010, 85, 17–27. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- De Schauwer, C.; Meyer, E.; Van de Walle, G.R.; Van Soom, A. Markers of stemness in equine mesenchymal stem cells: A plea for uniformity. Theriogenology 2011, 75, 1431–1443. [Google Scholar] [CrossRef]
- Jiang, A.; Chen, Y.; Shi, L.; Li, F. Differentiation of brown adipose-derived stem cells into cardiomyocyte-like cells is regulated by a combination of low 5-azacytidine concentration and bone morphogenetic protein 4. Int. J. Clin. Exp. Pathol. 2018, 11, 5514–5524. [Google Scholar]
- Kim, H.R.; Lee, J.; Byeon, J.S.; Gu, N.Y.; Lee, J.; Cho, I.S.; Cha, S.H. Extensive characterization of feline intra-abdominal adipose-derived mesenchymal stem cells. J. Vet. Sci. 2017, 18, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Guercio, A.; Di Bella, S.; Casella, S.; Di Marco, P.; Russo, C.; Piccione, G. Canine mesenchymal stem cells (MSCs): Characterization in relation to donor age and adipose tissue-harvesting site. Cell Biol. Int. 2013, 37, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Carslake, H.B.; Pinchbeck, G.L.; McGowan, C.M. Equine metabolic syndrome in UK native ponies and cobs is highly prevalent with modifiable risk factors. Equine. Vet. J. 2021, 53, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.E.; Frank, N.; McGowan, C.M.; Menzies-Gow, N.J.; Roelfsema, E.; Vervuert, I.; Feige, K.; Fey, K. ECEIM consensus statement on equine metabolic syndrome. J. Vet. Intern. Med. 2019, 33, 335–349. [Google Scholar] [CrossRef]
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of mesenchymal stem cells Isolated from metabolic syndrome and type 2 diabetic patients as result of oxidative stress and autophagy may limit their potential therapeutic use. Stem. Cell Rev. Rep. 2018, 14, 337–345. [Google Scholar] [CrossRef]
- Salehinejad, P.; Alitheen, N.B.; Ali, A.M.; Omar, A.R.; Mohit, M.; Janzamin, E.; Samani, F.S.; Torshizi, Z.; Nematollahi-Mahani, S.N. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton’s jelly. In Vitro Cell Dev. Biol. Anim. 2012, 48, 75–83. [Google Scholar] [CrossRef]
- Calloni, R.; Cordero, E.A.; Henriques, J.A.; Bonatto, D. Reviewing and updating the major molecular markers for stem cells. Stem. Cells Dev. 2013, 22, 1455–1476. [Google Scholar] [CrossRef]
- Später, D.; Hansson, E.M.; Zangi, L.; Chien, K.R. How to make a cardiomyocyte. Development 2014, 141, 4418–4431. [Google Scholar] [CrossRef]
- Esteves, C.L.; Sharma, R.; Dawson, L.; Taylor, S.E.; Pearson, G.; Keen, J.A.; McDonald, K.; Aurich, C.; Donadeu, F.X. Expression of putative markers of pluripotency in equine embryonic and adult tissues. Vet. J. 2014, 202, 533–535. [Google Scholar] [CrossRef]
- Kidder, B.L.; Yang, J.; Palmer, S. Stat3 and c-Myc genome-wide promoter occupancy in embryonic stem cells. PLoS ONE 2008, 3, e3932. [Google Scholar] [CrossRef]
- Ranera, B.; Remacha, A.R.; Álvarez-Arguedas, S.; Romero, A.; Vázquez, F.J.; Zaragoza, P.; Martín-Burriel, I.; Rodellar, C. Effect of hypoxia on equine mesenchymal stem cells derived from bone marrow and adipose tissue. BMC Vet. Res. 2012, 8, 142. [Google Scholar] [CrossRef]
- Kozlowska, U.; Krawczenko, A.; Futoma, K.; Jurek, T.; Rorat, M.; Patrzalek, D.; Klimczak, A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J. Stem. Cells 2019, 11, 347–374. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem. Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Kornicka, K.; Marycz, K.; Maredziak, M.; Tomaszewski, K.A.; Nicpon, J. The effects of the DNA methyltranfserases inhibitor 5-Azacitidine on ageing, oxidative stress and DNA methylation of adipose derived stem cells. J. Cell Mol. Med. 2017, 21, 387–401. [Google Scholar] [CrossRef]
- Kornicka-Garbowska, K.; Pedziwiatr, R.; Wozniak, P.; Kucharczyk, K.; Marycz, K. Microvesicles isolated from 5-azacytidine-and-resveratrol-treated mesenchymal stem cells for the treatment of suspensory ligament injury in horse-a case report. Stem. Cell Res. Ther. 2019, 10, 394. [Google Scholar] [CrossRef]
- Sliwa, A.; Balwierz, A.; Kiec-Wilk, B.; Polus, A.; Knapp, A.; Dembinska-Kiec, A. Differentiation of human adipose tissue SVF cells into cardiomyocytes. Genes Nutr. 2009, 4, 195–198. [Google Scholar] [CrossRef][Green Version]
- Song, K.; Wang, Z.; Li, W.; Zhang, C.; Lim, M.; Liu, T. In vitro culture, determination, and directed differentiation of adult adipose-derived stem cells towards cardiomyocyte-like cells induced by angiotensin II. Appl. Biochem. Biotechnol. 2013, 170, 459–470. [Google Scholar] [CrossRef]
- Antonitsis, P.; Ioannidou-Papagiannaki, E.; Kaidoglou, A.; Papakonstantinou, C. In vitro cardiomyogenic differentiation of adult human bone marrow mesenchymal stem cells. The role of 5-azacytidine. Interact. Cardiovasc. Thorac. Surg. 2007, 6, 593–597. [Google Scholar] [CrossRef]
- Martin-Rendon, E.; Sweeney, D.; Lu, F.; Girdlestone, J.; Navarrete, C.; Watt, S.M. 5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox Sang. 2008, 95, 137–148. [Google Scholar] [CrossRef]
- Van Dijk, A.; Niessen, H.W.; Zandieh Doulabi, B.; Visser, F.C.; van Milligen, F.J. Differentiation of human adipose-derived stem cells towards cardiomyocytes is facilitated by laminin. Cell Tissue Res. 2008, 334, 457–467. [Google Scholar] [CrossRef]
- Soltani, L.; Rahmani, H.R.; Daliri Joupari, M.; Ghaneialvar, H.; Mahdavi, A.H.; Shamsara, M. Ovine fetal mesenchymal stem cell differentiation to cardiomyocytes, effects of co-culture, role of small molecules; reversine and 5-azacytidine. Cell Biochem. Funct. 2016, 34, 250–261. [Google Scholar] [CrossRef]


| Gene | mRNA | Gene ID | Sequence 5′-3′ | Exon No | Intron Length | Product Length |
|---|---|---|---|---|---|---|
| ACTB1 | NM_001081838.1 | 100033878 | For: GCCAACCGCGAGAAGATGAC | 2 | 448 | 124 |
| Rev: AGTCCATCACGATGCCAGTG | 3 | |||||
| GAPDH1 | NM_001163856.1 | 100033897 | For: AAGAAGGTGGTGAAGCAGG | 9 | 86 | 116 |
| Rev: GCATCGAAGGTGGAAGAGTGGG | 10 | |||||
| RN18S1 | NW_019643269.1 | 100861557 | For: ACTCACACGGGAAACCTCAC | 1 | 0 | 122 |
| Rev: AACCAGACAAATCGCTCCAC | 1 | |||||
| MYC2 | XM_001497991.1 | 100068097 | For: CAGCGACTCTGAAGAAGAAC | 1 | 1069 | 241 |
| Rev: ACTGTCCAACTTAGCCCTC | 2 | |||||
| OCT4/ POU5F12 | XM_001490108 | 100050785 | For: AGCAATTTGCCAAGCTCC | 2 | 633 | 235 |
| Rev: GTCTCTGCTTTGCATATCTCC | 3–4 | |||||
| GATA43 | XM_023636259.1 | 100065126 | For: CAGAAAACGGAAGCCAAAGAAC | 4 | 2747 | 218 |
| Rev: ACATCGCACTGACCGAGAAC | 6 | |||||
| NKX2-53 | XM_005614765.3 | 100069632 | For: AAGGACCCTCGAGGCGATAA | 1 | 1508 | 247 |
| Rev: ACCAGATCTTGACCTGCGTG | 2 | |||||
| TNNI33 | NM_001081904.1 | 100034065 | For: TGGATGAGGAGAGATACGATG | 6 | 547 | 101 |
| Rev: CTTAAACTTGCCCCGAAGG | 7 | |||||
| MYH63 | XM_023622391.1 | 111767446 | For: GCGCATCGAGTTCAAGAAG | 18 | 1254 | 188 |
| Rev: TGATACGCCCAAACTCCTCC | 19 | |||||
| MYH73 | NM_001081758 | 791234 | For: TGAGAAGGGCAAAGGCAAG | 15 | 385 | 129 |
| Rev: ATGATGCAACGCACGAAG | 16 | |||||
| MYF64 | NM_001317257.1 | 100050603 | For: CAGCTACAGACCCAAGCAAGA | 1 | 539 | 202 |
| Rev: AGGAGAGTTTGCGTTCCTCC | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trachsel, D.S.; Stage, H.J.; Rausch, S.; Trappe, S.; Söllig, K.; Sponder, G.; Merle, R.; Aschenbach, J.R.; Gehlen, H. Comparison of Sources and Methods for the Isolation of Equine Adipose Tissue-Derived Stromal/Stem Cells and Preliminary Results on Their Reaction to Incubation with 5-Azacytidine. Animals 2022, 12, 2049. https://doi.org/10.3390/ani12162049
Trachsel DS, Stage HJ, Rausch S, Trappe S, Söllig K, Sponder G, Merle R, Aschenbach JR, Gehlen H. Comparison of Sources and Methods for the Isolation of Equine Adipose Tissue-Derived Stromal/Stem Cells and Preliminary Results on Their Reaction to Incubation with 5-Azacytidine. Animals. 2022; 12(16):2049. https://doi.org/10.3390/ani12162049
Chicago/Turabian StyleTrachsel, Dagmar S., Hannah J. Stage, Sebastian Rausch, Susanne Trappe, Katharina Söllig, Gerhard Sponder, Roswitha Merle, Jörg R. Aschenbach, and Heidrun Gehlen. 2022. "Comparison of Sources and Methods for the Isolation of Equine Adipose Tissue-Derived Stromal/Stem Cells and Preliminary Results on Their Reaction to Incubation with 5-Azacytidine" Animals 12, no. 16: 2049. https://doi.org/10.3390/ani12162049
APA StyleTrachsel, D. S., Stage, H. J., Rausch, S., Trappe, S., Söllig, K., Sponder, G., Merle, R., Aschenbach, J. R., & Gehlen, H. (2022). Comparison of Sources and Methods for the Isolation of Equine Adipose Tissue-Derived Stromal/Stem Cells and Preliminary Results on Their Reaction to Incubation with 5-Azacytidine. Animals, 12(16), 2049. https://doi.org/10.3390/ani12162049








