Cartilaginous Intrusion of the Atrioventricular Node in a Quarter Horse with a High Burden of Second-Degree AV Block and Collapse: A Case Report
Abstract
Simple Summary
Abstract
1. Introduction
2. Case Presentation
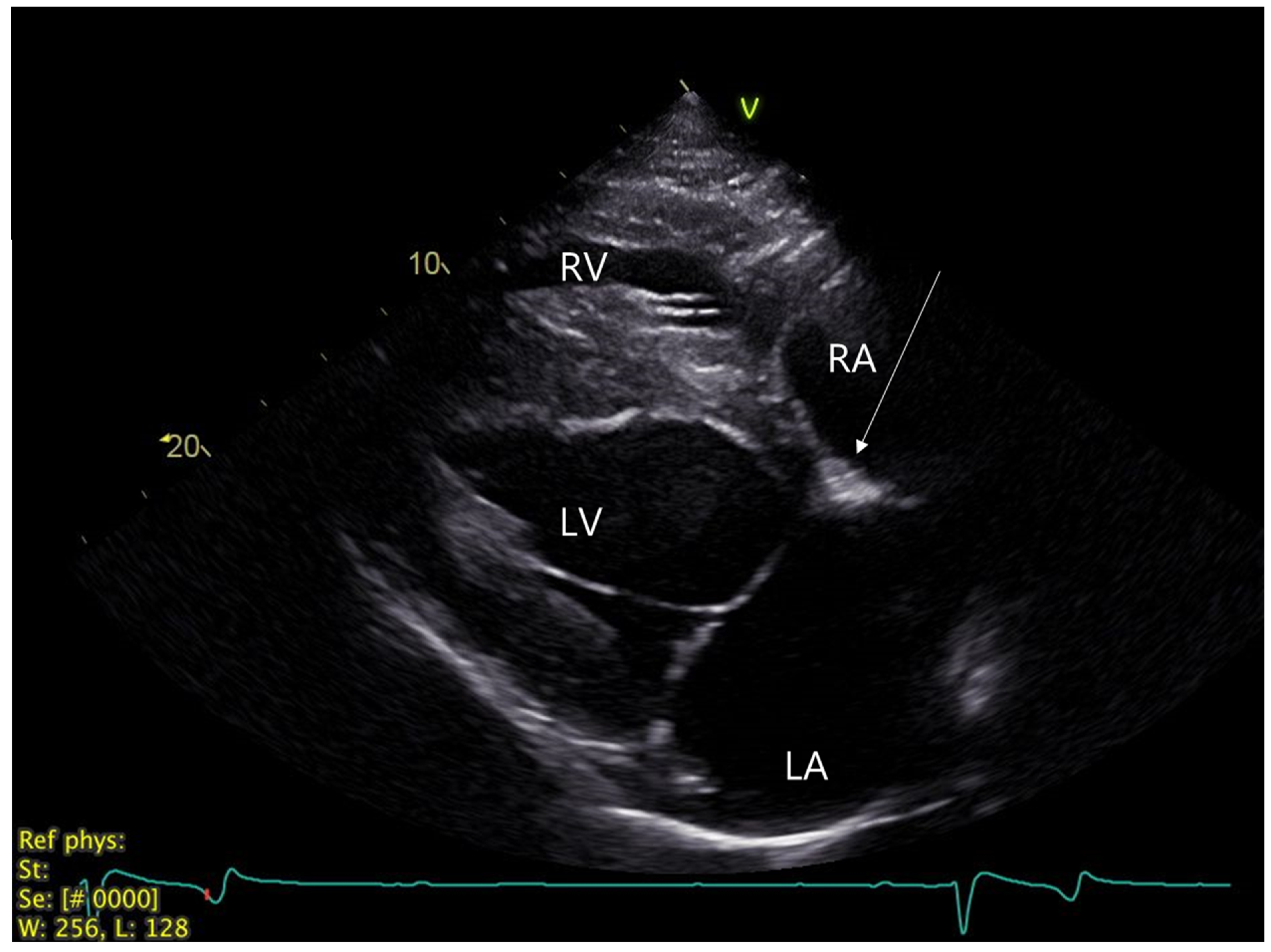
2.1. Clinical Investigation at Referral Center
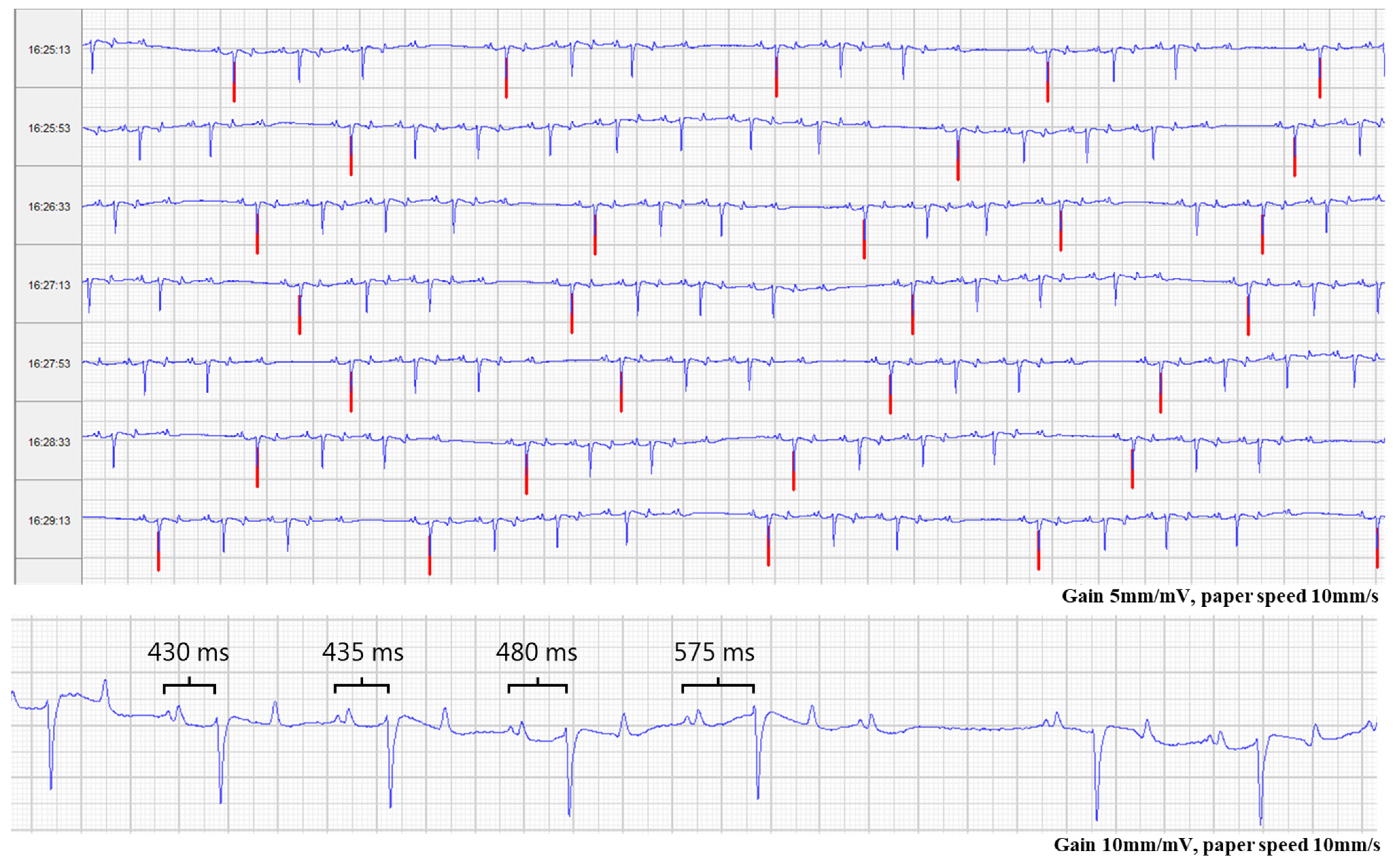
2.2. Monitoring Period after Discharge
2.3. Autonomic Nervous System
2.4. Macroscopic and Microscopic Tissue Investigations
2.5. Histological Results
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raekallio, M. Long term ECG recording with Holter monitoring in clinically healthy horses. Acta Vet. Scand. 1992, 33, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.D.; Weis, R.; Krag-Andersen, E.K.; Hesselkilde, E.M.; Isaksen, J.L.; Carstensen, H.; Kanters, J.K.; Linz, D.; Sanders, P.; Hopster-Iversen, C.; et al. Electrocardiographic characteristics of trained and untrained standardbred racehorses. J. Vet. Intern. Med. 2022, 36, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, G. Cardiac Arrhythmias in Horses. Vet. Clin. N. Am. Equine Pract. 2019, 35, 85–102. [Google Scholar] [CrossRef]
- Marr, C.; Bowen, M. Dysrhythmias: Assessment and medical management. In Cardiology of the Horse, 2nd ed.; Elsevier Health Sciences: Dinburgh, UK, 2011. [Google Scholar]
- Luethy, D.; Slack, J.; Kraus, M.S.; Gelzer, A.R.; Habecker, P.; Johnson, A.L. Third-Degree Atrioventricular Block and Collapse Associated with Eosinophilic Myocarditis in a Horse. J. Vet. Intern. Med. 2017, 31, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Bonagura, J.D.; Miller, M.S. Common conduction disturbances. J. Equine Vet. Sci. 1986, 6, 23–25. [Google Scholar] [CrossRef]
- Lawler, J.B.; Frye, M.A.; Bera, M.M.; Ehrhart, E.J.; Bright, J.M. Third-Degree Atrioventricular Block in a Horse Secondary to Rattlesnake Envenomation. J. Vet. Intern. Med. 2008, 22, 486–490. [Google Scholar] [CrossRef]
- Reef, V.; Marr, C.; Bowen, I. Cardiology of the Horse; Elsevier Health Sciences: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Keen, J.A. Pathological bradyarrhythmia in horses. Vet. J. 2020, 259, 105463. [Google Scholar] [CrossRef]
- Bosnic, L.; Rapic, S. The Adams-Stokes syndrome in partial heart block in a horse. Vet. Arch. 1941, 11, 1–17. [Google Scholar]
- Kashou, A.H.; Goyal, A.; Nguyen, T.; Chhabra, L. Atrioventricular Block. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Eggensperger, B.H.; Schwarzwald, C.C. Influence of 2nd-degree AV blocks, ECG recording length, and recording time on heart rate variability analyses in horses. J. Vet. Cardiol. 2017, 19, 160–174. [Google Scholar] [CrossRef]
- Buhl, R.; Nissen, S.D.; Winther, M.L.K.; Poulsen, S.K.; Hopster-Iversen, C.; Jespersen, T.; Sanders, P.; Carstensen, H.; Hesselkilde, E.M. Implantable loop recorders can detect paroxysmal atrial fibrillation in Standardbred racehorses with intermittent poor performance. Equine Vet. J. 2020, 53, 955–963. [Google Scholar] [CrossRef]
- Mesirca, P.; Nakao, S.; Nissen, S.D.; Forte, G.; Anderson, C.; Trussell, T.; Li, J.; Cox, C.; Zi, M.; Logantha, S.; et al. Intrinsic Electrical Remodeling Underlies Atrioventricular Block in Athletes. Circ. Res. 2021, 129, e1–e20. [Google Scholar] [CrossRef] [PubMed]
- Hamlin, R.L.; Klepinger, W.L.; Gilpin, K.W.; Smith, C.R. Autonomic control of heart rate in the horse. Am. J. Physiol. 1972, 222, 976–978. [Google Scholar] [CrossRef] [PubMed]
- Diab, S.S.; Poppenga, R.; Uzal, F.A. Sudden death in racehorses: Postmortem examination protocol. J. Vet. Diagn. Investig. 2017, 29, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Saljic, A.; Friederike Fenner, M.; Winters, J.; Flethøj, M.; Eggert Eggertsen, C.; Carstensen, H.; Dalgas Nissen, S.; Melis Hesselkilde, E.; van Hunnik, A.; Schotten, U.; et al. Increased fibroblast accumulation in the equine heart following persistent atrial fibrillation. IJC Heart Vasc. 2021, 35, 100842. [Google Scholar] [CrossRef]
- Zipes, D.P. Specific arrhythmias: Diagnosis and treatment. Heart Dis. 1992, 122, 792–797. [Google Scholar]
- Bharati, S.; Lev, M.; Dhingra, R.C.; Chuquimia, R.; Towne, W.D.; Rosen, K.M. Electrophysiologic and pathologic correlations in two cases of chronic second degree atrioventricular block with left bundle branch block. Circulation 1975, 52, 221–229. [Google Scholar] [CrossRef]
- Kaneshige, T.; Machida, N.; Yamamoto, S.; Nakao, S.; Yamane, Y. A Histological Study of the Cardiac Conduction System in Canine Cases of Mitral Valve Endocardiosis with Complete Atrioventricular Block. J. Comp. Pathol. 2007, 136, 120–126. [Google Scholar] [CrossRef]
- Waller, B.F.; Gering, L.E.; Branyas, N.A.; Slack, J.D. Anatomy, histology, and pathology of the cardiac conduction system—Part V. Clin. Cardiol. 1993, 16, 565–569. [Google Scholar] [CrossRef]
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac conduction Delay. J. Am. Coll. Cardiol. 2019, 74, e51–e156. [Google Scholar] [CrossRef]
- Bissett, J.K.; de Soyza, N.D.; Kane, J.J.; Murphy, M.L. Electrophysiology of atropine. Cardiovasc. Res. 1975, 9, 73–80. [Google Scholar] [CrossRef]
- Lyle, C.H.; Turley, G.; Blissitt, K.J.; Pirie, R.S.; Mayhew, I.G.; McGorum, B.C.; Keen, J.A. Retrospective Evaluation of Episodic Collapse in the Horse in a Referred Population: 25 Cases (1995–2009). J. Vet. Intern. Med. 2010, 24, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Calosso, E.; Mascioli, G.; Marangoni, E.; Donato, A.; Rossi, S.; Pala, M.; Foti, F.; Lunati, M. Utility of implantable loop recorder (Reveal Plus®) in the diagnosis of unexplained syncope. EP Eur. 2005, 7, 19–24. [Google Scholar] [CrossRef]
- Reef, V.B.; Bonagura, J.; Buhl, R.; McGurrin, M.K.J.; Schwarzwald, C.C.; van Loon, G.; Young, L.E. Recommendations for Management of Equine Athletes with Cardiovascular Abnormalities. J. Vet. Intern. Med. 2014, 28, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Dukes, H.H. A case of heart block in a horse. Cornell Vet. 1940, 30, 248–251. [Google Scholar]
- Bishop, S.P.; Cole, C.R. Morphology of the specialized conducting tissue in the atria of the equine heart. Anat. Rec. 1967, 158, 401–415. [Google Scholar] [CrossRef]
- Gómez-Torres, F.A.; Ballesteros-Acuña, L.E.; Ruíz-Sauri, A. Histological and morphometric study of the components of the sinus and atrioventricular nodes in horses and dogs. Res. Vet. Sci. 2019, 126, 22–28. [Google Scholar] [CrossRef]
- James, T.N. Structure and function of the sinus node, AV node and His bundle of the human heart: Part I-structure. Prog. Cardiovasc. Dis. 2002, 45, 235–267. [Google Scholar] [CrossRef]
- Michaëlsson, M.; Ho, S.Y. Congenital Heart Malformations in Mammals; Imperial College Press: London, UK, 2000; pp. 14–17. [Google Scholar] [CrossRef]
- McDonnell, K.M.W.; Shepard, R.K. Conduction Disorders after Transcatheter Aortic Valve Implantation: A Focused Review. Curr. Treat. Options Cardiovasc. Med. 2013, 15, 488–496. [Google Scholar] [CrossRef]
- Young Lee, M.; Chilakamarri Yeshwant, S.; Chava, S.; Lawrence Lustgarten, D. Mechanisms of Heart Block after Transcatheter Aortic Valve Replacement—Cardiac Anatomy, Clinical Predictors and Mechanical Factors that Contribute to Permanent Pacemaker Implantation. Arrhythm. Electrophysiol. Rev. 2015, 4, 81–85. [Google Scholar] [CrossRef]
- Kammler, J.; Blessberger, H.; Fellner, F.; Kypta, A.; Lambert, T.; Engl, M.; Hönig, S.; Lichtenauer, M.; Grund, M.; Kerschner, K.; et al. Implantation depth measured by 64-slice computed tomography is associated with permanent pacemaker requirement following transcatheter aortic valve implantation with the Core Valve® system. J. Cardiol. 2016, 67, 513–518. [Google Scholar] [CrossRef][Green Version]
- Reef, V.B.; Clark, E.S.; Oliver, J.A.; Donawick, W.J. Implantation of a permanent transvenous pacing catheter in a horse with complete heart block and syncope. J. Am. Vet. Med. Assoc. 1986, 189, 449–452. [Google Scholar] [PubMed]
- van Loon, G.; Fonteyne, W.; Rottiers, H.; Tavernier, R.; Deprez, P. Implantation of a dual-chamber, rate-adaptive pacemaker in a horse with suspected sick sinus syndrome. Vet. Rec. 2002, 151, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Saadi, M.; Tagliari, A.P.; Danzmann, L.C.; Bartholomay, E.; Kochi, A.N.; Saadi, E.K. Update in Heart Rhythm Abnormalities and Indications for Pacemaker After Transcatheter Aortic Valve Implantation. Braz. J. Cardiovasc. Surg. 2018, 33, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Decloedt, A. Cardiac arrhythmias as a potential sign of systemic disease: Which laboratory tests are useful? Equine Vet. Educ. 2022, 34, 347–350. [Google Scholar] [CrossRef]
- Van Steenkiste, G.; van Loon, G.; Decloedt, A.; Crevecoeur, G.; Delhaas, T. Equine Electrocardiography Revisited: 12-Lead Recording, Vectorcardiography and the Power of Machine Intelligence. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2020. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nissen, S.D.; Saljic, A.; Kjeldsen, S.T.; Jespersen, T.; Hopster-Iversen, C.; Buhl, R. Cartilaginous Intrusion of the Atrioventricular Node in a Quarter Horse with a High Burden of Second-Degree AV Block and Collapse: A Case Report. Animals 2022, 12, 2915. https://doi.org/10.3390/ani12212915
Nissen SD, Saljic A, Kjeldsen ST, Jespersen T, Hopster-Iversen C, Buhl R. Cartilaginous Intrusion of the Atrioventricular Node in a Quarter Horse with a High Burden of Second-Degree AV Block and Collapse: A Case Report. Animals. 2022; 12(21):2915. https://doi.org/10.3390/ani12212915
Chicago/Turabian StyleNissen, Sarah Dalgas, Arnela Saljic, Sofie Troest Kjeldsen, Thomas Jespersen, Charlotte Hopster-Iversen, and Rikke Buhl. 2022. "Cartilaginous Intrusion of the Atrioventricular Node in a Quarter Horse with a High Burden of Second-Degree AV Block and Collapse: A Case Report" Animals 12, no. 21: 2915. https://doi.org/10.3390/ani12212915
APA StyleNissen, S. D., Saljic, A., Kjeldsen, S. T., Jespersen, T., Hopster-Iversen, C., & Buhl, R. (2022). Cartilaginous Intrusion of the Atrioventricular Node in a Quarter Horse with a High Burden of Second-Degree AV Block and Collapse: A Case Report. Animals, 12(21), 2915. https://doi.org/10.3390/ani12212915





