Simple Summary
Escherichia coli is a bacterium which infects cow udders causing clinical mastitis, a potentially severe disease with welfare and economic consequences. During an infection, white blood cells (leukocytes) enter the udder to provide immune defence and assist tissue repair. We sequenced RNA derived from circulating leukocytes to investigate which genes are up- or down-regulated in dairy cows with naturally occurring cases of clinical mastitis in comparison with healthy control cows from the same farm. We also looked for genetic variations between infected and healthy cows. Blood samples were taken either EARLY (around 10 days) or LATE (after 4 weeks) during the recovery phase after diagnosis. Many genes (1090) with immune and inflammatory functions were up-regulated during the EARLY phase. By the LATE phase only 29 genes were up-regulated including six haemoglobin subunits, possibly important for the production of new red blood corpuscles. Twelve genetic variations which were associated with an increased or decreased expression of some important immune genes were identified between the infected and control cows. These results show that the initial inflammatory response to E. coli continued for at least 10 days despite the cows having received prompt veterinary treatment, but they had largely recovered within 4 weeks. Genetic differences between cows may predispose some animals to infection.
Abstract
The risk and severity of clinical infection with Escherichia coli as a causative pathogen for bovine mastitis is influenced by the hosts’ phenotypic and genotypic variables. We used RNA-Seq analysis of circulating leukocytes to investigate global transcriptomic profiles and genetic variants from Holstein cows with naturally occurring cases of clinical mastitis, diagnosed using clinical symptoms and milk microbiology. Healthy lactation-matched cows served as controls (CONT, n = 6). Blood samples were collected at two time periods during the recovery phase post diagnosis: EARLY (10.3 ± 1.8 days, n = 6) and LATE (46.7 ± 11 days, n = 3). Differentially expressed genes (DEGs) between the groups were identified using CLC Genomics Workbench V21 and subjected to enrichment analysis. Variant calling was performed following GATKv3.8 best practice. The comparison of E. coli(+) EARLY and CONT cows found the up-regulation of 1090 DEGs, mainly with immune and inflammatory functions. The key signalling pathways involved NOD-like and interleukin-1 receptors and chemokines. Many up-regulated DEGs encoded antimicrobial peptides including cathelicidins, beta-defensins, S100 calcium binding proteins, haptoglobin and lactoferrin. Inflammation had largely resolved in the E. coli(+) LATE group, with only 29 up-regulated DEGs. Both EARLY and LATE cows had up-regulated DEGs encoding ATP binding cassette (ABC) transporters and haemoglobin subunits were also up-regulated in LATE cows. Twelve candidate genetic variants were identified in DEGs between the infected and CONT cows. Three were in contiguous genes WIPI1, ARSG and SLC16A6 on BTA19. Two others (RAC2 and ARHGAP26) encode a Rho-family GTPase and Rho GTPase-activating protein 26. These results show that the initial inflammatory response to E. coli continued for at least 10 days despite prompt treatment and provide preliminary evidence for genetic differences between cows that may predispose them to infection.
Keywords:
E. coli mastitis; cow; mammary gland; antimicrobial peptides; ABC transporters; MHC system 1. Introduction
Mastitis is an inflammatory condition of the mammary gland which causes significant economic losses due to the cost of treatment, reduced milk production, discarding milk and the death or culling of infected cows [1]. Escherichia coli (E. coli) infection is generally associated with the rapid onset of acute mastitis, sometimes with severe systemic clinical symptoms including pyrexia, diarrhoea and dehydration, which have an adverse effect on animal welfare and may cause mortality [2]. The bacterial strain, cow genotype and physiological state and the farm environment can all interdependently affect mastitis susceptibility [3,4]. Studies have shown that the severity of E. coli mastitis is predominantly determined by cow factors rather than by E. coli pathogenicity [5,6], although some E. coli strains are better able to invade and replicate within mammary epithelial cells and can therefore cause a persistent intramammary infection [7].
The cell wall component lipopolysaccharide (LPS) is the main pathogen-associated molecular pattern (PAMP) for E. coli [6]. The LPS released within the mammary gland is recognised by the LPS receptor complex composed of the LPS-binding protein, Toll-like receptor (TLR) 4, myeloid differentiation protein 2 (MD-2) and cluster of differentiation (CD) 14. This recognition leads to the cascade activation of NFKB and other transcription factor pathways which induce a rapid and strong rise in the expression of various pro-inflammatory genes encoding cytokines, chemokines, prostaglandins and adhesion molecules which activate the cells of the innate and adaptive immune systems [8,9,10,11]. These signalling pathways also promote the production of oxygen and nitric oxide radicals and anti-inflammatory cytokines such as IL-10 and TGFß [9]. The initial response to LPS leads to the recruitment of circulating leukocytes, especially neutrophils, to the inflamed mammary gland where they play crucial roles in the initiation, development and resolution of mastitis [12]. In order for the individual cow to effectively deal with E. coli mastitis, the movement of leukocytes into the mammary gland must occur in a timely fashion and be properly controlled [13]. A mild response may fail to achieve pathogen elimination whereas an excessive and prolonged response is more likely to cause additional immune-response-induced tissue damage [14].
The majority of previous studies using transcriptomic profiles to investigate the response to mastitis-causing infections have analysed mammary gland biopsies or leukocytes collected from cows in which the infection was experimentally induced by intra-mammary inoculation with E. coli [6,15,16,17] or LPS [18]. Much less information is available concerning naturally occurring clinical cases. In the present study, we investigated changes in global transcriptomic profiles of circulating leukocyte in mid-lactation cows on a single farm experiencing clinical mastitis caused by E. coli using next-generation RNA sequencing and bioinformatics approaches. RNA-Seq-derived variants were also compared between the infected individuals and healthy control cows. The aims were firstly to understand more about how naturally infected cows continue to respond to the infection during the resolution phase and secondly to investigate whether there were any genetic differences between animals which are more or less susceptible to infection.
2. Materials and Methods
2.1. Animals
Suitable Holstein cows were recruited from a single 800 cow commercial dairy farm located in Mecklenburg-Pomerania, Germany, with an average 305 d milk yield exceeding 10,000 kg. All recruited cows were sampled in a single three-month summer period from May to July. All procedures were approved by the Animal Welfare Committee of Mecklenburg-Pomerania/Germany (LALLF 7221.3-18196-22-03). Ten cows with suspected cases of E. coli mastitis were examined by the attending veterinarian. One cow had no clinical symptoms and so was excluded from the study. Milk samples were taken before medical treatment and quick on-farm microbiological tests were performed. Additional milk samples were taken for submission to an approved Central Laboratory for microbiological testing (see below). All cows with severe symptoms (n = 8) were locally and systemically treated immediately with an aminoglykoside antibiotic and Metacam®, a nonsteroidal anti-inflammatory drug (NSAID) (Boehringer Ingelheim, Ingelheim am Rhein, Germany). In a few cases (n = 3), water drenching and stimulation of the rumen were also essential. Refer to Supplementary Table S1 for further cow details. Nine control E. Coli(−) cows (CONT) were recruited from the same farm and were matched as closely as possible with respect to lactation number, date and days in milk. The controls were therefore exposed to the same environmental conditions as the E. coli(+) cows. Milk samples were also taken from the control cows to exclude sub-clinical mastitis. The microbiological tests showed that three of them were infected with Streptococci or other bacteria. These cows were therefore excluded from analysis of the differential gene expression and the in vitro tests.
Blood samples were taken during a routine veterinary examination performed at a median time of 15 days (range 6–65 days) after the initial diagnosis. Heparinised fresh blood (15 mL) from each cow was collected by jugular venepuncture into a Falcon tube for in vitro testing and a second blood sample was taken into a Tempus blood collection tube (Thermo Fischer Scientific, Loughborough, UK), which was shaken vigorously for 15–20 s immediately upon collection, then frozen and stored at −80 °C for subsequent RNA extraction.
2.2. Microbiological Analysis
All milk samples were sent immediately to an approved Central Diagnostic Laboratory in Mecklenburg–Pomerania, Germany, for analysis by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MS), an approved routine method to identify specific microorganisms. Firstly, samples were inoculated in blood agar (BA; Caseinpepton-soya flour pepton-agar enriched with 5% blood from sheep), using a sterile swab. After inoculation, the BA plates were incubated at 37 °C for 48 h. Afterwards, colonies of the samples were selected for microbiological identification by MS. For protein extraction, a colony was selected from each isolate and applied to a steel plate containing 96 wells, prepared for identification by the MALDITOF Biotyper (MSP 96 Target polished steel, Bruker Daltonik GmbH, Bremen, Germany).
2.3. In Vitro Blood Tests
The fresh blood samples were taken to the laboratory at the Research Institute for Farm Animal Biology, layered onto Histopaque (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) and centrifuged at 1200× g for 30 min after which the opaque layer of peripheral blood mononuclear cells (PBMCs) was aspirated from the plasma/histopaque interface. These were washed ×3 in phosphate-buffered saline, counted and re-suspended in medium (RPMI 1640 with 10% FBS, 1% Glutamax, 1% pen/strep (10,000 U/mL penicillin, 10,000 µg/mL streptomycin)) to a concentration of 1 × 106 cells/mL. Aliquots of 1 × 105 cells were then seeded in triplicate into 96 well plates which were previously prepared with 90 µL/well of growth medium. After settling for 1 h, cells were stimulated with 500 ng/mL lipopolysaccharide (LPS from E. coli O111:B4, γ-irradiated, BioXtra, suitable for cell culture, L4391-1MG, Sigma-Aldrich Chemie GmbH) for 2 h and incubated at 37 °C and 5% CO2. At the end of the culture, the medium was transferred to another 96-well plate and frozen (−20 °C). The spent culture medium and the Tempus tubes containing blood samples were then shipped to the Royal Veterinary College (London, UK) frozen on dry ice for subsequent analysis.
2.4. Measurement of IL-1B and Nitric Oxide
Concentrations of IL-1B were measured in spent medium using an ELISA kit specific for boIL-1ß (Thermo Scientific, Rockford, IL, USA) as previously described [19]. The determination of nitric oxide (NO) concentration in medium was carried out using Griess reagents as previously described [19]. Briefly, a two-fold standard curve of 128 µM sodium nitrite in 2% FCS MØ media and sample supernatants were placed in a flat 96-well clear plate and mixed with equal volumes of Griess reagent (1:1 solutions A and B). After 10 min incubation, absorbance was analysed at 550 nm using a spectrophotometer (Spectramax M2, Molecular Devices, Wokingham, UK).
2.5. RNA Extraction
RNA was extracted from the whole blood samples using Tempus spin kits (Thermo Fisher Scientific, Hemel Hempstead, UK) following the supplied protocol. RNA quantity and integrity were assessed using an Agilent BioAnalyzer 2000 (Agilent, Milton Keynes, UK) and Agilent RNA 6000 Nano Kit (Agilent, UK). RNA measurements were also validated using a NanoDrop 1000 (Thermo Fischer Scientific, UK). All extracted RNA samples had a good integrity (RIN number >9.3) with concentrations between 40 and 163 ng/µL. They were kept at 80 °C for subsequent RNA sequencing. Quality control data are provided in Supplementary Table S2.
2.6. RNA-Sequencing, Mapping and Quantification
The extracted whole blood RNA samples were sent to Novogene Company Ltd. (Hong Kong, China) for RNA sequencing. After removing rRNA using a Globin-zero Gold rRNA removal kit (Illumina, San Diego, CA, USA), 400 ng total RNA was used for the preparation of RNA-Seq libraries with 250–300 bp insert strand specific library. The cDNA libraries were pooled and sequenced on Illumina NovaSeq platform with paired-end 150 bp sequencing (PE150) to reach over 30 million reads per sample. RNA-Seq analysis was carried out using a CLC Genomics Workbench V21 (Qiagen Digital Insights, Redwood City, CA 94063, USA) and its built-in workflows for RNA-Seq analysis. The poor quality reads were trimmed and the reads which passed quality control were mapped to a reference genome of Bos taurus assembly (ARS-UCD1.2, provided by GenBank). The gene expression (GE) values were quantified as reads per gene and reads per kilobase of transcript per million mapped reads (RPKM). These were stored as GE files in CLC Genomics Workbench for the analysis of differentially expressed genes (DEGs).
For variant analysis, reads were cleaned using Trimmomatic v. 0.36 (Trimmomatic: A flexible read trimming tool for Illumina NGS data. Available online: http://www.usadellab.org/cms/?page=trimmomatic. Accessed on 1 March 2021). The quality of raw and cleaned FASTQ files was assessed with FastQC (A quality control tool for high throughput sequence data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/. Accessed on 5 March 2021). Bos taurus assembly (ARS_UCD1.2), and its corresponding gene set was used as reference to map reads using the splice aware aligner HISAT2 [20]. Then, SAM files were converted to BAM files and coordinate sorted with SAMtools [21]. BAM files were further processed with Picard Tools (Picard. Available online: http://broadinstitute.github.io/picard/. Accessed on 15 March 2021) to mark PCR duplicates, add read group information, sort by chromosome and create indexes.
2.7. Analysis of Differentially Expression between Groups
DEGs between the groups were determined using an analysis of variance (ANOVA)-like model built in CLC Genomics Workbench V21. This included trimmed mean and Z-score normalisations across all samples and statistics based on a negative binomial generalised linear model. The cows were classified according to then lactation number as primiparous (PP, n = 6) or multiparous (MP, n = 9). The initial analysis showed that there were significant differences in leukocyte gene expression between the PP and MP cows. Therefore, the statistical model to examine the effect of E. coli mastitis on global gene expression of circulating leukocytes included mastitis group as a test variable and lactation group as confounding variable to control the differences of gene expression arising from number of lactations. Further information on group definitions is given in the Results section and Supplementary Table S1. Fold changes were derived from the RPKM values. The genes with an absolute fold change ≥1.5 in pairwise comparisons between the groups were selected for subsequent analysis. Benjamini–Hochberg (BH) procedure was used to adjust the p-values for multiple tests and significance was considered at p < 0.05.
2.8. Enrichment, Pathway and Cluster Analysis
Gene Ontology (GO) enrichment analysis was carried out using Partek Genomics Suite V7.1 (Partek Incorporation, Chesterfield, MO 63005, USA) with the genome version of ARS-UCD1.2 and the GO database formatted and updated by the software provider. The up- and down-regulated DEGs were separately analysed for GO enrichment with the focus on “Biological functions”. Fisher’s exact test with BH adjustment was used and statistical significance was considered at p (BH) < 0.05. The enrichment score (ES) was calculated as the negative natural logarithm of the enrichment p-value. The higher the enrichment score, the more over-represented this functional group was in the input gene list, with any ES >3 indicating significant enrichment. The DEG were also taken forward for pathway and cluster analysis using DAVID bioinformatics resources version 6.8 (The Database for Annotation, Visualization and Integrated Discovery (DAVID). Available online: https://david.ncifcrf.gov/. Accessed on 10 April 2022) [22,23] with Bos taurus as background. Fisher’s exact test with BH adjustment was used and statistical significance was considered at p (BH) < 0.05.
2.9. Variant Calling of Reads from RNA-Seq
Variant calling of the RNAseq data was performed following GATK best practice (GATK v3.8 [24]). Firstly, we used the GATK tool SplitNCigarReads to split reads into exon segments and hard-clip any sequences overhanging into the intronic regions; then, we used the Haplotype Caller to call variants in genomic blocks (gVCF mode) producing individual VCF files. A joint genotyping was performed on all gVCF files in order to create the variant call-set using GentoypeGBVFs tool. A series of hard allelic filters were applied as recommended by GATK germline best practices to prune low quality variants calls. The last step was to apply a hard filter (-window 35 -cluster 3 -filterName FS -filter “FS > 30.0” -filterName QD -filter “QD < 2.0”) to the joint file to optimise both high sensitivity and specificity. Variant calling was performed using the Ensembl VEP tool [25]. Linkage disequilibrium (LD) between autosomal variants up to 10 Mb apart across the genome extent was estimated in PLINK v1.9 [26] using the squared correlation between pairs of loci (r2) across autosomes and D’.
2.10. Statistical Analysis
The cow phenotype parameters (lactation number, yield, days in milk at sample collection) were expressed as mean ± SE. Differences between the groups were examined using a one-way ANOVA built in SPSS V28 (Chicago, IL, USA). The level for statistical significance was set at p < 0.05.
3. Results
3.1. Group Characteristics
The phenotype data from individual cows including their clinical results are given in Supplementary Table S1. One cow initially classified as E. coli(+) (A7) was omitted from the study as it had no clinical symptoms. This left nine cows per group which did or did not suffer from E. coli mastitis during the recruitment period, and all these animals were subsequently taken forward for the genetic variant analysis. All except one E. coli(+) cow were treated with antibiotics and NSAIDs as described above. Comparing the E. coli(+) and E. coli(−) control cows, their lactation numbers were 2.2 ± 0.5 and 3.0 ± 0.8, 305 day milk yields were 9835 ± 263 and 10,519 ± 205 kg and the days in milk at sample collection were 133 ± 18.5 and 112 ± 17.8 days, respectively (mean ± SEM). None of these measurements were significantly different.
Further sample selection was made prior to the RNAseq DEG analysis. Firstly, the E. coli(+) cows were divided into two subgroups based on the number of days from initial diagnosis until blood sample collection: these were EARLY (10.3 ± 1.8 days, range 6–17 days, n = 6) and LATE (46.7 ± 11 days, range 27–65 days, n = 3). Secondly, the three control cows with infected milk samples including the presence of Streptococci and Enterobacter (cows A6, A18 and A19, see Supplementary Table S1) were also excluded, leaving the control group (CONT) with n = 6 cows.
3.2. In Vitro PBMC Responses
Isolated PBMCs cultured in vitro and stimulated with LPS increased the production of IL-1ß as expected. There were no differences detected in the measurements of NO or IL-1ß produced by isolated PBMCs without LPS stimulation with respect to the E. coli status of the cows (Table 1). After LPS stimulation, there was a trend (p = 0.058) for the E. coli(+) LATE cows to produce a slightly higher concentration of NO than the CONT cows.

Table 1.
Production of NO and IL1B in vitro by PBMCs isolated from E. coli(−) and E. coli(+) cows with or without stimulation with 500 ng/mL LPS for 2 h.
3.3. Differential Gene Expression between the Groups
After mapping sequencing reads to the Bos taurus reference genome (ARS-UCD 1.2), 20,110 out of 35,158 genes/transcripts were represented. The three groups of E. coli(+) (EARLY), E. coli(+) (LATE) and E. coli(−) (CONT) were then compared. Volcano plots showing the differential expression between groups is given in Figure 1. The numbers of DEG for each condition and those shared between them are illustrated in a Venn diagram (Figure 2) and the full gene lists are given in Supplementary Table S3A–C. The Venn diagram shows that there were 1251 DEGs between the CONT and EARLY cows but only 45 DEGs between CONT and LATE. For the two groups of E. coli(+) cows, there were 710 DEGs between the EARLY and LATE animals. The majority of these (535/710, 75%) were found in common between the CONT vs. EARLY and the EARLY vs. LATE comparisons. This indicates that the EARLY cows, sampled at 6–17 days after diagnosis, showed a very different leucocyte expression profile to the CONT animals but this was no longer the case in the LATE cows, sampled 27–65 days after diagnosis.
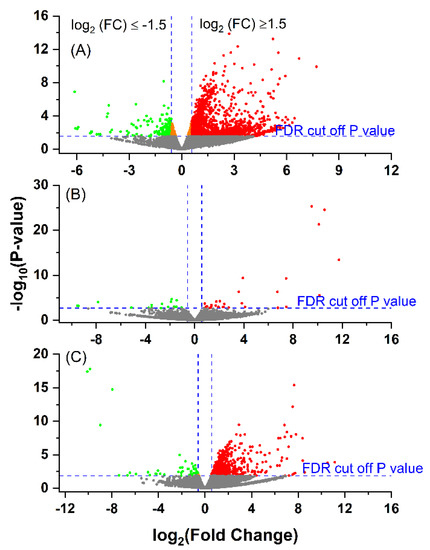
Figure 1.
Volcano plot showing the gene expression profiles following an E. coli mastitis infection between: (A) EARLY (n = 6) and Control (CONT, n = 6); (B) LATE (n = 3) and CONT and (C) EARLY and LATE. The reads were quantified as reads per kilobase million (RPKM) and normalised with trimmed mean and Z-score across all samples. The fold changes were log2-tramsformed. The p-values were transformed with −log10. Cut-off point was p < 0.05 and absolute fold change ≥1.5. The cut-off p (raw) value for false discovery rate control (FDR) at p < 0.05 was 0.0275 for EARLY vs. CONT, 0.0019 for LATE vs. CONT and 0.0134 for EARLY vs. LATE. The green dots indicate significantly down-regulated genes; the red dots indicate significantly up-regulated genes; and the orange dots are the genes with FDR p < 0.05, but with absolute fold changes <1.5.
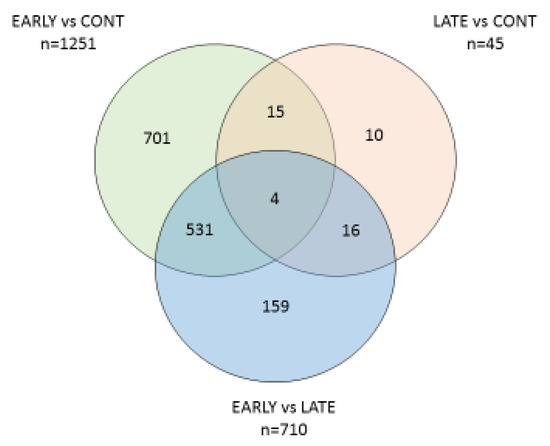
Figure 2.
Venn diagram showing the numbers of DEGs identified between the three groups of cows.
3.4. Comparison between E. coli(+) (EARLY) and E. coli(−) (CONT) Cows
In the comparison between the CONT and EARLY cows, there were 1090 up-regulated DEG in the E. coli(+) cows but only 161 were down-regulated (Supplementary Table S3A). Many of the top 20 up-regulated DEGs, with fold changes ranging from 38 to 209, were involved in a clear theme of immune functions and inflammation, including five antimicrobial peptides (AMP) (CATHL1, CATHL2, CATHL4, CATHL5, CATHL6) and three genes from the serpin family (SERPINB4, LOC112445470, LOC511106). Several top 20 genes encoded proteins with putative roles in cell motility and adhesion (ACTA1, ACTBL2, CCN1, CDH13, MFAP5, PVALB). The pro-inflammatory cytokines IL1B, IL12B and IL18 were significantly up-regulated by 2.5–2.7-fold. A number of interleukin receptors (IL1R1, IL1R2, IL1RL1, IL2RA, IL6R, IL13RA1, IL15RA, IL17RD, IL18R1, IL20RB, IL21R), their associated proteins (IL1RAP, IL18RAP) and the interleukin 1 receptor antagonist (IL1RN) were also significantly up-regulated. In the TNF family, only TNFSF14 (TNF superfamily member 14), TNFAIP6 (TNF alpha induced protein 6) and TNFRSF1A (TNF receptor superfamily member 1A) were in the up-regulated list. The biological functions of the top 20 down-regulated DEGs were more diverse, and the majority encoded proteins whose functions are poorly characterised. Those with known immune activity included C1R, LILRA4 and LOC534578. Three of the most highly down-regulated genes (LOC505052, MGC151921, and LOC101904044, with 29–63% reduction in expression) are predicted to encode odorant binding proteins.
3.5. Comparison between E. coli(+) (LATE) and E. coli(-) (CONT) Cows
The LATE cows were sampled on average 47 days after their initial diagnosis of E. coli mastitis and by this time, there was relatively little difference in their leucocyte gene expression in comparison with the CONT cows, with 29 up-regulated DEG and only 16 down-regulated (Supplementary Table S3B). Six of the most up-regulated genes encoded haemoglobin sub-units (HBA1, HBB, HBE1, HBE4, HBG, and LOC528470) while eight were predicted to encode for multidrug resistance-associated proteins (LOC112449109, LOC509854, LOC107131247, LOC101902462, LOC107131271, LOC107131273, and LOC100848700). The most down-regulated gene LOC505052 encodes for an odorant binding protein and was also identified in the EARLY vs. CONT comparison. Four other highly down-regulated genes were predicted to encode for leukocyte immunoglobulin-like receptors (LILRA4, LOC100852090, LOC790181, and LOC790255).
3.6. Comparison between E. coli(+) (EARLY) and E. coli(+) (LATE) Cows
This comparison yielded 710 DEG, among which 662 were up-regulated and 48 down-regulated in the EARLY group cows (Supplementary Table S3C). The top 20 up-regulated genes again included four encoding cathelicidins and five serpins indicating that these were more highly expressed in the early period after infection. Of the other genes, AZU1, KLRF2 and PGLYRP1 also have antimicrobial activity while MMP8, PLAT and PCOLCE2 have roles in remodelling extracellular matrix. Most of the down-regulated genes in this comparison (i.e., having a higher expression in the LATE cows) were the same as those which were up-regulated in the LATE vs. CONT comparison. The top 20 list included all six of the genes encoding haemoglobin subunits and three of the same genes encoding multidrug resistance-associated proteins. Of the DEG identified in the EARLY vs. LATE comparison, 535 were common with the EARLY vs. CONT comparison (Supplementary Table S3A). For all except four of these, the change was in the same direction, i.e., those genes which were up-regulated in the EARLY cows were more highly expressed than in either CONT or LATE cows. The exceptions were DNER, LOC101902462, LOC112449072 and LOC112449109. These were all up-regulated in the EARLY vs. CONT comparison but down-regulated in EARLY vs. LATE. These encode the Delta/notch-like EGF-related receptor and three multidrug resistance-associated protein 4-like proteins.
3.7. GO Enrichment and Cluster Analysis for Gene Functions
The preliminary analysis suggested that the cows with E. coli mastitis were still exhibiting a high degree of inflammatory response around 10 days after their initial diagnosis and treatment. This response had largely ceased in the cows sampled between 4 and 9 weeks after diagnosis, but these animals nevertheless showed some longer-term changes in leukocyte gene expression. These two aspects were therefore taken forward for a more detailed investigation, mainly focusing on the genes which were up-regulated following infection.
GO enrichment analysis of the up-regulated genes in the E. coli(+) EARLY cows vs. CONT identified 16 biological functions with an ES >3 (Figure 3, Supplementary Table S4A). Biological adhesion, containing 48 genes, had the highest ES of 15.96. Cell adhesion was also the top subcategory within cellular process (ES 11.49), in which the other top terms were the cellular response to stimulus, cell killing, cell activation and cell cycle process. There were 53 genes associated with locomotion (ES 10.39), in which the only two subcategories were taxis and cell motility. Within immune system process (ES 9.83, 66 genes) the top subcategory was leucocyte activation. In interspecies interaction between organisms (ES 9.24, 52 genes), the top subcategory was the killing of cells of other organisms. Genes involved in both adhesion and locomotion included ADAM8, CD24, CD44, CDH13, CDK5R1, FN1, PARVA, RELN, S100A8, S100A9 and SCARB1. Those directly contributing to the killing of pathogens included those encoding antimicrobial peptides (CATHL1, CATHL3, CATHL4, CATHL6, DEFB1, ELANE, HP, LTF, PGLYRP1, PGLYRP4, PTX3, S100A12, S100A8, S100A9 and TREM1), components of neutrophil azurophil granules (AZU1 and MPO), macrophage activation (ARG1, ARG2 and TMEM229B) and SLC11A1, an iron and magnesium cation transporter. Full gene lists relating to the identified subcategories are in Supplementary Table S4B.
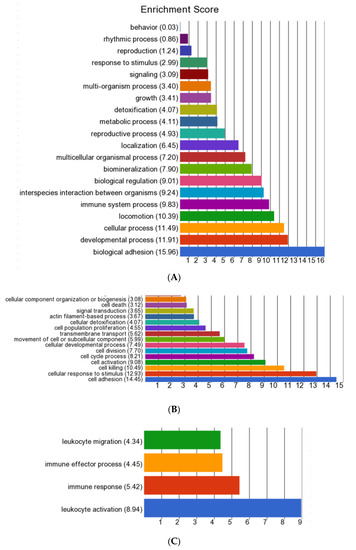
Figure 3.
Summary of Gene Ontology (GO) enrichment analysis of the biological functions activated in the E. coli(+) EARLY cows in comparison with the E. coli(-) CONT cows (each n = 6). (A) All biological functions identified from the list of all up-regulated differentially expressed genes (DEGs, n = 1090); (B) sub-categories with an enrichment score (ES) >3 in the main pathway “Cellular process”; and (C) sub categories with an ES > 3 in the main pathway “Interspecies interaction”.
GO enrichment analysis of the down-regulated genes in the EARLY vs. CONT comparison only yielded one significant biological function. This was an immune system process, with an ES of 12.79 and containing 20 DEG. These genes were mainly involved in antigen processing and presentation (BLA-DQB, BOLA-DMA, BOLA-DOA, BOLA-DRA, BOLA-DRB3, CD40, CD79B, DSB, FCRL1, IFI30) and leucocyte activation (C1R, CD19, CXCL10, TLR10, TNFRSF21).
The up-regulated DEG in the comparison between EARLY vs. CONT cows were also uploaded onto the DAVID Bioinformatics Resources website to generate functional annotation charts and clusters. The functional annotation chart displayed 89 functions and pathways significant with p (BH) < 0.05 and a fold enrichment >1.25. The full list is given in Supplementary Table S4C. The genes associated with the top terms are listed in Table 2. Functional annotation clustering identified 14 clusters with an ES > 2.5. These are listed in full in Supplementary Table S4D. These two approaches yielded similar results and are thus considered together. The main signalling pathways involved were bta04621:NOD-like receptor signalling pathway; bta04062: chemokine signalling pathway; bta05146: amoebiasis; and GO:0004908~interleukin-1 receptor activity. Pathway activation led to the increased expression of a number of genes encoding antimicrobial agents including six cathelicidins, four beta-defensins, three S100 calcium binding proteins, haptoglobin and lactoferrin. The genes identified under KEGG pathway bta1523-antifolate resistance included FOLR3 (encoding folate receptor alpha), IL1B, four genes encoding ATP binding cassette subfamily members (ABCA6, ABCA7, ABCA13 and ABCB11) and ten genes predicted to encode multidrug resistance-associated protein 4-like proteins, which are also members of the ABC superfamily.

Table 2.
Gene lists from the main terms identified by DAVID chart analysis associated with differentially up-regulated genes in leukocytes collected from cows with E. coli(+) (EARLY) compared with healthy CONT cows (n = 6 cows/group) #.
Finally, the two lists of DEGs, which were more highly expressed in the LATE cows compared with either CONT (n = 29) or EARLY (n = 48) cows, were merged to generate a list of 62 DEGs (of which 15 were in common) which were more highly up-regulated in the leucocytes of cows in the LATE recovery phase from an E. Coli infection (Supplementary Table S3D). This list was uploaded into DAVID. Cluster analysis yielded four clusters with p (BH) < 0.05 and a fold enrichment >1.25 (Supplementary Table S4E). Cluster 1 included five genes encoding haemoglobin subunits; Cluster 2 contained five multidrug resistance-associated protein 4-like transporters; and Cluster 3 contained genes mainly involved in cholesterol biosynthesis. Cluster 4 contained a wider selection of 20 genes encoding proteins with at least one transmembrane domain. GO enrichment analysis and DAVID chart analysis revealed similar findings (data not shown).
3.8. Variant Calling
Variant calling from RNA-seq data can be unreliable due to missing genotypes arising from gaps in sequencing coverage. A stringent filtering process was therefore applied to avoid this issue. The initial output generated over 1.3 million variants. E. coli(+) and E. coli(−) cows had 828,016 variants in common spanning over 14,810 genes. A further 552,615 variants (overlapping 15,697 genes) were uniquely present in E. coli(+) cows and 514,179 (overlapping 15,008 genes) were found in E. coli(−) cows. Only variants found in both E. coli(+) and E. coli(−) cows were taken forward and further filtering was applied. Firstly, they were required to have a minor allele frequency (MAF) > 0.8. This implied that, in either the E. coli(+) or E. coli(−) group, over 80% of the cows had to have a variant differing from the allele most commonly present in the Bos taurus population. Secondly, the variant had to be present in ≥8 of the 9 cows in one group but in ≤1 of the 9 cows in the other group. This generated a list of 94 genetic variants found in 38 different genes which were almost fixed in either the E. coli(+) or E. coli(−) cows (Supplementary Table S5A).
Each variant was then linked to the corresponding transcript to investigate whether the variants influenced gene expression. This showed that 12 genes containing the identified variants were differentially expressed between E. coli(+) EARLY and CONT cows (Table 3). Among these, 11 were up-regulated in E. coli(+) cows with fold changes between 1.5 and 3.3. Just one gene, BOLA-DOA, had a 1.5-fold reduction in expression. Using assembly ARS-UCD1.2, three DEGs (WIPI1, NC_037346.1 (61752501..61782685); ARSG NC_037346.1 (61781773..61886526, complement and SLC16A6, NC_037346.1 61867803..61877805)) were in adjacent positions on BTA 19 (https://www.ncbi.nlm.nih.gov/gene/528410, accessed 26 April 2022) and these were found to be in linkage disequilibrium (r2 = 0.58 and D’ = 0.86).

Table 3.
Genes with variants present in at least 8 out of 9 samples of E. coli(+) or E. coli(−) cows which were also differentially expressed between the EARLY and CONT groups of cows.
More information on the variants identified in genes having differential expression between E. coli(+) and E. coli(−) cows is given in Supplementary Table S5B. The majority were intron variants, which can impact alternative splicing by interfering with splice site recognition. The variants in WIPI1 and BOLA-DOA were located 3’ of the gene. All the variants had an impact classification of “Modifier”. This indicates that they may affect protein production, but predictions are difficult to make.
In addition, all 76 genes containing variants listed in Supplementary Table S5A were input as a gene list into a DAVID functional annotation analysis. Significance was not reached when an FDR correction was applied but this analysis did provide some indicative information, summarised in Table 4. Three genes (DPYD, ACO2, NARFL) are involved in iron–sulphur (Fe-S) clusters. A further four genes (GSK3B, PPP3R1, RAC2 and AKT1) act at various points in the B-cell receptor signalling pathway.

Table 4.
DAVID functional annotation analysis of 38 genes identified containing variants which differed between E. coli(+) and E. coli(−) cows, showing the top 6 terms.
4. Discussion
E. coli is a widespread environmental pathogen which is one of the major causes of clinical mastitis. Severe local effects are often multiplied by an aberrant immune response, and infection sometimes leads to the development of serious systemic symptoms [27]. Most previous studies using transcriptomic profiles to investigate the response to E. coli infections have used intra-mammary inoculation [6,15,16,17]. This approach has the advantage of being easy to control. The susceptibility to disease and its subsequent severity are, however, also influenced by the cow’s defence status [6] and genotype [4,28]. These aspects may be more closely reflected in naturally occurring cases compared with experimental models. The present study therefore compared the transcriptomic gene expression profiles in circulating leukocytes between cows with naturally occurring clinical mastitis caused by E. coli and paired samples from healthy cows on the same commercial farm.
These samples were necessarily collected at varying intervals of 6–65 days after the initial diagnosis. E. coli infections causing acute clinical mastitis are generally of short duration [11]. It was estimated that acute infections could usually be cleared within 5–12 days [29]. Blum et al. [30] divided the responses to E. coli into two stages. The acute phase, involving establishment of the infection, leucocyte infiltration and mammary gland inflammation started within a few hours, lasted for 1–2 weeks and was associated with local tissue damage. This was followed by a chronic resolution phase, during which milk parameters gradually recovered over several more weeks, although there might be a longer-term reduction in milk quality and yield [30]. Continued leukocyte infiltration into the mammary gland during this second phase involved both mononuclear cells and polymorphonuclear leukocytes, contributing to tissue repair processes. In support of this, the somatic cell count was previously shown to reduce to basal levels within 88 h of a mammary inflammation induced by LPS [18]. We therefore anticipated that the cows in our study, which received appropriate veterinary treatment following their diagnosis, would have largely recovered from the acute phase of infection at the time of blood sample collection. On the other hand, some investigators reported persistent cases of E. coli infection, in which bacteria were still recoverable after 40 days [29]. This is most likely due to longer survival within an intracellular reservoir in mammary epithelial cells, which could be associated with the strain of bacteria and/or to intrinsic differences in immune responses between cows [7,29]. The EARLY cows in the present study, sampled on average 10 days post infection, still showed clear evidence of a strong ongoing inflammatory response at this time. This does not necessarily imply that infective bacteria were still present, as the response could be associated with the removal of necrotic epithelial cells. This inflammation had, however, largely resolved in the LATE cows, sampled after at least 27 days had elapsed.
4.1. Evidence for an Ongoing Inflammatory Response during the Resolution Stage of an E. coli Infection
We identified 1090 up-regulated DEGs (but only 161 down-regulated DEGs) in leucocytes obtained from the E. coli(+) EARLY cows in comparison with CONT animals. The GO function and cluster analyses showed that the majority of up-regulated DEGs were involved in the activation of systemic immunity and inflammation. This was mainly achieved through the activation of the NOD-like receptor, chemokine and TLR4/IL1 signalling pathways. This closely agreed with previous studies on mammary gene expression using intramammary inoculation with E. coli which similarly reported the upregulation of the pathways of TLR signalling, NOD-like receptor (NLR) signalling, chemokine signalling and cytokine–cytokine interaction [16]. NLRs are a family of cytosolic pattern recognition receptors which detect specific PAMPs or host-derived damage signals (DAMPs) in the cytosol and can cooperate and interact with TLRs to regulate inflammatory processes such as NF-kappa B-/AP-1-dependent expression of pro-inflammatory cytokines and apoptosis [31]. In this study, genes encoding IL1 and IL18 receptors and the peptidoglycan recognition protein PGLYRP1 were all up-regulated. The latter stimulates TREM1 (triggering receptor expressed on myeloid cells). TREM1 also detects DAMPs and PAMPs and is a key activator of cytokine production, T-cell proliferation and activation of antigen-presenting cells [32]. Chemokines are an essential component of an inflammatory immune response to an infection by providing directional cues to recruit leukocytes to the site of inflammation. They also regulate many biological processes relating to the cellular activation, differentiation and survival of hematopoietic cells. Their role in recruiting leucocytes to the mammary gland following an E. coli mastitis infection has been recognised previously (e.g., [33,34]).
AMPs are multifunctional effector molecules which can kill pathogens through a variety of mechanisms [35]. Our recent study illustrated that the increased production of a variety of AMPs was related to the severity of mammary inflammation and was one of the main distinguishing differences in the way that circulating leukocytes responded to clinical compared with subclinical mastitis [36]. In the present study, E. coli infection induced the significant up-regulation of many AMPs including cathelicidins, beta defensins, S100A calcium binding proteins, lactoferrin and haptoglobin. Five cathelicidins were all in the top 20 up-regulated DEGs, with high fold changes. Cathelicidins and beta defensins are both families of small, cationic peptides which have a variety of bactericidal actions against both Gram-negative and Gram-positive bacteria [37]. Individual members of the S100 protein family are multifunctional proteins which readily form complexes including calprotectin, a heterodimer of S100A8 and S100A9. S100A proteins are implicated in regulating many intracellular and extracellular activities including cell differentiation, the stimulation of pro-inflammatory cytokines, induction of matrix metalloproteinases and the support of phagocytic properties through cytoskeletal rearrangement [38,39]. Both haptoglobin and lactoferrin are acute phase proteins which are well-established biomarkers of clinical mastitis, having direct bactericidal activity against E. coli [39,40]. SLC11A1 (NRAMP1) is another key protein in antibacterial defence via its role as an iron and magnesium cation transporter, which is crucial in iron homeostasis [41]. These various AMPs can act synergistically to clear infections by improving epithelial defences and prevent pathogen colonisation through both their direct antimicrobial actions and by fine-tuning other host immune responses and inflammation.
The KEGG pathway bta1523-Antifolate resistance was also up-regulated in the E. coli(+) EARLY cows. The genes identified included FOLR3 (encoding folate receptor alpha), IL1B, four genes encoding ATP binding cassette subfamily members (ABCA6, ABCA7, ABCA13 and ABCB11) and ten genes predicted to encode multidrug resistance-associated protein 4 (MRP4)-like proteins, which are also members of the ABC superfamily. These are low affinity, high-capacity ATP-driven transporters which move a wide variety of substrates including sugars, ions, amino acids, complex peptides, and hydrophobic (lipophilic) molecules across cell membranes [42] and they can therefore act as positive or negative regulators of metabolic pathways. Eight genes identified as encoding MRP4-like proteins were also among the top 20 most highly up-regulated genes in the E.coli(+) LATE cows. We also previously reported that the ABC transporter pathway was up-regulated in the leukocytes of a population of Holstein dairy cows with a low-energy balance status in early lactation [43]. These results suggest that ABC transporters are likely to play an important role in influencing leucocyte metabolic activity in response to increased ATP concentrations/consumption. However, the increase in these transporters could be a double-edged sword as ATP released by bacteria has recently been shown to limit IgA production as well as aiding the transport of nutrients, not only for the host cells but also for the bacteria [44,45]. The roles of different ABC superfamily members in response to mastitis therefore warrants further investigation.
The most up-regulated genes in the E. coli(+) EARLY cows also contained a number of DEGs predicted to encode for serpin-B3 and serpin-B4-like molecules (SERPINB4, LOC511695, LOC786348, LOC112445470, LOC107131803 and LOC511106). We recently reported that three of these same genes were also amongst the most highly up-regulated in the same earlier study of cows with a low energy balance status, many of which were experiencing either mastitis or endometritis [43]. The serpin superfamily are serine/cysteine protease inhibitors which are strongly associated with a variety of inflammatory conditions, particularly through targeting areas with active thrombosis and/or thrombolysis [46,47]. The term GO:0002020~protease binding was also identified as up-regulated in E. coli(+) cows in the present study. Of the genes identified, A2M encodes alpha-2-macroglobulin which can act as an inhibitor of both thrombosis and fibrinolysis, involving the degradation of a fibrin clot by plasmin. These proteins are therefore likely to be playing a part in the response to vascular injury within the mammary glands of E. coli infected cows.
Far fewer genes (45 in total) were differentially expressed between the E. coli LATE and CONT cows, indicating that the cows had, by this time, largely recovered from their infection. The up-regulated list in the LATE cows mainly contained genes encoding haemoglobin subunits (HBA1, HBB, HBE1, HBE4, HBG) and multidrug resistance-associated proteins. In the present study, we used Tempus tubes and its isolation system. This extracts all the RNA present in blood and we did not separate the cell types. In addition to leukocytes, whole blood also contains reticulocytes. These are immature red blood cells derived from the bone marrow which usually circulate for about a day in the blood stream before developing into mature red blood corpuscles (RBCs). They account for 1–4% of the erythrocytes present in healthy adult humans [48]. Reticulocytes contain a network of ribosomal RNA which can be used for continued haemoglobin synthesis [49]. The increase in haemoglobin gene expression in the circulating blood of the LATE group of cows, several weeks after their initial E. coli infection, suggests an increase in the generation of new RBCs from bone marrow at this time. This may be needed to replace any damaged or lost RBCs during the earlier stages of infection.
4.2. Evidence for Genetic Differences between E. coli Infected and Healthy Cows
The likelihood of a particular cow developing clinical mastitis is mainly determined by the host defence status rather than by the pathogenicity of E. coli [6]. The bacteria need to overcome barriers of innate immunity to enter and proliferate in the mammary grand [50]. Many factors may contribute to the impairment of immune function and Rainard et al. [18] found marked differences between cows in their responses to low-dose LPS infusion. Previous evidence has indicated that genetic differences between cows results in varying susceptibility to infection [4,28]. Macrophages are key players in the body’s defence, having both pro-inflammatory and inflammation resolution activity [51,52,53]. Following the activation of the inflammasome complex, reactive oxygen species (ROS) are produced via a series of oxidative reactions [54,55]. The enzyme inducible nitric oxide synthase (iNOS) is also up-regulated, which then interacts with NADPH and ROS intermediaries to generate reactive nitrogen species (RNS) [56]. The bactericidal activity of macrophages mainly involves this interlinked production of ROS and RNS causing damage to cells and DNA [57,58]. Our previous work has shown that macrophages from Brown Swiss cattle produce significantly more RNS and less IL-1B in response to bacterial stimuli when compared to those from Holstein Friesian cattle and they also exhibited more efficient phagocytic activity and bacterial killing [19]. The present study therefore compared the basal and LPS-stimulated production of IL-1B and NO between the macrophages obtained from Holstein cows which did or did not succumb to an E. coli infection. LPS stimulated NO concentrations were slightly higher in the E. coli(+) LATE cows but there was no difference between the E. coli(+) EARLY and CONT groups. This study therefore did not provide any evidence that monocytes from cows of the same breed which did not catch an E. coli infection had a higher NO production capacity.
The clinical mastitis cases used in the present study occurred naturally and were paired in the analysis with samples collected from healthy cows. This enabled us to compare the prevalence of variants in genes expressed in leukocytes between cows which did or did not succumb to an E. coli infection when was exposed to the same environment. From the stringent analysis used, we identified 94 variants which were consistently present in at least 8 out of 9 cows in either the E. coli(+) or E. coli(−) groups but were rarely present or absent in the other group. The genes containing 12 of these variants were then found to be differentially expressed in leucocytes between the E. coli(+) EARLY and CONT cows. Among these, three DEGs (WIPI1, ARSG and SLC16A6) located together on BTA 19 came from a genomic region with an extended run of homozygosity (ROH) which was detected in a population of Shanghai Holstein cattle [59]. Analyses of ROH allow the identification of genomic regions with possible selection signatures for the breed, as their size and frequency vary according to population diversity and selection pressure. WIPI1 encodes a member of the WD40 repeat family, and has been implicated in nucleophagy in differentiating keratinocytes during epidermal differentiation, with deficiency associated with skin disease [60]. This could potentially be important in maintaining good teat health. ARSG encodes a lysosomal sulfatase which hydrolyses sulphate esters and is involved in hormone biosynthesis, the modulation of cell signalling, and degradation of macromolecules. SLC16A6 encodes a protein which transports lactate, pyruvate and beta-hydroxybutyrate across the cell membrane and is required for the hepatocyte secretion of ketone bodies during fasting [61]. These variants were in LD, and it remains to be determined which, if any, of these proteins may be relevant in the context of E. coli susceptibility.
Of the other variants identified, RAC2 encodes a Rho-family GTPase that contributes to the B cell receptor signalling pathway and is involved in the phagocytosis of microbes and oxidative burst microbial killing. Mutations in this gene are associated with neutrophil immunodeficiency syndrome in humans [62]. Another SNP was present in ARHGAP26, which encodes Rho GTPase activating protein 26. This protein binds to focal adhesion kinase and mediates the activity of the GTP binding proteins RhoA and Cdc42 [63]. It was recently identified as a candidate gene for clinical mastitis in Sahiwal cattle, a Bos indicus breed [64].
Of the other variants in genes which showed an up-regulated differential expression between E. coli(+) and E. coli(−) cows, EBF1 and EYA3 are both involved in regulation of gene transcription. GNG7 encodes guanine nucleotide-binding protein γ−7, whose expression level was associated with the infiltration of multiple immune cells into human colorectal cancer samples [65]. Little information is available concerning FAM129A (also called NIBAN1), although expression is up-regulated as part of the integrated stress response [66]. PFKFB4 encodes an enzyme which regulates the concentration of the glycolytic by-product fructose-2,6-bisphosphate (F2,6BP) and there is evidence that it is important for the stimulation of glycolysis and activation of T-cells [67]. CCND3 encodes cyclin D3, which contributes to the cell cycle G1/S-phase transition and is a key regulator of B-cell proliferation [68]. A further four genes containing variants (GSK3B, PPP3R1, RAC2 and AKT1), but which were not differentially expressed, also act at various points in the B-cell receptor signalling pathway. Three further genes (DPYD, ACO2, NARF1) are involved in iron–sulphur (Fe-S) clusters. These act as small metallocofactors which are used to perform complex chemical reactions within several important cellular pathways, including redox catalysis, fatty acid oxidation and DNA repair and replication [69].
The highly polymorphic major histocompatibility complex (MHC) has been implicated in the resistance and susceptibility to a broad range of diseases including mastitis [70]. BOLA-DOA encodes the major histocompatibility complex, class II, DO alpha. This was the only variant identified in a gene with significantly lower expression in the E. coli(-) cows. Class II molecules are expressed on antigen-presenting cells and are important for driving T-cell development and differentiation. The bovine MHC region of BTA23 has been under significant selection pressure during the development of the Holstein breed, with significantly decreased heterozygosity in contemporary animals [71]. A high proportion of the relatively few genes which were down-regulated in E. coli infected cows were enriched in those encoding proteins important for antigen processing and presentation in both the EARLY (BLA-DQB, BOLA-DMA, BOLA-DOA, BOLA-DRA, BOLA-DRB3) and LATE cows (four genes encoding leukocyte immunoglobulin-like receptor subfamily A members). The latter are widely expressed throughout the body and interact with collagen and MHC class 1 molecules. They may influence the signalling pathways of both the innate and adaptive immune systems [72] and were implicated in causing an immunosuppressive environment [73]. Together, these results therefore support earlier work in suggesting that cows which are more susceptible to E. coli mastitis have potentially important alterations in their MHC system.
4.3. Study Limitations
This was an observational study performed on a commercial farm, which meant that some aspects of the investigation could not be as well controlled as would be possible at a research establishment. Firstly, all cows diagnosed with E. coli mastitis were treated as deemed most appropriate by the attending veterinarian, with most receiving both NSAID and antibiotic therapy. The inflammatory response observed in blood samples obtained at the follow-up visit some days later would presumably have been even more pronounced in the absence of treatment. This should not, however, have influenced the comparison of the genetic variants between cases and control cows. Secondly, we did not assess whether there were still viable bacteria present in the milk at the time of blood sample collection and thirdly the timing from diagnosis to blood sample collection varied from 6 to 17 days for the E. coli(+) EARLY cows. We therefore could not know how quickly individual animals recovered from infection. Nevertheless, the EARLY cows were still clearly undergoing an inflammatory response. This could have been associated with continuing clearance of necrotic tissue from their mammary gland. There is, however, evidence of E. coli survival in the mammary gland for up to at least 40 days in some cows which develop a persistent infection [7,29]. While speculative, this situation would possibly be more likely to occur in commercial animals experiencing natural infections in comparison with studies using challenge models performed on previously healthy cows kept under research conditions.
5. Conclusions
This study found that leukocytes from E. coli-infected cows showed an up-regulation of NOD-like, interleukin-1 receptor and chemokine signalling pathways resulting in the increased expression of genes encoding a broad spectrum of antimicrobial peptides. This provided a clear indication that the cows were still actively engaged in immune and inflammatory responses to infection in their second week post diagnosis, despite having received standard veterinary treatment. Inflammation had, however, largely resolved within four weeks. A search for genetic variants between cows which did or did not become infected with E. coli during the same period identified 94 genetic variants in 38 different genes, of which 12 showed a differential expression between control and infected cows. These findings supported existing evidence that mastitis susceptibility is influenced by genes encoding Rho-family GTPases and the major histocompatibility complex, affecting antigen presentation and processing. A better understanding of how cows respond to infection is essential for both short-term improvements in treatment options and the longer- term goal of breeding more disease-resistant cows.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12162146/s1, Supplementary Table S1.: Animal Information; Supplementary Table S2.: Concentration and quality of leukocyte RAN samples analysed with Agilent BioAnalyzer and NanoDrop; Supplementary Table S3A–D.: (A) DEG in comparison of E. coli(+) EARLY vs. CONT cows; (B) DEG in comparison of E. coli(+) LATE (n = 3) vs. CONT cows; (C) DEG in comparison of E. coli(+) EARLY vs. E. coli(+) LATE cows; (D) Combined list of up-regulated genes in E. coli(+) LATE cows (n = 3) versus either CONT or E. coli(+) EARLY; Supplementary Table S4A–E.: (A) GO enrichment analysis of the up-regulated genes in the E. coli(+) EARLY cows vs. E.coli(−) CONT cows, each n = 6; (B) Full gene lists relating to the subcategories identified from the GO enrichment analysis illustrated in Figure 3 of the up-regulated genes in the E. coli(+) EARLY cows vs. E.coli(−) CONT cows, each n = 6; (C) DAVID Bioinformatic Analysis. Functional annotation chart of up-regulated genes in comparison with E. coli(+)EARLY vs. CONT cows, each n = 6 cows; (D) DAVID Bioinformatic Analysis. Functional annotation clustering of up-regulated genes in comparison of E. coli(+) EARLY vs. CONT cows, each n = 6 cows; (E) Functional annotation clustering in comparison of all up-regulated genes which showed significant differential expression in leukocytes in the cows with E. coli(+) LATE compared with either the healthy CONT cows or the E. coli(+) EARLY group; Supplementary Table S5.: Common genetic variants between E. coli +ve and E. coli -ve cows; Supplementary Table S6: Annotation of variants identified in genes having differential expression between E. coli(+) and E. coli(−).
Author Contributions
Conceptualization, Z.C. and D.C.W.; methodology, Z.C., S.P.-V. and L.B.; software, Z.C. and L.B.; validation, D.C.W., Z.C., F.B. and D.W.; formal analysis, Z.C., L.B. and M.S.; investigation, S.P.-V., F.B., D.C.W. and D.W.; resources, F.B., D.C.W., D.W. and Z.C.; data curation, Z.C. and L.B.; writing—original draft preparation, Z.C. and D.C.W.; writing—review and editing, D.C.W., Z.C., L.B., D.W., F.B., S.P.-V. and M.S.; visualization, Z.C. and L.B.; supervision, D.C.W., F.B. and D.W.; project administration, D.C.W., F.B. and D.W.; funding acquisition, D.C.W., D.W., F.B. and member of the GplusE Consortium. All authors have read and agreed to the published version of the manuscript.
Funding
This project received funding from the European Union’s Seventh Framework Programme (Brussels, Belgium) for research, technological development, and demonstration under grant agreement no. 613689. The views expressed in this publication are the sole responsibility of the authors and do not necessarily reflect the views of the European Commission.
Institutional Review Board Statement
The data in this study were collected as a part of the Genotype plus Environment (GplusE) FP7-Project (http://www.gpluse.eu (accessed on 20 November 2021). This was an on-farm study in which all sample collection and treatments were classified as routine veterinary procedures as approved by the Animal Welfare Committee of Mecklenburg-Pomerania/Germany (LALLF 7221.3-18196-22-03).
Informed Consent Statement
Not applicable.
Data Availability Statement
The RNA-seq fastq data can be obtained from NCBI Sequence Read Archive (https://submit.ncbi.nlm.nih.gov/about/sra/, accessed on 8 April 2022) BioProject PRJNA837869 with BioSample Accession number SAMN28232994.
Acknowledgments
The following are members of the GplusE Consortium (http://www.gpluse.eu/index.php/contributors/, accessed on 6 Jan 2022): Mark Crowe, Niamh McLoughlin, Alan Fahey, Fiona Carter, Elizabeth Matthews, Andreia Santoro, Colin Byrne, Pauline Rudd, Roisin O’Flaherty, Sinead Hallinan, D Claire Wathes, Laura Buggiotti, Mazdak Salavati, Zhangrui Cheng, Ali Fouladi, Geoff Pollott, Dirk Werling, Beatriz Sanz Bernardo, Conrad Ferris, Alistair Wylie, Matt Bell, Mieke Vaneetvelde, Kristof Hermans, Miel Hostens, Geert Opsomer, Sander Moerman, Jenne De Koster, Hannes Bogaert, Jan Vandepitte, Leila Vandevelde, Bonny Vanranst, Klaus Ingvartsen, Martin Tang Sorensen, Johanna Hoglund, Susanne Dahl, Soren Ostergaard, Janne Rothmann, Mogens Krogh, Else Meyer, Leslie Foldager, Charlotte Gaillard, Jehan Ettema, Tine Rousing, Torben Larsen, Victor H. Silva de Oliveira, Cinzia Marchitelli, Federica Signorelli, Francesco Napolitano, Bianca Moioli, Alessandra Crisà, Luca Buttazzoni, Jennifer McClure, Daragh Matthews, Francis Kearney, Andrew Cromie, Matt McClure, Shujun Zhang, Xing Chen, Huanchun Chen, Junlong Zhao, Liguo Yang, Guohua Hua, Chen Tan, Guiqiang Wang, Michel Bonneau, Marlène Sciarretta, Armin Pearn, Arnold Evertson, Linda Kosten, Anders Fogh, Thomas Andersen, Matthew Lucy, Chris Elsik, Gavin Conant, Jerry Taylor, Deborah Triant, Nicolas Gengler, Michel Georges, Frederic Colinet, Marilou Ramos Pamplona, Hedi Hammami, Catherine Bastin, Haruko Takeda, Aurelie Laine, Anne-Sophie Van Laere, Rodrigo Mota, Saied Naderi Darbagshahi, Frederic Dehareng, Clement Grelet, Amelie Vanlierde, Eric Froidmont, Frank Becker, Martin Schulze, Sergio Palma Vera a Andrea Pompozzi.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Aghamohammadi, M.; Haine, D.; Kelton, D.F.; Barkema, H.W.; Hogeveen, H.; Keefe, G.P.; Dufour, S. Herd-Level Mastitis-Associated Costs on Canadian Dairy Farms. Front. Vet. Sci. 2018, 5, 100. [Google Scholar] [CrossRef]
- Petzl, W.; Zerbe, H.; Gunther, J.; Seyfert, H.M.; Hussen, J.; Schuberth, H.J. Pathogen-specific responses in the bovine udder. Models and immunoprophylactic concepts. Res. Vet. Sci. 2018, 116, 55–61. [Google Scholar] [CrossRef]
- Harmon, R.J. Physiology of mastitis and factors affecting somatic cell counts. J. Dairy Sci. 1994, 77, 2103–2112. [Google Scholar] [CrossRef]
- Miles, A.M.; Huson, H.J. Graduate Student Literature Review: Understanding the genetic mechanisms underlying mastitis. J. Dairy Sci. 2021, 104, 1183–1191. [Google Scholar] [CrossRef]
- Bradley, A. Bovine mastitis: An evolving disease. Vet. J. 2002, 164, 116–128. [Google Scholar] [CrossRef]
- Burvenich, C.; Van Merris, V.; Mehrzad, J.; Diez-Fraile, A.; Duchateau, L. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 2003, 34, 521–564. [Google Scholar] [CrossRef]
- Dogan, B.; Klaessig, S.; Rishniw, M.; Almeida, R.A.; Oliver, S.P.; Simpson, K.; Schukken, Y.H. Adherent and invasive Escherichia coli are associated with persistent bovine mastitis. Vet. Microbiol. 2006, 116, 270–282. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- De Schepper, S.; De Ketelaere, A.; Bannerman, D.D.; Paape, M.J.; Peelman, L.; Burvenich, C. The toll-like receptor-4 (TLR-4) pathway and its possible role in the pathogenesis of Escherichia coli mastitis in dairy cattle. Vet. Res. 2008, 39, 5. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Schukken, Y.H.; Gunther, J.; Fitzpatrick, J.; Fontaine, M.C.; Goetze, L.; Holst, O.; Leigh, J.; Petzl, W.; Schuberth, H.J.; Sipka, A.; et al. Host-response patterns of intramammary infections in dairy cows. Vet. Immunol. Immunopathol. 2011, 144, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Li, R.W.; Capuco, A.V. Mastitis associated transcriptomic disruptions in cattle. Vet. Immunol. Immunopathol. 2010, 138, 267–279. [Google Scholar] [CrossRef]
- Bruckmaier, R.M.; Wellnitz, O. TRIENNIAL LACTATION SYMPOSIUM/BOLFA: Pathogen-specific immune response and changes in the blood-milk barrier of the bovine mammary gland. J. Anim. Sci. 2017, 95, 5720–5728. [Google Scholar] [CrossRef]
- Blum, S.E.; Heller, E.D.; Jacoby, S.; Krifucks, O.; Leitner, G. Comparison of the immune responses associated with experimental bovine mastitis caused by different strains of Escherichia coli. J. Dairy Res. 2017, 84, 190–197. [Google Scholar] [CrossRef]
- Buitenhuis, B.; Rontved, C.M.; Edwards, S.M.; Ingvartsen, K.L.; Sorensen, P. In depth analysis of genes and pathways of the mammary gland involved in the pathogenesis of bovine Escherichia coli-mastitis. BMC Genom. 2011, 12, 130. [Google Scholar] [CrossRef]
- Loor, J.J.; Moyes, K.M.; Bionaz, M. Functional adaptations of the transcriptome to mastitis-causing pathogens: The mammary gland and beyond. J. Mammary Gland. Biol. Neoplasia 2011, 16, 305–322. [Google Scholar] [CrossRef]
- Lawless, N.; Reinhardt, T.A.; Bryan, K.; Baker, M.; Pesch, B.; Zimmerman, D.; Zuelke, K.; Sonstegard, T.; O’Farrelly, C.; Lippolis, J.D.; et al. MicroRNA regulation of bovine monocyte inflammatory and metabolic networks in an in vivo infection model. G3 Genes Genomes Genet. 2014, 4, 957–971. [Google Scholar] [CrossRef]
- Rainard, P.; Cunha, P.; Gilbert, F.B. Innate and Adaptive Immunity Synergize to Trigger Inflammation in the Mammary Gland. PLoS ONE 2016, 11, e0154172. [Google Scholar] [CrossRef]
- Gibson, A.J.; Woodman, S.; Pennelegion, C.; Patterson, R.; Stuart, E.; Hosker, N.; Siviter, P.; Douglas, C.; Whitehouse, J.; Wilkinson, W.; et al. Differential macrophage function in Brown Swiss and Holstein Friesian cattle. Vet. Immunol. Immunopathol. 2016, 181, 15–23. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing S. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef] [PubMed]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Vaarst, M.; Enevoldsen, C. Patterns of clinical mastitis manifestations in Danish organic dairy herds. J. Dairy Res. 1997, 64, 23–37. [Google Scholar] [CrossRef]
- Warner, C.M.; Meeker, D.L.; Rothschild, M.F. Genetic control of immune responsiveness: A review of its use as a tool for selection for disease resistance. J. Anim. Sci. 1987, 64, 394–406. [Google Scholar] [CrossRef]
- White, L.J.; Schukken, Y.H.; Dogan, B.; Green, L.; Dopfer, D.; Chappell, M.J.; Medley, G.F. Modelling the dynamics of intramammary E. coli infections in dairy cows: Understanding mechanisms that distinguish transient from persistent infections. Vet. Res. 2010, 41, 13. [Google Scholar] [CrossRef][Green Version]
- Blum, S.E.; Heller, D.E.; Jacoby, S.; Krifuks, O.; Merin, U.; Silanikove, N.; Lavon, Y.; Edery, N.; Leitner, G. Physiological response of mammary glands to Escherichia coli infection:A conflict between glucose need for milk production and immune response. Sci. Rep. 2020, 10, 9602. [Google Scholar] [CrossRef]
- Saxena, M.; Yeretssian, G. NOD-Like Receptors: Master Regulators of Inflammation and Cancer. Front. Immunol. 2014, 5, 327. [Google Scholar] [CrossRef]
- Roe, K.; Gibot, S.; Verma, S. Triggering receptor expressed on myeloid cells-1 (TREM-1): A new player in antiviral immunity? Front. Microbiol. 2014, 5, 627. [Google Scholar] [CrossRef]
- Gilbert, F.B.; Cunha, P.; Jensen, K.; Glass, E.J.; Foucras, G.; Robert-Granie, C.; Rupp, R.; Rainard, P. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Vet. Res. 2013, 44, 40. [Google Scholar] [CrossRef]
- Younis, S.; Javed, Q.; Blumenberg, M. Meta-Analysis of Transcriptional Responses to Mastitis-Causing Escherichia coli. PLoS ONE 2016, 11, e0148562. [Google Scholar] [CrossRef]
- Afacan, N.J.; Yeung, A.T.; Pena, O.M.; Hancock, R.E. Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr. Pharm. Des. 2012, 18, 807–819. [Google Scholar] [CrossRef]
- Cheng, Z.; Buggiotti, L.; Salavati, M.; Marchitelli, C.; Palma-Vera, S.; Wylie, A.; Takeda, H.; Tang, L.; Crowe, M.A.; Wathes, D.C.; et al. Global transcriptomic profiles of circulating leucocytes in early lactation cows with clinical or subclinical mastitis. Mol. Biol. Rep. 2021, 48, 4611–4623. [Google Scholar] [CrossRef]
- Blyth, G.A.D.; Connors, L.; Fodor, C.; Cobo, E.R. The Network of Colonic Host Defense Peptides as an Innate Immune Defense Against Enteropathogenic Bacteria. Front. Immunol. 2020, 11, 965. [Google Scholar] [CrossRef]
- Donato, R. Intracellular and extracellular roles of S100 proteins. Microsc. Res. Tech. 2003, 60, 540–551. [Google Scholar] [CrossRef]
- Pyorala, S.; Hovinen, M.; Simojoki, H.; Fitzpatrick, J.; Eckersall, P.D.; Orro, T. Acute phase proteins in milk in naturally acquired bovine mastitis caused by different pathogens. Vet. Rec. 2011, 168, 535. [Google Scholar] [CrossRef]
- Kutila, T.; Pyorala, S.; Saloniemi, H.; Kaartinen, L. Antibacterial effect of bovine lactoferrin against udder pathogens. Acta Vet. Scand. 2003, 44, 35–42. [Google Scholar] [CrossRef]
- Ratledge, C.; Dover, L.G. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 2000, 54, 881–941. [Google Scholar] [CrossRef]
- Thurm, C.; Schraven, B.; Kahlfuss, S. ABC Transporters in T Cell-Mediated Physiological and Pathological Immune Responses. Int. J. Mol. Sci. 2021, 22, 9186. [Google Scholar] [CrossRef]
- Wathes, D.C.; Becker, F.; Buggiotti, L.; Crowe, M.A.; Ferris, C.; Foldager, L.; Grelet, C.; Hostens, M.; Ingvartsen, K.L.; Marchitelli, C.; et al. Associations between Circulating IGF-1 Concentrations, Disease Status and the Leukocyte Transcriptome in Early Lactation Dairy Cows. Ruminants 2021, 1, 147–177. [Google Scholar] [CrossRef]
- Inami, A.; Kiyono, H.; Kurashima, Y. ATP as a Pathophysiologic Mediator of Bacteria-Host Crosstalk in the Gastrointestinal Tract. Int. J. Mol. Sci. 2018, 19, 2371. [Google Scholar] [CrossRef]
- Proietti, M.; Perruzza, L.; Scribano, D.; Pellegrini, G.; D’Antuono, R.; Strati, F.; Raffaelli, M.; Gonzalez, S.F.; Thelen, M.; Hardt, W.D.; et al. ATP released by intestinal bacteria limits the generation of protective IgA against enteropathogens. Nat. Commun. 2019, 10, 250. [Google Scholar] [CrossRef]
- Sun, Y.; Sheshadri, N.; Zong, W.X. SERPINB3 and B4: From biochemistry to biology. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2017; Volume 62, pp. 170–177. [Google Scholar] [CrossRef]
- Yaron, J.R.; Zhang, L.; Guo, Q.; Haydel, S.E.; Lucas, A.R. Fibrinolytic Serine Proteases, Therapeutic Serpins and Inflammation: Fire Dancers and Firestorms. Front. Cardiovasc. Med. 2021, 8, 648947. [Google Scholar] [CrossRef]
- Greer, J.P.; Arber, D.A.; Glader, B.E.; List, A.F.; Means, R.M.; Rodgers, G.M. Wintrobe’s Clinical Hematology; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Lee, E.; Choi, H.S.; Hwang, J.H.; Hoh, J.K.; Cho, Y.H.; Baek, E.J. The RNA in reticulocytes is not just debris: It is necessary for the final stages of erythrocyte formation. Blood Cells Mol. Dis. 2014, 53, 1–10. [Google Scholar] [CrossRef]
- Isobe, N. Control mechanisms for producing antimicrobial factors in ruminant mammary gland. Anim. Sci. J. 2017, 88, 937–943. [Google Scholar] [CrossRef]
- Aguilar-Ruiz, S.R.; Torres-Aguilar, H.; Gonzalez-Dominguez, E.; Narvaez, J.; Gonzalez-Perez, G.; Vargas-Ayala, G.; Meraz-Rios, M.A.; Garcia-Zepeda, E.A.; Sanchez-Torres, C. Human CD16+ and CD16- monocyte subsets display unique effector properties in inflammatory conditions in vivo. J. Leukoc. Biol. 2011, 90, 1119–1131. [Google Scholar] [CrossRef]
- Hussen, J.; Duvel, A.; Sandra, O.; Smith, D.; Sheldon, I.M.; Zieger, P.; Schuberth, H.J. Phenotypic and functional heterogeneity of bovine blood monocytes. PLoS ONE 2013, 8, e71502. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Bokoch, G.M.; Knaus, U.G. NADPH oxidases: Not just for leukocytes anymore! Trends Biochem. Sci. 2003, 28, 502–508. [Google Scholar] [CrossRef]
- Quinn, M.T.; Gauss, K.A. Structure and regulation of the neutrophil respiratory burst oxidase: Comparison with nonphagocyte oxidases. J. Leukoc. Biol. 2004, 76, 760–781. [Google Scholar] [CrossRef]
- Jungi, T.W.; Adler, H.; Adler, B.; Thony, M.; Krampe, M.; Peterhans, E. Inducible nitric oxide synthase of macrophages. Present knowledge and evidence for species-specific regulation. Vet. Immunol. Immunopathol. 1996, 54, 323–330. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Forman, H.J.; Torres, M. Redox signaling in macrophages. Mol. Aspects Med. 2001, 22, 189–216. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Z.; Zhao, W.; Guo, L.; Sun, H.; Zhu, K.; Liu, G.; Shen, X.; Zhao, X.; Wang, Q.; et al. Genome-wide selection signatures detection in Shanghai Holstein cattle population identified genes related to adaption, health and reproduction traits. BMC Genom. 2021, 22, 747. [Google Scholar] [CrossRef]
- Akinduro, O.; Sully, K.; Patel, A.; Robinson, D.J.; Chikh, A.; McPhail, G.; Braun, K.M.; Philpott, M.P.; Harwood, C.A.; Byrne, C.; et al. Constitutive Autophagy and Nucleophagy during Epidermal Differentiation. J. Investig. Dermatol. 2016, 136, 1460–1470. [Google Scholar] [CrossRef]
- Hugo, S.E.; Cruz-Garcia, L.; Karanth, S.; Anderson, R.M.; Stainier, D.Y.; Schlegel, A. A monocarboxylate transporter required for hepatocyte secretion of ketone bodies during fasting. Genes Dev. 2012, 26, 282–293. [Google Scholar] [CrossRef]
- Lougaris, V.; Baronio, M.; Castagna, A.; Tessarin, G.; Rossi, S.; Gazzurelli, L.; Benvenuto, A.; Moratto, D.; Chiarini, M.; Cattalini, M.; et al. Paediatric MAS/HLH caused by a novel monoallelic activating mutation in p110delta. Clin. Immunol. 2020, 219, 108543. [Google Scholar] [CrossRef]
- Valtcheva, N.; Primorac, A.; Jurisic, G.; Hollmen, M.; Detmar, M. The orphan adhesion G protein-coupled receptor GPR97 regulates migration of lymphatic endothelial cells via the small GTPases RhoA and Cdc42. J. Biol. Chem. 2013, 288, 35736–35748. [Google Scholar] [CrossRef] [PubMed]
- Kour, A.; Deb, S.M.; Nayee, N.; Raina, V.S.; Yadav, V.; Niranjan, S.K. Understanding the genomic architecture of clinical mastitis in Bos indicus. 3 Biotech 2021, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhong, R.; Qiu, C.; Zou, B.B. The Prognostic Value of GNG7 in Colorectal Cancer and Its Relationship With Immune Infiltration. Front. Genet. 2022, 13, 833013. [Google Scholar] [CrossRef] [PubMed]
- Evstafieva, A.G.; Kovaleva, I.E.; Shoshinova, M.S.; Budanov, A.V.; Chumakov, P.M. Implication of KRT16, FAM129A and HKDC1 genes as ATF4 regulated components of the integrated stress response. PLoS ONE 2018, 13, e0191107. [Google Scholar] [CrossRef]
- Telang, S.; Clem, B.F.; Klarer, A.C.; Clem, A.L.; Trent, J.O.; Bucala, R.; Chesney, J. Small molecule inhibition of 6-phosphofructo-2-kinase suppresses t cell activation. J. Transl. Med. 2012, 10, 95. [Google Scholar] [CrossRef]
- Ramezani-Rad, P.; Chen, C.; Zhu, Z.; Rickert, R.C. Cyclin D3 Governs Clonal Expansion of Dark Zone Germinal Center B Cells. Cell. Rep. 2020, 33, 108403. [Google Scholar] [CrossRef]
- Hinton, T.V.; Batelu, S.; Gleason, N.; Stemmler, T.L. Molecular characteristics of proteins within the mitochondrial Fe-S cluster assembly complex. Micron 2022, 153, 103181. [Google Scholar] [CrossRef]
- Rupp, R.; Boichard, D. Genetics of resistance to mastitis in dairy cattle. Vet. Res. 2003, 34, 671–688. [Google Scholar] [CrossRef]
- Ma, L.; Sonstegard, T.S.; Cole, J.B.; VanTassell, C.P.; Wiggans, G.R.; Crooker, B.A.; Tan, C.; Prakapenka, D.; Liu, G.E.; Da, Y. Genome changes due to artificial selection in U.S. Holstein cattle. BMC Genom. 2019, 20, 128. [Google Scholar] [CrossRef]
- Brown, D.; Trowsdale, J.; Allen, R. The LILR family: Modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens 2004, 64, 215–225. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Shiota, G. Immune evasion by cancer stem cells. Regen. Ther. 2021, 17, 20–33. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).