Four Mx Genes Identified in Andrias davidianus and Characterization of Their Response to Chinese Giant Salamander Iridovirus Infection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. RNA Isolation and Gene Cloning
2.3. Bioinformatics Analysis
2.4. Tissue Distribution of the adMx Gene
2.5. Virus Infection and the adMx Expression in Spleen
2.6. Statistical Analysis
3. Results
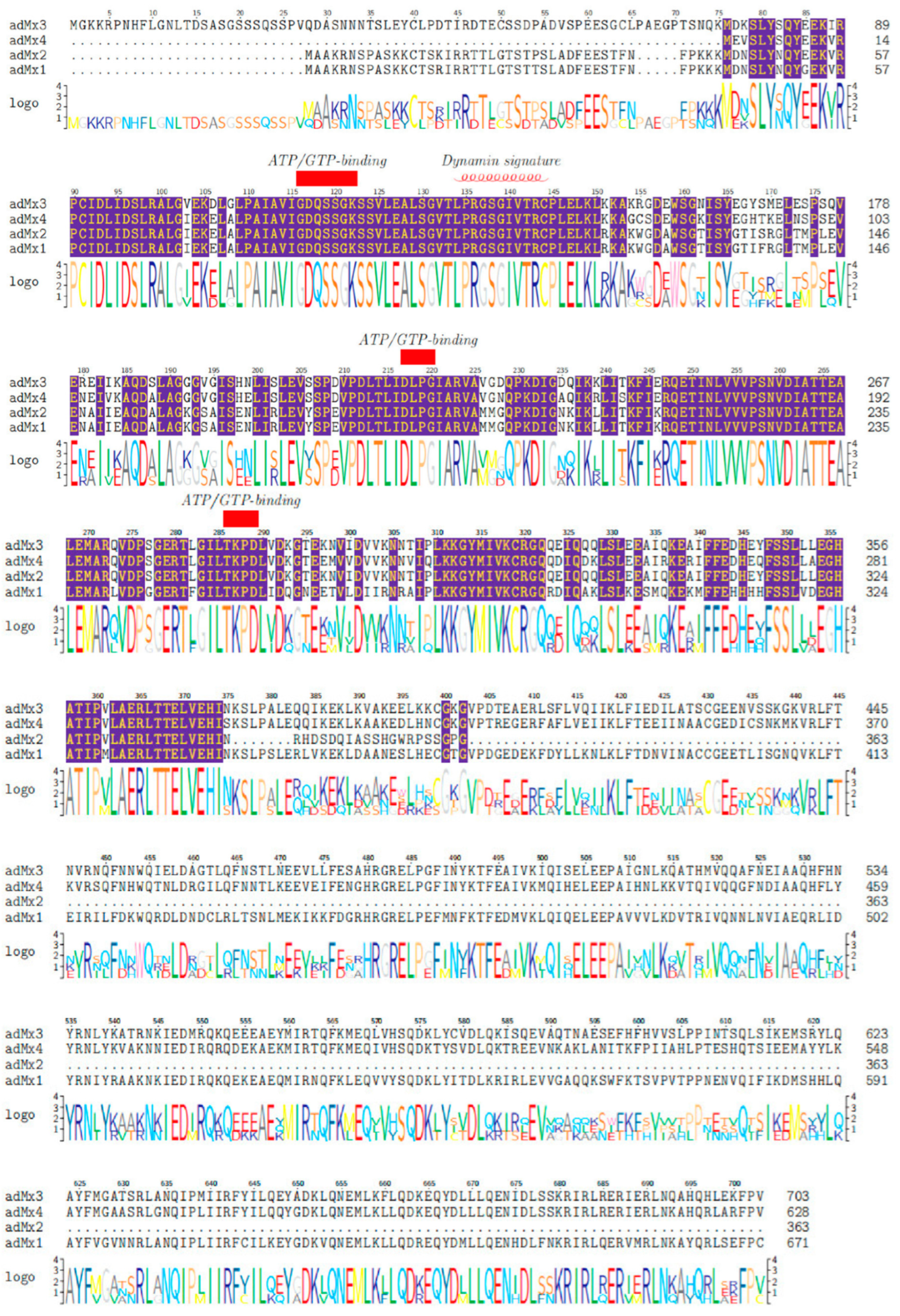
3.1. Sequence and Domain Architecture Analysis
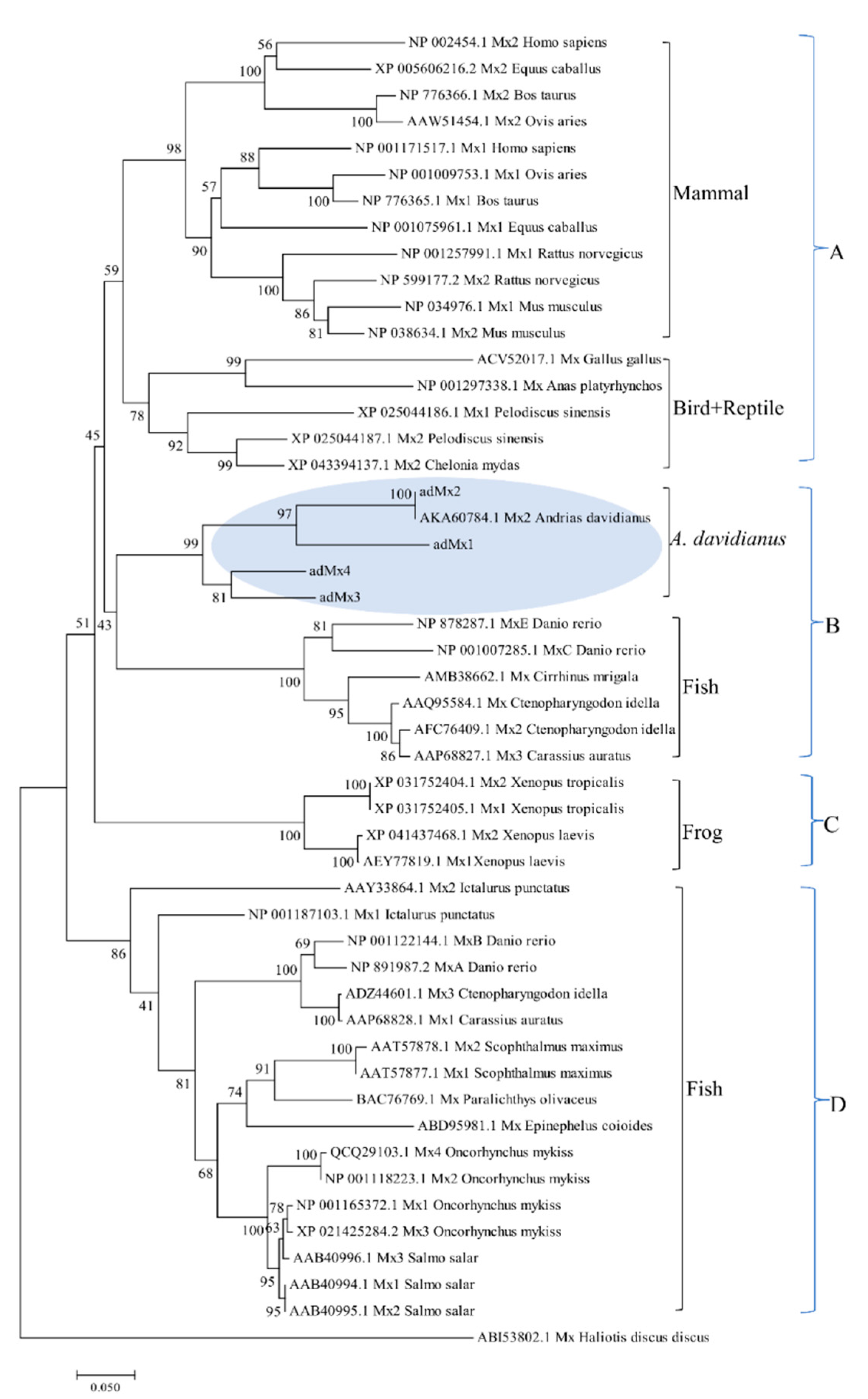
3.2. Phylogenetic Analysis
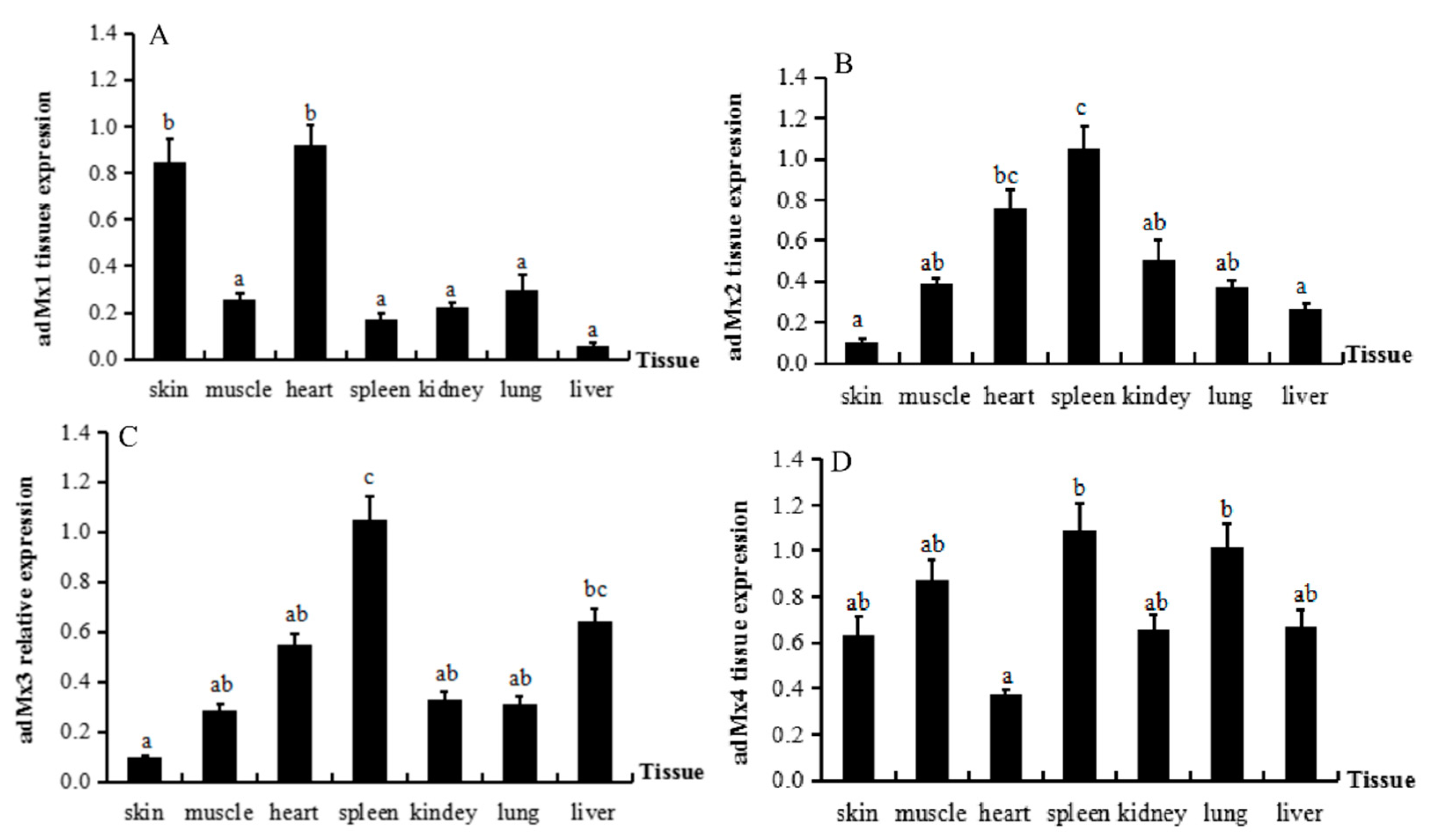
3.3. Expression of adMx Genes in Normal Tissues
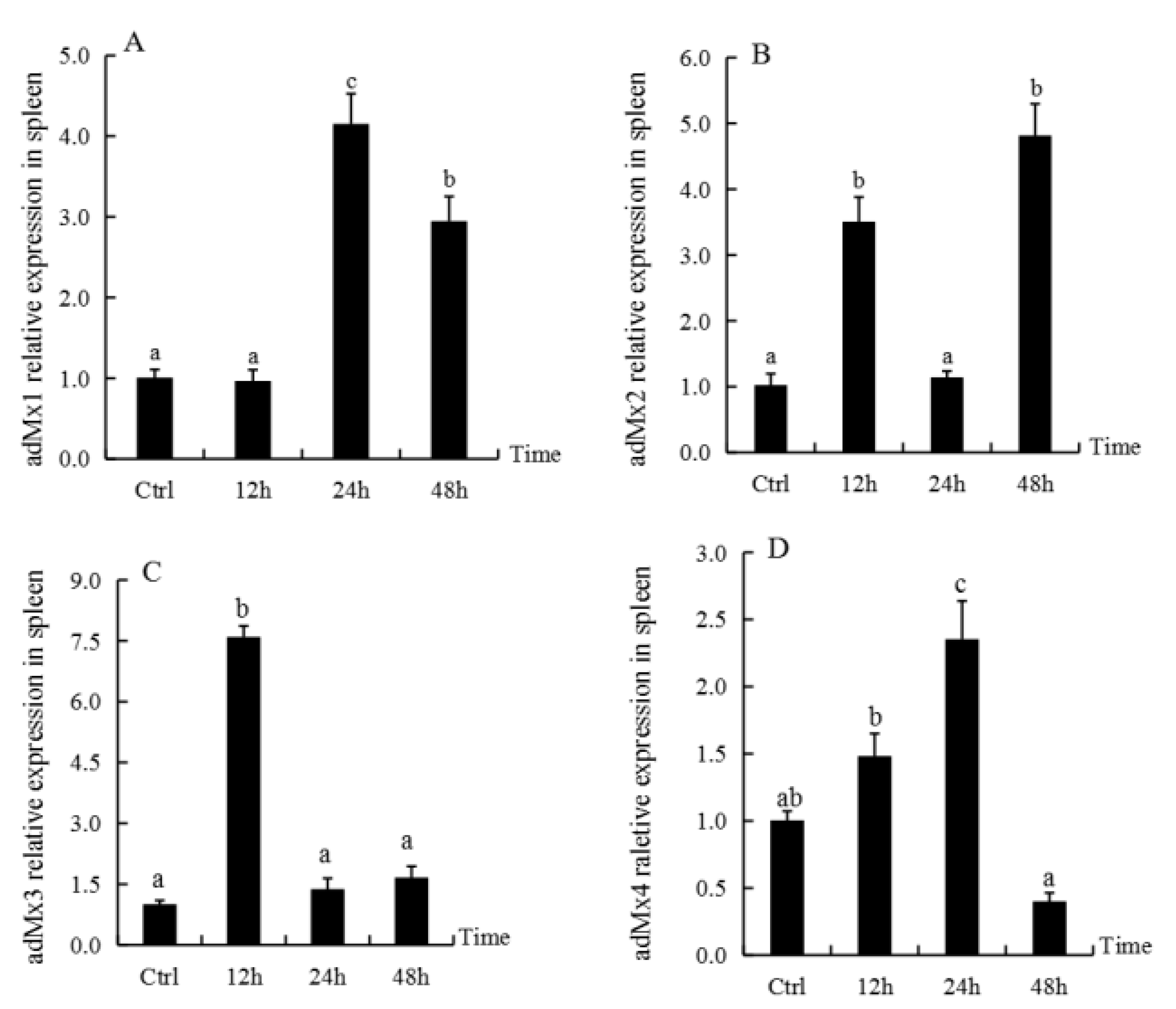
3.4. Expression of adMx Genes in the Spleen Following Treatment with GSIV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mesev, E.V.; LeDesma, R.A. Decoding type I and III interferon signaling during viral infection. Nat. Microbiol. 2019, 4, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Katze, M.G.; He, Y.P. Viruses and interferon: A fight for supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Malsburg, V.D.A. Structure of myxovirus resistance protein a reveals intra- and intermolecular domain interactions required for the antiviral function. Immunity 2011, 35, 514–525. [Google Scholar] [CrossRef]
- Roy, P.; Rout, A.K. Molecular characterization, constitutive expression and GTP binding mechanism of Cirrhinus mrigala (Hamilton, 1822) Myxovirus resistance (Mx) protein. Int. J. Biol. Macromol. 2019, 136, 1258–1272. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.R.; Yang, A.R. Birth and death of Mx genes and the presence/absence of genes regulating Mx transcription are correlated with the diversity of antipathogenicity in vertebrate species. Mol. Genet. Genom. 2019, 294, 121–133. [Google Scholar] [CrossRef]
- Kojima, T.; Oshima, K. The bovine Mx1 gene: Characterization of the gene structure, alternative splicing, and promoter region. Biochem. Genet. 2003, 41, 375–390. [Google Scholar] [CrossRef]
- Berlin, S.; Qu, L. Positive diversifying selection in avian Mx genes. Immunogenetics 2008, 60, 689. [Google Scholar] [CrossRef]
- Lee, S.H.; Vidal, S.M. Functional diversity of Mx proteins: Variations on a theme of host resistance to infection. Genome Res. 2002, 12, 527–530. [Google Scholar] [CrossRef]
- Novel, P.; Fernández-Trujilloa, M.A. Two Mx genes identified in European sea bass (Dicentrarchus labrax) respond differently to VNNV infection. Vet. Immunol. 2013, 153, 240–248. [Google Scholar] [CrossRef]
- Fernández-Trujillo, M.A.; García-Rosado, E. Antiviral activity of Mx proteins from gilthead seabream (Sparus aurata) against lymphocystis disease virus (LCDV). Fish Shellfish Immunol. 2013, 34, 1651. [Google Scholar] [CrossRef][Green Version]
- Solbakken, M.H.; Rise, M.L. Successive losses of central immune genes characterize the gadiformes’ alternate immunity. Genome Biol. Evol. 2016, 8, 3508–3515. [Google Scholar] [CrossRef] [PubMed]
- Bϕrre, R.; Linn, G.T. Analysis of the Atlantic salmon genome reveals a cluster of Mx genes that respond more strongly to IFN gamma than to type I IFN. Dev. Comp. Immunol. 2019, 90, 80–89. [Google Scholar]
- Wang, T.Y.; Liu, F.G. Lineage/species-specific expansion of the Mx gene family in teleosts: Differential expression and modulation of nine Mx genes in rainbow trout Oncorhynchus mykiss. Fish Shellfish Immunol. 2019, 90, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Torres, D.; Béjar, J. Structural and functional characterization of the Senegalese sole (Solea senegalensis) Mx promoter. Fish Shellfish Immunol. 2013, 35, 1642–1648. [Google Scholar] [CrossRef]
- Caipang, C.M.; Hirono, I. In vitro inhibition of fish rhabdoviruses by Japanese flounder, Paralichthys olivaceus Mx. Virology 2003, 317, 373–382. [Google Scholar] [CrossRef]
- Haller, O.; Heinz, A. The discovery of the antiviral resistance gene Mx: A story of great ideas, great failures, and some success. Annu. Rev. Virol. 2018, 5, 33–51. [Google Scholar] [CrossRef]
- Larsen, R.; Røkenes, T.P. Inhibition of infectious pancreatic necrosis virus replication by Atlantic salmon Mx1 protein. J. Virol. 2004, 78, 7938–7944. [Google Scholar] [CrossRef]
- Lin, C.H.; Christopher John, J.A. Inhibition of nervous necrosis virus propagation by fish Mx proteins. Biochem. Biophys. Res. Commun. 2006, 351, 534–539. [Google Scholar] [CrossRef]
- Zeng, M.; Chen, S. Molecular identification and comparative transcriptional analysis of myxovirus resistance GTPase (Mx) gene in goose (Anser cygnoide) after H9N2 AIV infection. Comp. Immunol. Microbiol. 2016, 47, 32–40. [Google Scholar] [CrossRef]
- Staeheli, P.; Haller, O. Mx protein: Constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to Influenza virus. Cell 1986, 44, 147–158. [Google Scholar] [CrossRef]
- Netherton, C.L.; Simpson, J. Inhibition of a large Double-Stranded DNA virus by MxA Protein. J. Virol. 2009, 83, 2310–2320. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Niklasch, M. Interferon-inducible MX2 is a host restriction factor of hepatitis B virus replication. J. Hepatol. 2020, 72, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Forzán, M.J.; Heatley, J.; Russell, K.E.; Horney, B. Clinical pathology of amphibians: A review. Vet. Clin. Pathol. 2017, 46, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Gui, J.F. Genome architecture changes and major gene variations of Andrias davidianus ranavirus (ADRV). Vet. Res. 2013, 44, 101. [Google Scholar] [CrossRef]
- Dong, W.Z.; Zhang, M. Iridovirus outbreak in Chinese giant salamanders, China, 2010. Emerg. Infect. Dis. 2010, 17, 2388–2389. [Google Scholar] [CrossRef]
- Meng, Y.; Ma, J. Pathological and microbiological findings from mortality of the Chinese giant salamander (Andrias davidianus). Arch. Virol. 2014, 159, 1403–1412. [Google Scholar] [CrossRef]
- Fan, Y.; Chang, M.X. Transcriptomic analysis of the host response to an iridovirus infection in Chinese giant salamander, Andrias davidianus. Vet. Res. 2015, 46, 126–156. [Google Scholar] [CrossRef]
- Cui, D.; Lan, Q.J. Stability evaluation of reference genes in tissues of Andrias davidianus at different development stages. J. Northwest AF Univ. (Nat. Sci. Ed.) 2017, 45, 1–6. [Google Scholar]
- Sirisena, D.M.K.P.; Tharuka Neranjan, M.D. An interferon-induced GTP-binding protein, Mx, from the redlip mullet, Liza haematocheila: Deciphering its structural features and immune function. Fish Shellfish Immunol. 2020, 96, 279–289. [Google Scholar] [CrossRef]
- Liu, Y.N.; Li, Y.Q. Characterization, expression pattern and antiviral activities of Mx gene in Chinese Giant Salamander, Andrias davidianus. Int. J. Mol. Sci. 2020, 21, 2246. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, J. Identification of Type I IFN in Chinese giant salamander (Andrias davidianus) and the response to an iridovirus infection. Mol. Immunol. 2015, 65, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.; Graf, L. Role of nucleotide binding and GTPase domain dimerization in dynamin-like myxovirus resistance protein a for GTPase activation and antiviral activity. J. Biol. Chem. 2015, 290, 12779–12792. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Staeheli, P. Mx GTPases: Dynamin-like antiviral machines of innate immunity. Trends Microbiol. 2015, 23, 154–163. [Google Scholar] [CrossRef]
- Verhelst, J.; Hulpiau, P. Mx proteins: Antiviral gatekeepers that restrain the uninvited. Microbiol. Mol. Biol. Rev. 2013, 77, 551–566. [Google Scholar] [CrossRef]
- Ramachandran, R.; Schmid, S.L. The dynamin superfamily. Curr. Biol. 2018, 28, 367–420. [Google Scholar] [CrossRef]
- Collet, B. Innate immune responses of salmonid fish to viral infections. Dev. Comp. Immunol. 2014, 43, 160–173. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Wluka, A.; Olszewski, W.L. Innate and adaptive processes in the spleen. Ann. Transplant. 2006, 11, 22–29. [Google Scholar] [PubMed]
- Grayfer, L.; Andino, F.D.J. The amphibian (Xenopus laevis) type I interferon response to Frog Virus 3: New insight into ranavirus pathogenicity. J. Virol. 2014, 88, 5766–5777. [Google Scholar] [CrossRef]
| Primer | Sequence (5′–3′) | Purpose |
|---|---|---|
| Mx1 3P | TGGAGGTTGTTGGAGCACAACAGAA | 3′-RACE |
| Mx2 3P | TTGTGGTGCCAAGTAATGTGGATAT | |
| Mx3 3P | GGTTGCTCAAACAAATGCAGAGTCTGAA | |
| Mx4 3P | GCAGCCTGTGGAGAAGACATTTGTTCTAA | |
| Mx1 5P | TCTCTCCGCCGGGATCCACCAGTCG | 5′-RACE |
| Mx2 5P | GCAATCCCAGGAAGGTCAATCAGTG | |
| Mx3 5P | CCACATTTCTTTAGTTCCTCTTTCGCTAC | |
| Mx4 5P | ACAAATGTCTTCTCCACAGGCTGCGTT | |
| Mx1 qF | ATCCCGCTGAAGAAGGGTTAC | qPCR |
| Mx1 qR | CGTTTGCTGCGTCAAGTTTCT | |
| Mx2 qF | TTCCCAGAGGCAGTGGTATTG | |
| Mx2 qR | ACTTCTAAGGGCATTGTCAGG | |
| Mx3 qF | CGAGGAGATGAATGGAGTGGG | |
| Mx3 qR | CCACTCCTCCACCAGCCAGA | |
| Mx4 qF | AGAGGCACTAGAAATGGCACG | |
| Mx4 qR | TGTCCTGGATGTCCTGTTGC | |
| EF1-αF | GGACAGACCCGTGAACATGC | Internal reference |
| EF1-αR | CTTCCTTAGTGATCTCCTCGTAGC |
| Gene | Length (bp) | 5′-UTR (bp) | ORF (bp) | 3′-UTR (bp) | Amino Acids (AA) | MW (Daltons) | pI |
|---|---|---|---|---|---|---|---|
| adMx1 | 2808 | 1–54 | 55–2070 | 2071–2808 | 671 | 76,688.82 | 8.09 |
| adMx2 | 2635 | 1–53 | 54–1145 | 1146–2635 | 363 | 39,786.77 | 6.53 |
| adMx3 | 2840 | 1–124 | 125–2236 | 2237–2840 | 703 | 79,105.06 | 5.23 |
| adMx4 | 2008 | 1–38 | 39–1925 | 1926–2008 | 628 | 71,301.06 | 6.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Fan, Y.; Jiang, N.; Xue, M.; Li, Y.; Liu, W.; Zeng, L.; Zhou, Y. Four Mx Genes Identified in Andrias davidianus and Characterization of Their Response to Chinese Giant Salamander Iridovirus Infection. Animals 2022, 12, 2147. https://doi.org/10.3390/ani12162147
Meng Y, Fan Y, Jiang N, Xue M, Li Y, Liu W, Zeng L, Zhou Y. Four Mx Genes Identified in Andrias davidianus and Characterization of Their Response to Chinese Giant Salamander Iridovirus Infection. Animals. 2022; 12(16):2147. https://doi.org/10.3390/ani12162147
Chicago/Turabian StyleMeng, Yan, Yuding Fan, Nan Jiang, Mingyang Xue, Yiqun Li, Wenzhi Liu, Lingbing Zeng, and Yong Zhou. 2022. "Four Mx Genes Identified in Andrias davidianus and Characterization of Their Response to Chinese Giant Salamander Iridovirus Infection" Animals 12, no. 16: 2147. https://doi.org/10.3390/ani12162147
APA StyleMeng, Y., Fan, Y., Jiang, N., Xue, M., Li, Y., Liu, W., Zeng, L., & Zhou, Y. (2022). Four Mx Genes Identified in Andrias davidianus and Characterization of Their Response to Chinese Giant Salamander Iridovirus Infection. Animals, 12(16), 2147. https://doi.org/10.3390/ani12162147







