Simple Summary
Sheep meat in Greece is considered a by-product of dairy production, associated with undefined quality and low revenues. Production is based on slaughtering of young lambs and consists of light carcasses. Heavy carcasses are scarce and mainly derived from rams and ewes that are no longer appropriate for dairy farming due to age, health or productivity issues; no dietary treatments or fattening protocols are implemented prior to slaughter. Improving production sustainability implies more efficient use of available resources, including local fat-tailed sheep that are reared in high numbers for their milk, although their carcasses are undervalued; supportive research data are scarce. Hence, we used objective methods to assess the quality of carcasses of fat-tailed sheep slaughtered at different live weights (representing five developmental stages, from young lambs to adult sheep) and to compare them with carcasses of thin-tailed sheep, which are considered superior. We found that quality of fat-tailed sheep carcasses was equal or higher compared to thin-tailed. Differences between sexes and developmental stages existed. Slaughtering of fat-tailed sheep at higher live weights (50–70% of the average adult sheep weight) will benefit production quality and quantity, improving profitability and sustainability of the sector.
Abstract
Sheep farming in Greece is focused on milk production. Meat is considered a by-product and consists mainly light carcasses of undefined quality. The main challenge of the sector is to ensure sustainability, and hence efforts are towards efficient use of available resources, including undervalued carcasses of local fat-tailed sheep. The objective here was twofold: (i) to assess the carcass quality of fat-tailed sheep slaughtered at different live weights and (ii) to compare them with carcasses from thin-tailed sheep. In total, 146 fat-tailed and 97 thin-tailed dairy sheep were used. They belonged to five live-weight categories (LWC), representing 25%, 35%, 50%, 70% and 100% of mature body weight. Carcass length/weight/yield/pH and wither height were recorded. Muscle fiber minimum Feret’s diameter and meat color/tenderness/moisture/lipid and protein content were determined. Sex and LWC differences in fat-tailed sheep were assessed. Parametric and non-parametric tests were used to compare with thin-tailed sheep, considering the effects of LWC, sex and their interactions with sheep population (fat-tailed/thin-tailed). Most traits were significantly different (p < 0.05) between groups of fat-tailed sheep. Carcass yield of fat-tailed sheep was significantly higher compared to thin-tailed (p < 0.01). Interactions of sheep population with LWC or sex affected wither height, carcass pH, meat color and tenderness (p < 0.05). Fat-tailed sheep meat quality is equal or higher compared to thin-tailed. Finishing weights corresponding to 50 and 70% LWC may improve capitalization of fat-tailed carcasses.
1. Introduction
Sheep meat production in Greece comprises more than one-fourth (27.2%) of domestic non-poultry meat production [1] and one-tenth (11.7%) of total EU sheep meat production. Despite the size of the sector, sheep meat in Greece is considered a by-product of dairy production associated with very low revenues [2]. Most slaughtered animals are young lambs (approximately one to three months old). Other categories include ewes and rams removed from production due to age or health problems without any specific dietary treatment prior to slaughter. Hence, the quality of their carcasses is poor, with large variation regarding classification. In practice, such carcasses are sold whole or in halves. Smaller cuts and marketable sheep meat products are scarce. Hence, consumption is mostly seasonal and related to religious or festive occasions [3,4].
In Greece, consumption of sheep meat shows a constant decline and is being replaced by other types of meat (bovine, swine, poultry), for which Greece is less self-sufficient and their production is less sustainable [5]. One of the major goals of the EU agricultural outlook for the next decade is to achieve more efficient production systems [6]. Regarding meat production in Greece, the notion is that efficient use of available resources will increase self-sufficiency and limit imports. In this context, production of heavier sheep carcasses will allow year-round market supply and will increase the marketability of sheep meat [2,7]. However, production of heavier sheep carcasses requires implementation of special management practices and designated nutrition, considering the large variation in breeds and their crosses comprising the national flock in Greece that is oriented towards milk production. Moreover, the national flock involves fat-tailed dairy sheep in significant numbers (mostly animals of Chios and Lesvos breeds) that show remarkable resilience in challenging habitats in terms of food availability and temperature fluctuations [8], but their meat production is generally undervalued.
To achieve efficient use and better capitalization of sheep meat production in Greece, it is necessary to define the baseline quality of produced carcasses, without any dietary or other interventions. Special attention should be drawn to fat-tailed sheep carcasses, which are largely considered of lower quality compared to thin-tailed, a perception that affects their marketability, despite the scarcity of supportive scientific evidence. Investigation of possible differences between the two sheep populations of the national flock, fat-tailed and thin-tailed, also considering sex and weight variation, will highlight the strong and weak points of meat production in each case and reveal potential for improvement through the implementation of customized management practices. In this study our objective was twofold: (i) to characterize and assess the carcass quality of fat-tailed sheep reared in Greece and slaughtered at different live weights and (ii) to perform a comparison with carcasses of thin-tailed sheep.
2. Materials and Methods
2.1. Animals and Study Design
A total of 146 fat-tailed and 97 thin-tailed dairy sheep of both sexes were used. Animals were selected with the aim of better representing the sheep populations reared in Greece. Fat-tailed sheep were mainly Chios purebreds or their crosses, whereas thin-tailed sheep were crossbreds of the various thin-tailed dairy sheep breeds that are common in Greece. The population of the latter is greatly diverse, with undefined genetic background; although in some Greek dairy sheep farms, thin-tailed sheep of specific breeds are present or even exclusively bred, most farms use sheep of more than one breed and their crosses. The selected animals came from semi-intensively reared flocks, which represent the most common sheep farming system in Greece; sheep were grazing daily during spring and summer months in natural grasslands, and their diet also included roughage (wheat straw and alfalfa hay) and a concentrate mix of 16% total protein content, consisting mainly of soya and corn. They were slaughtered at different live weights and allocated into five different live-weight categories (LWC), representing 25%, 35%, 50%, 70% and 100% of mature weight, which ranges approximately from 55–80 kg, considering both sexes (Table 1).

Table 1.
Weight range per live-weight category (LWC).
Slaughtering took place in two approved commercial abattoirs in Greece, according to EU standards [9]. Prior to slaughter, all animals were weighted (live weight), and their wither height was measured. Carcasses were commercially dressed; light carcasses (25%, 35% and 50% of mature weight) included head and viscera (heart, lungs, liver, internal fat and kidneys). Following carcass dressing and prior to refrigeration, hot carcass weight was recorded; then, carcass yield was calculated as the ratio of carcass weight to live weight. With carcasses suspended by the hind legs, carcass length from the base of the tail to the base of the neck was measured [10]. One hour after refrigeration, carcass pH was measured non-destructively, using a portable pH meter (FiveGo pH meter F2, Mettler Toledo, Zaventem, Belgium), on the medial side of the right leg, following muscle incision. The average of two consecutive measurements on the same point of incision was calculated. Prior to use, the instrument was calibrated following the manufacturer’s instructions, with two buffer solutions of standard pH, 4.00 and 7.00.
2.2. Sample Collection
Samples of randomly selected carcasses from each LWC and sex were obtained for further analysis. Specifically, samples from a standardized area of the psoas major muscle were collected immediately after slaughter, for histological and morphometrical evaluation. In total, 76 samples were collected, 34 of which were from fat-tailed sheep. Furthermore, approximately 24 h after slaughter, meat samples 1–2 cm thick were obtained from the 13th rib of the left side of cold carcasses and from a standardized area of the quadriceps muscle in the left hind leg. A total of 99 meat samples from the 13th rib (58 of which were obtained from fat-tailed sheep carcasses) were vacuum-packed and stored at 4 °C for six days until they were subjected to quality assessment. Last, meat samples from the thigh region (M. quadriceps femoris), 43 in total (27 of which were from fat-tailed sheep carcasses) and approximately 100 g each, were vacuum-packed and stored at −20 °C until they were used for physicochemical analyses.
2.3. Muscle Histomorphometry
Formalin-fixed psoas major muscle samples were embedded in paraffin, cut at 4 μm, and stained with hematoxylin and eosin. For morphometry, fifteen 10× magnification muscle cross-section images were captured from each sample. Then, two to eight images were randomly selected for morphometrical analysis; a total of 1001 to 1766 muscle fibers per animal were analyzed. The muscle fiber minimum Feret’s diameter, which represents a geometrical parameter that remains largely unaffected by miscalculations due to orientation and sectioning angles [11], was automatically calculated in each image. Morphometry was performed using the “analyze particles” command of the ImageJ 1.53k software (NIH, Bethesda, MD, USA) based on a previously described methodology [12].
2.4. Meat Quality Assessment
Quality assessment of meat samples from the 13th rib included texture profile analysis (TPA), pH measurement and colorimetry. Meat color parameters (lightness—L*, redness—a*, yellowness—b*) were measured on freshly exposed meat samples directly after unpacking, using a colorimeter (CR-410 Chroma meter, Konica Minolta, New Jersey, USA) with 50 mm aperture size, illuminant C and 2° observer. Prior to scanning, calibration of the colorimeter was performed according to the manufacturer’s instructions with a white tile of standard parameters (Y: 94.8/X: 0.3130/y: 0.3190). Three consecutive scans perpendicular to the myofibrils were performed at the same position on each sample; between scans, the measuring head of the colorimeter was rotated 90 degrees clockwise. Measured values of L*, a* and b* were averaged over the three scans of each sample, and the mean values were considered for statistical analyses. Mean a* and b* values of each sample were used to calculate chroma and hue angle according to the following formulae, as described by the American Meat Society Association [13]:
Chroma (saturation index) = (a*2 + b*2)1/2
Meat pH measurement and TPA were performed as described previously by [14]. Briefly, a portable pH meter (FiveGo pH meter F2, Mettler Toledo, Zaventem, Belgium) was used. The average value of two consecutive measurements on the same point of each sample was used for statistical analyses. A Stable Micro Systems TA.HD plus Texture Analyser with a flat-faced cylindrical 1.27 cm diameter probe and the Exponent software (version 6.1.16.0, Stable Micro Systems Ltd., Surrey, UK) were used for TPA. Oval-shaped pieces (1–2 cm thick) were extracted from the center of meat samples and used in a double-compression cycle test. Probe pre-test speed of 1.00 mm/s and test and post-test speed of 5.00 mm/s were implemented, achieving a 40% deformation in each cycle. Time between cycles was 2.02 s. The calculated parameters included “Hardness 1”, “Hardness 2”, “Cohesiveness”, “Springiness” and “Chewiness” as previously described by Skaperda et al. [14]. The first two represented the hardness of meat samples during the first and second bite, respectively. Cohesiveness and springiness indicated resistance to deformation and capacity of recovering after deformation, respectively, whereas chewiness represented the energy required to chew samples until they can be swallowed [15,16].
2.5. Physicochemical Properties of Meat
Physicochemical analyses of meat samples from the thigh region included measurements of total lipid, protein and moisture content. Samples were first comminuted with a Warring laboratory blender. For the estimation of moisture content, 3 g of meat were placed on an aluminum pan and analyzed at 105 °C with a dedicated moisture analyzer (MB27, Ohaus, Parsippany, NJ, USA), according to the manufacturer’s instructions. Total lipid content was estimated with the Weibull–Stoldt method, with hydrolysis as the first step and extraction in Soxtherm, according to AOAC method 991.36. In brief, a meat sample of 10 g was placed into a beaker. The protein of the sample was digested with boiling hydrochloric acid (4 mol/L) in order to break the lipo-protein bonds. Immediately after hydrolysis, the digestion mixture was filtrated with a pleated filter moistened with water. After drying, the remaining fat was extracted from the filter with petroleum ether of 40 to 60 °C boiling range. Following solvent evaporation, the samples were dried and weighed. Total lipid content was calculated based on the difference between the initial sample weight and the weight at the end of analysis. Total protein was determined according to AOAC Official Method 928.08. In brief, 1.5 g of meat was placed in digestion tubes. The organically bound nitrogen of the sample was digested at 400 °C, with concentrated sulphuric acid and a catalyst, and broken down to ammonium sulphate. After the addition of 32% sodium hydroxide, ammonia was released by water stream distillation and trapped in a 2% solution of boric acid. The solution was then titrated against a 0.1 mL/L hydrochloric acid solution. The total protein content of the sample was calculated by multiplying the total nitrogen content by 6.25.
2.6. Statistical Analyses
Data were analyzed with R programming language (software version 4.1.2, R core team, Vienna, Austria) [17]. Descriptive statistics were calculated using “psych” and “dplyr” statistical packages [18,19]. Regarding body (live weight, wither height, muscle fiber minimum Feret’s diameter), carcass (hot carcass weight, carcass yield/length/pH) and meat quality traits (meat pH/moisture/protein content/lipid content, TPA parameters—hardness 1, hardness 2, springiness, cohesiveness, chewiness—and meat color traits—L*, a*, b*, chroma, hue angle) of fat-tailed sheep, differences between sexes and LWCs were assessed with non-parametric Mann–Whitney U tests and Kruskal–Wallis one-way analyses of variance, respectively. Following Kruskal–Wallis analyses, Dunn’s tests with Bonferroni correction were used to detect statistically significant differences between groups. The latter analyses were not performed on muscle fiber minimum Feret’s diameter due to the limited number of observations in some LWCs of fat-tailed sheep (less than four per LWC).
Mann–Whitney U tests were also performed to compare fat-tailed and thin-tailed sheep concerning the above traits. The effect of sheep population (fat-tailed or thin-tailed) on the above traits, when accounting also for the effects of LWC or sex and their interaction with sheep population, was assessed with two-way analyses of variance (ANOVA). The models for the latter analyses were of the following general form:
where Yghi is the dependent variable (one of the body, carcass or meat quality traits, as presented above), μ is the overall population mean, SPg is the fixed effect of sheep population (2 levels: fat-tailed, thin-tailed), LSh is the fixed effect of LWC (5 levels: 25, 35, 50, 70, 100% of mature weight) or sex (2 levels: male, female), SPgLSh is the fixed effect of the interaction between sheep population and LWC or sex, and eghi is the residual error.
Yghi = μ + SPg + LSh + SPgLSh + eghi,
Tukey’s range tests were performed for post hoc testing to allow for pairwise comparisons between groups. ANOVA and Tukey’s tests including LWC and the interaction effects were not performed on meat moisture, protein content, lipid content and muscle fiber minimum Feret’s diameter, due to lack of observations (less than or equal to 1) in some groups. Homogeneity of variances and normality of distributions were assessed with Levene’s test and Kolmogorov–Smirnov test, respectively (p > 0.05). Residuals versus fitted and Q–Q plots of the analyses were considered, as well. In the case that the assumptions of homoscedasticity and normality of ANOVA residuals were not met, a non-parametric equivalent Scheirer–Ray–Hare test was performed. Specifically, the effects of LWC, sheep population and their interaction on live weight, carcass length, hot carcass weight, carcass yield, carcass pH, L*, a*, chroma, meat pH and cohesiveness were assessed with Scheirer–Ray–Hare tests. The same applied for the effects of sex, sheep population and their interaction on live weight, carcass length, hot carcass weight, carcass yield, carcass pH, muscle fiber minimum Feret’s diameter, meat pH, hardness 1 and 2, chewiness, moisture and lipid and protein content. Statistical packages “stats” and “rcompanion” [17,20] were used for the above analyses, and level of statistical significance was set at p = 0.05.
3. Results
3.1. Descriptive Statistics and Quality Assessment of Fat-Tailed Sheep Carcasses
Descriptive statistics of all studied traits for both fat-tailed and thin-tailed sheep per LWC and sex are presented in Table 2 (a and b, respectively). Mann–Whitney U tests revealed statistically significant differences between female and male fat-tailed sheep regarding live weight (W = 3184, p < 0.05), carcass yield (W = 1803, p < 0.001), muscle fiber minimum Feret’s diameter (W = 59, p < 0.01), L* (W = 271.5, p < 0.05) and hue angle (W = 274, p < 0.05). The medians of the above traits were lower for females than males; the only exception was live weight. Furthermore, larger interquartile ranges were observed for females, except for muscle fiber minimum Feret’s diameter (Table 3). Results of Kruskal–Wallis one-way analyses of variance show statistically significant differences between different LWCs of fat-tailed sheep (Table 4) regarding all body (live weight: H(4) = 138.39, wither height: H(4) = 120.37—p < 0.001) and most carcass (carcass length: H(4) = 118.27, hot carcass weight: H(4) = 132.36, carcass yield: H(4) = 85.46—p < 0.001) and meat quality traits (L*: H(4) = 24.526—p < 0.001, hue angle: H(4) = 10.646—p < 0.05, meat pH: H(4) = 12.333—p < 0.05, hardness 1: H(4) = 14.017—p < 0.01, hardness 2: H(4) = 13.978—p < 0.01, springiness: H(4) = 11.405—p < 0.05, chewiness: H(4) = 16.796—p < 0.01). Statistically significant (p < 0.05) pairwise comparisons between mean ranks of LWC for each of the above traits are presented in Figure 1. Regarding body and carcass traits, almost all differences between LWC were significant; the exceptions were 50% with 70% and 70% with 100% LWC. Moreover, wither height, carcass length and weight of 35% were not significantly different from that of 50% LWC, nor were carcass yields of 25% and 35% LWC. Few significant pairwise comparisons were observed regarding meat color and tenderness traits, most of which were observed for L* and meat chewiness; L* of 25% and 35% LWC differed from that of 70% and 100%, whereas meat chewiness of 25% was different from 35% and 50% LWC and that of 50% from 100% LWC. Differences regarding physicochemical characteristics of meat were not statistically significant (p > 0.05).

Table 2.
(a). Descriptive statistics for body, carcass and meat quality traits of fat-tailed and thin-tailed sheep per live-weight category (LWC). (b) Descriptive statistics for body, carcass and meat quality traits of fat-tailed and thin-tailed sheep per sex.

Table 3.
Results of non-parametric Mann–Whitney U tests performed to compare between sexes of fat-tailed sheep (rejection of null hypothesis when p < 0.05: Differences between medians of groups are statistically significant, hence groups come from different populations).

Table 4.
Results of non-parametric Kruskal–Wallis one-way analyses of variance for assessing the statistical significance of the differences between mean ranks of the studied traits for different live-weight categories (LWC—% of mature live weight) of fat-tailed sheep (rejection of null hypothesis when p < 0.05: There are statistically significant differences between mean ranks of groups).
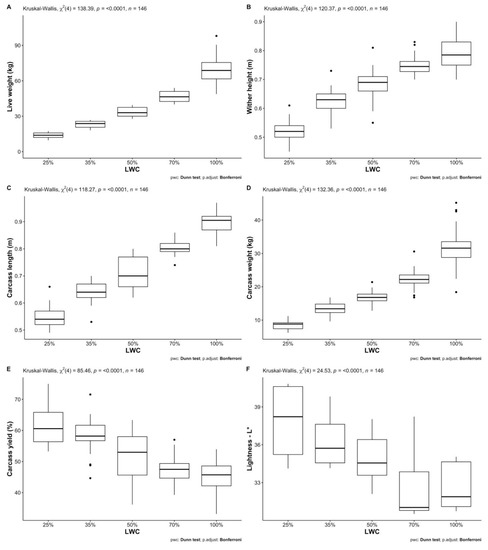
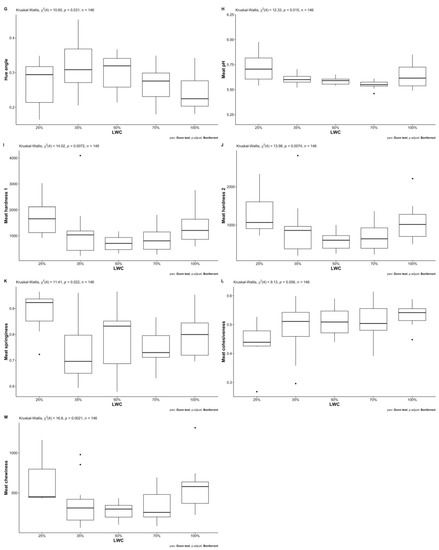
Figure 1.
Dunn’s post hoc analysis for pairwise comparisons between live-weight categories (LWC) of fat-tailed sheep regarding the tested traits: (A) Live weight—in kg; (B) Wither height—in m; (C) Carcass length—in m; (D) Carcass weight—in kg; (E) Carcass yield—as %; (F) Lightness—L*; (G) Hue angle; (H) Meat pH; (I) Meat hardness 1; (J) Meat hardness 2; (K) Meat springiness; (L) Meat cohesiveness; (M) Meat chewiness.
3.2. Comparison of Fat-Tailed and Thin-Tailed Sheep Carcasses
3.2.1. The Effect of Sheep Population
Mann Whitney U tests revealed statistically significant differences between fat-tailed and thin-tailed sheep regarding carcass yield (W = 8544.5, p < 0.01) and a* (W = 923.5, p < 0.05). The median carcass yield of fat-tailed sheep was 53.74% compared to 48.77% for thin-tailed sheep, whereas for a*, the respective values were 12.44 and 13.13 (Table 5).

Table 5.
Results of non-parametric Mann–Whitney U tests performed to compare fat-tailed sheep to thin-tailed sheep (rejection of null hypothesis when p < 0.05: Differences between medians of groups are statistically significant, hence groups come from different populations).
3.2.2. Effects of the Interaction of Sheep Population with LWC
Statistically significant results of the two-way ANOVA, including the effects of the interactions of sheep population with LWC on the studied traits, are presented in Table 6. A detailed version of the table also containing non-significant results can be found in the supplementary materials section (Table S1a). In the aforementioned analyses, the effect of sheep population was statistically significant for wither height (f(1) = 5.53, p < 0.05); compared to fat-tailed sheep, thin-tailed sheep were on average shorter by 0.01 m. The respective significant effects of the Scheirer–Ray–Hare tests are presented in Table 7 (detailed version of the table provided in the supplementary materials section—Table S2a); results show that sheep population significantly affected carcass yield (H(1) = 7.44, p < 0.01) and a* (H(1) = 4.23, p < 0.05).

Table 6.
(a) Statistically significant effects (p < 0.05) of sheep population (fat-tailed or thin-tailed sheep), live-weight category (LWC—% of mature live weight) and their interaction on studied traits, as estimated based on two-way ANOVA. (b) Statistically significant effects (p < 0.05) of sheep population (fat-tailed or thin-tailed sheep), sex and their interaction on studied traits, as estimated based on two-way ANOVA.

Table 7.
(a). Statistically significant effects (p < 0.05) of sheep population (fat-tailed or thin-tailed sheep), live-weight category (LWC) and their interaction on studied traits, as estimated by Scheirer–Ray–Hare tests. (b) Statistically significant effects (p < 0.05) of sheep population (fat-tailed or thin-tailed sheep), sex and their interaction on studied traits, as estimated by Scheirer–Ray–Hare tests.
ANOVA results indicate also that the interaction of sheep population with LWC had significant effects on wither height (f(4) = 3.45, p < 0.01), b* (f(4) = 2.56, p < 0.05), hue angle (f(4) = 4.63, p < 0.01) and springiness (f(4) = 3.77, p < 0.01). Tukey’s post hoc tests revealed statistically significant pairwise differences between groups, as shown in Figure 2. Scheirer–Ray–Hare tests showed that meat cohesiveness (H(4) = 11.84, p < 0.05) and carcass pH (H(4) = 9.80, p < 0.05) were significantly affected by the interaction of sheep population with LWC.
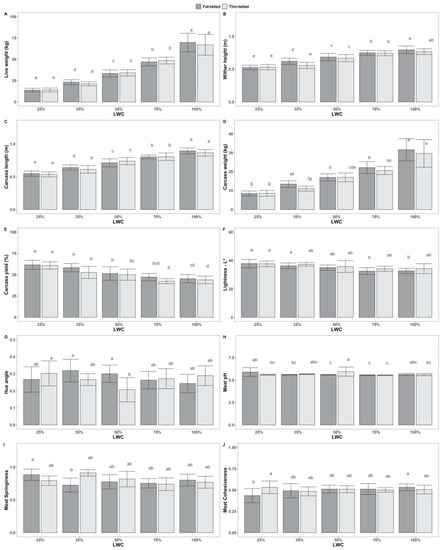
Figure 2.
Tukey’s post hoc tests for pairwise comparisons between groups, following two-way ANOVA, accounting for the effect of the interaction between live-weight categories (LWC—25, 35, 50, 70 and 100% of mature live weight) and sheep populations (fat-tailed, thin-tailed) on the tested traits: (A) Live weight—in kg; (B) Wither height—in m; (C) Carcass length—in m; (D) Carcass weight—in kg; (E) Carcass yield—as %; (F) Lightness—L*; (G) Hue angle; (H) Meat pH; (I) Meat Springiness; (J) Meat Cohesiveness. Superscripts not sharing any common letter between groups indicate statistically significant differences (p < 0.05).
3.2.3. Effects of the Interaction of Sheep Population with Sex
When accounting for the effects of the interactions of sheep population with sex, ANOVA results (significant effects presented in Table 6—full version including all effects in Table S1b) show that sheep population had no significant effect on the studied traits (p > 0.05). Scheirer–Ray–Hare tests (Table 7 and Table S2b present significant effects only and total effects, respectively) showed that thin-tailed sheep had significantly lower carcass yield (H(1) = 7.44, p < 0.01).
Regarding the interaction of sheep population with sex, significant effects were found on a* (f(1) = 4.03, p < 0.05) and chroma (f(1) = 4.37, p < 0.05), based on ANOVA. Results of Tukey’s post hoc tests that were statistically significant are presented in Figure 3. Moreover, a significant effect of the interaction was found on carcass pH (H(1) = 4.67, p < 0.05) according to Scheirer–Ray–Hare tests.
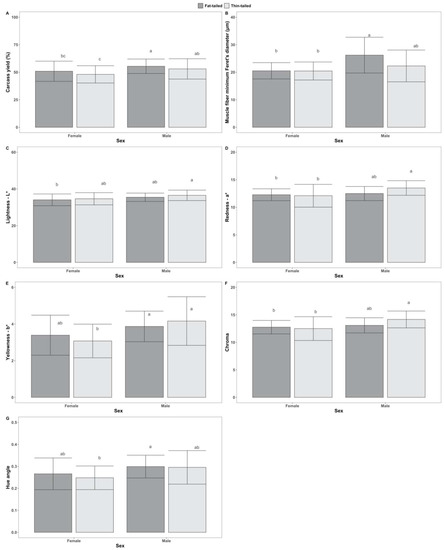
Figure 3.
Tukey’s post hoc tests for pairwise comparisons between groups, following two-way ANOVA, accounting for the effect of the interaction between sex (male, female) and sheep populations (fat-tailed, thin-tailed) on the tested traits: (A) Carcass yield—as %; (B) Muscle fiber minimum Feret’s diameter—in μm; (C) Lightness—L*; (D) Redness—a*; (E) Yellowness—b*; (F) Chroma; (G) Hue angle. Superscripts not sharing any common letter between groups indicate statistically significant differences (p < 0.05).
4. Discussion
4.1. Quality Assessment of Fat-Tailed Sheep Carcasses
Mean values of body and carcass traits of fat-tailed sheep reported herein present both similarities and differences compared to respective estimations of relevant studies on Chios and other fat-tailed sheep breeds. Wither height was similar to that reported in literature (ca. 0.60 m) for Chios crosses of live weight corresponding to 35% LWC of the present study [21,22]. Present results of mean wither height for 70 and 100% LWC are also in general accordance with those of Awassi (Jordan, Turkey), Red Karaman (Turkey), Barki (Libya, Egypt), Ossimi and Rahmani (Egypt); averages ranged from 0.72 to 0.74 m [23,24,25]. However, adult sheep of other fat-tailed breeds reared in the Mediterranean basin, Middle East and Northern Africa had lower mean wither height (0.54–0.70 m) [26].
Regarding carcass traits, Obeidat et al. [21] reported lower mean hot carcass weight (9.2 kg) and yield (47.0%) of Chios crosses corresponding to 35% LWC, whereas Ekiz et al. [27] presented lower mean carcass yield (54.6%) but slightly higher mean carcass weight (14.6 kg) and length (0.69 m). Chios crosses with a mean live weight corresponding to 50% LWC presented lower mean carcass weights (12.5–13.2 kg, depending on management practices) and yields (44.2–45.6%) compared to present results [28]. Differences regarding carcass dressing between the present study and the previous studies may be the underlying cause of the observed deviations. Herein, light and midweight carcasses (25%, 35% and 50% LWC) included head and some viscera, resulting in higher yields, whereas, in the other studies, these carcass parts were removed during dressing. Implementation of a uniform carcass dressing across all studies would possibly limit the observed discrepancies. Earlier studies [29,30], concerning Chios lambs slaughtered at approximately 50% of mature live weight and dressed as herein, reported similar (16.5 kg) and slightly higher mean carcass weights (20.3 kg) and yields (54.6% and 59.5%, respectively) compared to ours. Although these studies are about sheep of the same breed and similar live weight as the present ones, they are quite dated; hence, it is likely that the studied population has evolved, causing the observed discrepancies. Soycan Önenç et al. [31] have found that intensively reared Chios sheep of live weight corresponding to 70% LWC produced carcasses marginally lighter (20.4 kg) and shorter in length (0.61 m) that yielded less (40.8%). The observed differences with the present study most likely reflect true variability between populations and management practices, given that mean live weights and carcass dressing were similar.
Concerning carcasses of other fat-tailed sheep breeds, highly variable length averages (0.53–1.04 m for adult sheep and 0.44–0.99 m for lambs) have been reported [26]; such results are not directly comparable with ours, since values of studied traits were averaged based on the age of the animals, whereas in the present study, data were distinguished based on sheep live weight or sex. Studies reviewed by Mohapatra and Shinde [32] reported that Awassi, Karaman and indigenous Iranian fat-tailed sheep in 50 and 70% LWC presented high variability regarding mean carcass weights (12.2–18.9 kg and 19.1–26.4 kg, respectively) and yields (39.7–49.7% and 46.0–50.8%, respectively). In the present study, respective values of 70% LWC are within the above ranges, whereas mean yield of 50% LWC is slightly higher, probably due to carcass dressing differences discussed above. Awassi carcasses corresponding to the present study’s 35, 50 and 70% LWC had lower mean weights (8.5, 14.1 and 18.4, respectively) and higher yield (ca. 50%), except for the 35% LWC, as expected due to the dissimilar dressing [33]. Fat-tailed Iranian Chaal and Zandi and Egyptian Ossimi, Barki and Rahmani sheep, fattened up to live weights within 70% LWC, presented higher mean carcass yield (53.5–58.2% for Iranian and 53.1–56.2% for Egyptian breeds) and ranging mean carcass weight (slightly lower for Iranian, 20.1–21.5 kg, and higher for Egyptian, 24.3–26.1 kg) [25,34]. Since carcass dressing was similar to that of the present study, the higher yield of Iranian and Egyptian fat-tailed sheep carcasses can be attributed at least partly to the fattening periods that preceded slaughter; it is likely that fattening of Greek fat-tailed Chios sheep and their crosses may also increase carcass yields.
Studies regarding carcass pH and meat quality parameters (pH/color/tenderness) of Chios sheep are rather scarce. In agreement with the present results, Ekiz et al. [27] reported a mean carcass pH of ca. 6.5, measured approximately 45 min after slaughter, for Chios sheep with mean live weight corresponding to 35% LWC. They also report slightly higher L* and a* and ranging b* values. Relevant studies have reported higher L*, b* and chroma values of meat samples from Awassi, indigenous Turkish and Iranian fat-tailed sheep corresponding to 35%, 50% and 70% LWC of the present study [35,36,37]. Higher L* and a* and lower b* values were reported for Kangal Akkaraman sheep [38]. Slightly higher values of all color parameters were reported for meat of Awassi sheep in 70% LWC [39]. In all the above cases, measurements were performed in a smaller time interval after slaughter (24 h) and different sample preparation, illuminant, aperture size and observer angle were implemented compared to the present study. Such differences may partly explain the observed discrepancies. This hypothesis is further supported by the fact that in our study, meat color parameters changed among LWCs following trends similar to other studies, in which heavier carcasses produced darker meat (higher L*), whereas a* and b* were approximately the same regardless of carcass weight [36]. Thus, color parameter differences of other studies and the present one may reflect different methods and equipment settings used. In most of the above studies, meat tenderness was assessed, as well; Warner–Bratzler shear force tests and cooked meat were used, and hence results are not comparable to the present ones.
Muscle fiber minimum Feret’s diameter, a trait commonly used in human and veterinary medical studies involving investigation of muscle fiber microstructure, is rarely used for meat quality assessment. However, increased muscle fibers’ diameter is important to the meat production industry, since it has been correlated with higher meat yield and more tender meat [40]. Relevant studies on pigs, beef and avian species have been published recently [40,41,42,43,44,45]; to our knowledge, no relevant studies on sheep are available. Results of the present study show a tendency for increased diameter of muscle fibers in heavier carcasses. Fat-tailed sheep in 70% LWC presented the largest diameter. The latter suggests that young adult sheep that have not reached the end of their dairy productive life are more likely to produce carcasses of higher quality; normally, sheep at this productive stage have not been exposed to stress and challenges related to consecutive reproductive periods and lactations, and hence they retain a healthier, balanced and robust body conformation.
Concerning the physicochemical characteristics of meat, slightly higher protein and lipid contents and lower moisture were observed in our study compared to other relevant ones [36,37,39]; light carcasses (corresponding to 35% LWC) were the exception, in which case present results indicated lower protein content. In all cases, differences regarding protein content were slight (ca. 1–2%), whereas lipid contents differed more strongly (ranging roughly from 2 to 8%). The lower moisture content is most likely caused by the thawing process to which the present samples were subjected; in the other studies, fresh samples were used. Given the lower moisture content of our samples, the higher protein and fat contents may be attributed at least partly to condensing. Nevertheless, fat content differences are relatively large, possibly indicating an actual trend of higher fat content in Chios sheep carcasses compared to other breeds.
In agreement with studies on Chios or other fat-tailed sheep [46,47], statistically significant differences between male and female sheep were detected, regarding live weight, carcass yield, muscle fiber minimum Feret’s diameter, L* and hue angle. Interestingly, females presented higher live weight; this could be associated with the fact that carcasses of female sheep in the heavier LWC slightly outnumbered those of male sheep. On the contrary, male carcass yield and muscle fiber minimum Feret’s diameter were larger. This agrees with the fact that sex-related anatomical differences generally render male sheep more muscular than female. Color parameter differences suggest that meat of female sheep is darker (lower L*) and more reddish (lower hue angle); however, they are very slight and need to be interpreted with caution. Likewise, Yousefi et al. [36] reported that female Chaal lambs had statistically significant higher a*, which also results in more reddish meat.
Overall, in previous studies, largely ranging values were observed for most studied traits. Categorization of sheep and their carcasses according to their weight facilitated comparisons between the present study and other studies. The importance of this categorization is underpinned by the fact that the observed differences among LWCs of the present study were statistically significant for most traits. Considering the absence of quality-improving feed interventions prior to slaughter and given that all studied animals are considered “by-products” of dairy production, slaughtered at different live weights and productive stages, large variability was expected regarding the studied traits. However, this indicates that there may be space for improving fat-tailed sheep carcass and meat quality. The different LWCs defined in the present study equally represent very light lamb carcasses (25% LWC) and heavy carcasses of adult sheep (100% LWC), which are mainly produced in Greece, as well as midweight carcasses, which are generally scarce (35, 50, 70% LWC). Comparison with the respective data of other mentioned studies implies improvement potential through implementing appropriate fattening protocols.
Considering all tested traits, fat-tailed sheep in 50 and 70% LWC produce carcasses of the highest yield and best quality across all LWC. Specifically, they are characterized by muscle fibers with larger diameter, lower L* and hue angle, higher a* and chroma, moderate b*, lower values of TPA parameters, higher moisture and protein content and lower lipid content. For all the above traits, except for chroma, a*, b*, meat cohesiveness and meat composition traits, the effect of LWC as well as some differences between groups were statistically significant (p < 0.05). Effects that did not reach statistical significance showed a positivity tendency, implying higher quality of these carcasses; hence, further investigation on a larger sample is warranted. Carcasses of 50 and 70% LWC had higher moisture content, thicker muscle fibers, lower values of TPA parameters and meat composition that complied with consumer demands for leaner [4] and more tender meat [40,48]. Meat color profile indicated that meat was more reddish and less yellowish, with a more intense and vivid hue, which are relevant to the ideal bright red color that consumers associate with freshness [13,49]. Therefore, implementation of appropriate fattening protocols up to finishing weights corresponding to 50 and 70% LWC may increase meat yields (compared to currently common light carcasses of ca. 9 kg), improve meat quality and capitalization of fat-tailed sheep carcasses and overall boost the sheep meat industry. This is further supported by other studies reporting optimum finishing weights of 44 and 30 kg (corresponding to 70% and 50% LWC) for fat-tailed Kangal Akkaraman and Awassi sheep, respectively, that maximize meat quantity without compromising quality [33,50].
4.2. Comparison of Fat-Tailed and Thin-Tailed Sheep Carcasses
To support the validity of our inferences, two different statistical approaches were involved in the comparison of fat-tailed and thin-tailed sheep: Mann–Whitney U tests and either two-way ANOVA or its non-parametric equivalent Scheirer–Ray–Hare tests, depending on compliance of the distribution of each studied trait with the assumptions of ANOVA. Results of Mann–Whitney U tests were further assessed with two-way ANOVA that accounted also for the effects of LWC or sex and their interaction with sheep population; the latter explained part of the observed variation, otherwise attributed only to the sheep population effect. In cases where ANOVA assumptions were violated, Scheirer–Ray–Hare tests were performed to avoid misleading results; as non-parametric, the latter tests were considered more reliable for the interpretation of effects in such cases.
Results from the above analyses suggest that fat-tailed sheep carcass yield was higher than that of thin-tailed. This is in accordance with the study of Panopoulou et al. [29], in which the carcass yield of Chios sheep was higher (by 2.2%) compared to a thin-tailed indigenous Greek sheep breed, Karagouniko. On the contrary, Ekiz et al. [27] have found that Chios reared in Turkey yielded less than Merino and Kivircik thin-tailed sheep (by 2.6%). Regarding other fat-tailed breeds, Akkaraman sheep presented consistently higher carcass yield compared to thin-tailed Anatolian Merino under different feeding systems [51]. Furthermore, Iranian fat-tailed Chaal and Zandi sheep presented higher carcass yield than thin-tailed Zel sheep or their crosses; however, these differences were not always statistically significant [34,52]. Based on the above, fat-tailed sheep are not consistently presenting higher yields compared to thin-tailed. This could potentially be attributed to underlying differences among studied populations regarding management practices, sampling methods or sample size variance. However, the present results, supported by the evidence of another study concerning Greek sheep [29], suggest that Chios and their crosses achieve higher carcass yields compared to local thin-tailed populations.
Meat of the studied fat-tailed sheep had statistically significant lower a* compared to that of thin-tailed, based on statistical analyses performed. These findings are in accordance with other studies that have also reported respective differences between Chios and thin-tailed Kivircik and Imroz sheep [27] and between fat-tailed Chaal and thin-tailed Zel sheep [52]. Lower a* represents meat color that is more brownish than reddish, hence less desirable to consumers. However, in order to make safe assumptions, all color parameters should be considered. Present results indicate that chroma, b* and hue angle were affected by the interaction of sheep population with sex or LWC (p < 0.05). This observation is in accordance with Yousefi et al. [52], who have reported significantly different b* between fat-tailed and thin-tailed sheep. Overall, in the present study, sheep population affects meat color; fat-tailed sheep present a trend towards less desirable meat color; however, differences from thin-tailed are minimal. Further investigation of a larger sample size may facilitate quantifying the overall impact.
Wither height was significantly affected by sheep population and its interaction with LWC, emphasizing unique morphological features between the two populations; fat-tailed sheep were taller than thin-tailed by approximately 1 cm. However, due to the scarcity of similar studies concerning this trait, assessing the relevance of our observations is rather difficult.
Statistically significant effects of the interaction of sheep population and LWC on meat springiness and cohesiveness were also detected, suggesting that meat of fat-tailed sheep is more tender (lower springiness and cohesiveness) compared to that of thin-tailed. Yousefi et al. [52] reported the opposite regarding Iranian thin-tailed Zel and fat-tailed Chaal lambs; however, assessment methods were different than those of the present study. Unique features of each population may be the underlying cause for the observed discrepancies.
The interactions with LWC and sex had statistically significant effects on carcass pH. Similar significant differences (p < 0.05) between carcasses of animals slaughtered at different live weights have been reported in literature for both fat-tailed and thin-tailed sheep, however, in these cases pH was measured at a larger time interval of 24 h; differences between sexes were not statistically significant [36,53].
Meat physicochemical characteristics did not differ between sheep populations. On the contrary, Aksoy et al. [37] have reported such differences between fat-tailed and thin-tailed sheep for one or more of the studied traits. In our study, the small sample size used for the relevant analyses may be insufficient for the detection of possible significant differences between groups or overall effects. In future studies, assessment of the above effects with a larger sample size may facilitate safe assumptions. Furthermore, evaluation of the fatty acid profile, which is commonly performed in relevant studies [36,37,52,54], may provide further insight.
5. Conclusions
The present results indicate that carcasses and meat of Chios fat-tailed sheep (and their crosses) reared in Greece present desirable characteristics, in most cases exceeding the performance of local thin-tailed sheep, which are generally considered to produce leaner meat of higher quality. Most of the studied traits were significantly affected by LWC and/or sex. Live weight at slaughter is key to maximizing meat production, capitalizing and minimizing waste. Present results suggest that fattening of lambs until finishing weights corresponding to 50 and 70% LWC could improve the otherwise low marketability of fat-tailed sheep carcasses, therefore contributing to the overall production efficiency and sustainability of the sector.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12151998/s1, Table S1a: Effects of sheep population (fat-tailed or thin-tailed sheep), live-weight category (LWC—% of mature live weight) and their interaction on studied traits, as estimated based on two-way ANOVA; Table S1b: Effects of sheep population (fat-tailed or thin-tailed sheep), sex and their interaction on studied traits, as estimated based on two-way ANOVA; Table S2a: Effects of sheep population (fat-tailed or thin-tailed sheep), live-weight category (LWC) and their interaction on studied traits, as estimated by Scheirer–Ray–Hare tests; Table S2b: Effects of sheep population (fat-tailed or thin-tailed sheep), sex and their interaction on studied traits, as estimated by Scheirer–Ray–Hare tests.
Author Contributions
Conceptualization, A.A., A.T., I.S., T.K., V.E. and G.A.; methodology, A.A., A.T., I.S., S.V., T.K., V.E. and G.A.; validation, A.A., A.T., I.S., V.E. and G.A.; formal analysis, A.A.; investigation, A.A., A.T., I.S., S.V. and T.K.; resources, T.K., V.E. and G.A.; data curation, A.A.; writing—original draft preparation, A.A.; writing—review and editing, A.A., A.T., I.S., S.V., T.K., V.E. and G.A.; visualization, A.A.; supervision, V.E. and G.A.; project administration, G.A.; funding acquisition, G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH—CREATE—INNOVATE (project code: T1EDK-05479—project: Greek Quality Meat—GreQuM).
Institutional Review Board Statement
The study was part of the project Grequm (T1EDK-05479) that was approved for its experimental methodologies and ethics by a designated committee of the Greek General Secretary for Research and Innovation following national and European regulations.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hellenic Statistical Authority. Livestock and Livestock Products 2019 (in Greek). 2019. Available online: https://www.statistics.gr/el/statistics/-/publication/SPK33/- (accessed on 30 March 2022).
- Zygoyiannis, D.; Kyriazakis, I.; Stamataris, C.; Friggens, N.C.; Katsaounis, N. The growth and development of nine European sheep breeds. 2. Greek breeds: Boutsko, Serres and Karagouniko. Anim. Sci. 1997, 65, 427–440. [Google Scholar] [CrossRef]
- Mandolesi, S.; Naspetti, S.; Arsenos, G.; Caramelle-Holtz, E.; Latvala, T.; Martin-Collado, D.; Orsini, S.; Ozturk, E.; Zanoli, R. Motivations and barriers for sheep and goat meat consumption in Europe: A means–end chain study. Animals 2020, 10, 1105. [Google Scholar] [CrossRef]
- Tsitsos, A.; Economou, V.; Arsenos, G.; Kalitsis, T.; Argyriadou, A.; Theodoridis, A. Greek and European consumer behaviour towards beef, lamb and Mutton Meat Safety and quality: A Review. Int. J. Agric. Resour. Gov. Ecol. 2021, 17, 414–431. [Google Scholar] [CrossRef]
- Van Zanten, H.H.E.; Herrero, M.; Van Hal, O.; Röös, E.; Muller, A.; Garnett, T.; Gerber, P.J.; Schader, C.; De Boer, I.J.M. Defining a land boundary for sustainable livestock consumption. Glob. Change Biol. 2018, 24, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- European Commission. EU Agricultural Outlook for Markets, Income and Environment, 2021–2031; European Commission, DG Agriculture and Rural Development: Brussels, Belgium; Available online: https://ec.europa.eu/info/sites/default/files/food-farming-fisheries/farming/documents/agricultural-outlook-2021-report_en.pdf (accessed on 30 March 2022).
- Zygoyiannis, D.; Stamataris, K.; Kouimtzis, S.; Doney, J.M. Carcass composition in lambs of Greek dairy breeds of sheep. Anim. Sci. 1990, 50, 261–269. [Google Scholar] [CrossRef]
- Mastrangelo, S.; Moioli, B.; Ahbara, A.; Latairish, S.; Portolano, B.; Pilla, F.; Ciani, E. Genome-wide scan of fat-tail sheep identifies signals of selection for fat deposition and adaptation. Anim. Prod. Sci. 2019, 59, 835–848. [Google Scholar] [CrossRef]
- European Parliament and Council. Council Regulation (EC) No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing. Available online: https://eur-lex.europa.eu/eli/reg/2009/1099 (accessed on 12 May 2022).
- Álvarez, J.M.; Rodríguez Iglesias, R.M.; García Vinent, J.; Giorgetti, H.; Rodríguez, G.; Baselga, M. Introduction of sheep meat breeds in extensive systems: Lamb carcass characteristics. Small Rumin. Res. 2013, 109, 9–14. [Google Scholar] [CrossRef]
- Briguet, A.; Courdier-Fruh, I.; Foster, M.; Meier, T.; Magyar, J.P. Histological parameters for the quantitative assessment of muscular dystrophy in the MDX-mouse. Neuromuscul Disord. 2004, 14, 675–682. [Google Scholar] [CrossRef]
- Varian, B.J.; Goureshetti, S.; Poutahidis, T.; Lakritz, J.R.; Levkovich, T.; Kwok, C.; Teliousis, K.; Ibrahim, Y.M.; Mirabal, S.; Erdman, S.E. Beneficial bacteria inhibit cachexia. Oncotarget 2016, 7, 11803–11816. [Google Scholar] [CrossRef] [Green Version]
- AMSA. AMSA Meat Color Measurement Guidelines; AMSA: Champaign, IL, USA, 2012; ISBN 61820 800-517-2672.
- Skaperda, Z.; Argyriadou, A.; Nechalioti, P.M.; Alvanou, M.; Makri, S.; Bouroutzika, E.; Kyriazis, I.D.; Tekos, F.; Veskoukis, A.S.; Kallitsis, T.; et al. Redox biomarker baseline levels in cattle tissues and their relationships with meat quality. Antioxidants 2021, 10, 958. [Google Scholar] [CrossRef]
- Trinh, T. On The Texture Profile Analysis Test. In Proceedings of the Chemeca 2012, Wellington, New Zealand, 23–26 September 2012. [Google Scholar]
- Peleg, M. The instrumental texture profile analysis revisited. J. Texture Stud. 2019, 50, 362–368. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, version 4.1.2; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 31 March 2022).
- Revelle, W. psych: Procedures for Personality and Psychological Research, version 2.1.9; Northwestern University: Evanston, IL, USA, 2021; Available online: https://CRAN.R-project.org/package=psych (accessed on 31 March 2022).
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation, R Package version 1.0.8; 2022. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 31 May 2022).
- Mangiafico, S. Rcompanion: Functions to Support Extension Education Program Evaluation, R Package version 2.4.13; 2022; Available online: https://CRAN.R-project.org/package=rcompanion (accessed on 31 May 2022).
- Obeidat, M.D.; Abdullah, A.Y.; Kolib, S.; Obeidat, B.S.; Jawasreh, K.; Subih, H.S. Pre-weaning growth performance and carcass traits of Awassi and Chios-awassi lambs. S. Afr. J. Anim. Sci. 2019, 49, 140. [Google Scholar] [CrossRef]
- Irim, I.; Akbas, A.A.; Saatci, M. Determination of fertility traits of sheep and growth characteristics of Chios crossbred male lambs reared under local breeder conditions. J. Istanb. Vet. Sci. 2020, 4, 102–108. [Google Scholar] [CrossRef]
- Topal, M.; Yildiz, N.; Esenbuğa, N.; Aksakal, V.; Macit, M.; Özdemir, M. Determination of best fitted regression model for estimation of body weight in Awassi sheep. J. Appl. Anim. Res. 2003, 23, 201–208. [Google Scholar] [CrossRef]
- Topai, M.; Macit, M. Prediction of body weight from body measurements in Morkaraman sheep. J. Appl. Anim. Res. 2004, 25, 97–100. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.Y. Body and carcass characteristics of Ossimi, Barki and Rahmani ram lambs raised under intensive production system. Egypt. J. Sheep Goat Sci. 2009, 4, 1–16. [Google Scholar]
- Pourlis, A.F. A review of morphological characteristics relating to the production and reproduction of fat-tailed sheep breeds. Trop. Anim. Health Prod. 2011, 43, 1267–1287. [Google Scholar] [CrossRef]
- Ekiz, B.; Yilmaz, A.; Ozcan, M.; Kaptan, C.; Hanoglu, H.; Erdogan, I.; Yalcintan, H. Carcass measurements and meat quality of Turkish Merino, Ramlic, Kivircik, Chios and Imroz Lambs raised under an intensive production system. Meat Sci. 2009, 82, 64–70. [Google Scholar] [CrossRef]
- Kocak, O.; Ekiz, B.; Yalcintan, H.; Yilmaz, A. Slaughter and carcass quality characteristics of Chios × Tahirova crossbred lambs under intensive, traditional and organic production systems. Ank. Üniv. Vet. Fak. Derg. 2016, 63, 187–193. [Google Scholar]
- Panopoulou, E.; Deligeorgis, S.G.; Papadimitriou, T.; Rogdakis, E. Carcass composition, size of fat cells and NADPH-generating dehydrogenases activity in adipose tissue of the fat-tailed Chios and the thin-tailed Karagouniko sheep breed. J. Anim. Breed. Genet. 1989, 106, 51–58. [Google Scholar] [CrossRef]
- Hadjipanayiotou, M.; Koumas, A. Carcass characteristics of equally mature Chios lambs and Damascus kids. Small Rumin. Res. 1994, 13, 71–77. [Google Scholar] [CrossRef]
- Soycan Önenç, S.; Özdoğan, M.; Erdoğan Ataç, F.; Taşkın, T. Fattening performance and carcass traits of Chios male lambs fed under traditional and intensive feeding conditions. Trop. Anim. Health Prod. 2012, 44, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Shinde, A.K. Fat-tailed sheep-an important sheep genetic resource for meat production in tropical countries: An overview. Indian J. Small Rumin. 2018, 24, 1–17. [Google Scholar] [CrossRef]
- Abdullah, A.Y.; Qudsieh, R.I. Carcass characteristics of Awassi ram lambs slaughtered at different weights. Livest. Sci. 2008, 117, 165–175. [Google Scholar] [CrossRef]
- Kashan, N.E.J.; Manafi Azar, G.H.; Afzalzadeh, A.; Salehi, A. Growth performance and carcass quality of fattening lambs from fat-tailed and tailed sheep breeds. Small Rumin. Res. 2005, 60, 267–271. [Google Scholar] [CrossRef]
- Abdullah, A.Y.; Qudsieh, R.I.; Nusairat, B.M. Effect of crossbreeding with exotic breeds on meat quality of Awassi lambs. Livest. Sci. 2011, 142, 121–127. [Google Scholar] [CrossRef]
- Yousefi, A.R.; Sadeghipanah, A.; Kohram, H.; Zare Shahneh, A.; Dadashpour Davachi, N.; Aghashahi, A.; Ponnampalam, E.N. Determination of optimum carcass weight for meat quality and fatty acid composition in fat-tailed male and female Chall lambs. Trop. Anim. Health Prod. 2019, 51, 545–553. [Google Scholar] [CrossRef]
- Aksoy, Y.; Çiçek, Ü.; Şen, U.; Şirin, E.; Uğurlu, M.; Önenç, A.; Kuran, M.; Ulutaş, Z. Meat production characteristics of Turkish native breeds: II. meat quality, fatty acid, and cholesterol profile of lambs. Arch. Anim. Breed. 2019, 62, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Ekiz, B.; Dilara Kecici, P.; Ziya Ograk, Y.; Yalcintan, H.; Yilmaz, A. Evaluation of the functionality of EUROP carcass classification system in thin-tailed and fat-tailed lambs. Meat Sci. 2021, 181, 108603. [Google Scholar] [CrossRef]
- Suliman, G.M.; Al-Owaimer, A.N.; El-Waziry, A.M.; Hussein, E.O.S.; Abuelfatah, K.; Swelum, A.A. A comparative study of sheep breeds: Fattening performance, carcass characteristics, meat chemical composition and quality attributes. Front. Vet. Sci. 2021, 8, 647192. [Google Scholar] [CrossRef]
- Liu, D.; Fan, W.; Xu, Y.; Yu, S.; Liu, W.; Guo, Z.; Huang, W.; Zhou, Z.; Hou, S. Genome-wide association studies demonstrate that TASP1 contributes to increased muscle fiber diameter. Heredity 2021, 126, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Duconseille, A.; François, O.; Bruno, P.; Lambert, C.; Peyron, M.A.; Hennequin, M. Measuring the effects of in vitro mastication on bolus granulometry of shredded meat: A proposal for a new methodological procedure. Food Res. Int. 2019, 116, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Kokoszyński, D.; Stęczny, K.; Żochowska-Kujawska, J.; Sobczak, M.; Kotowicz, M.; Saleh, M.; Fik, M.; Arpášová, H.; Hrnčár, C.; Włodarczyk, K. Carcass Characteristics, Physicochemical Properties, and Texture and Microstructure of the Meat and Internal Organs of Carrier and King Pigeons. Animals 2020, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Chian, F.M.; Kaur, L.; Astruc, T.; Vénien, A.; Stübler, A.S.; Aganovic, K.; Loison, O.; Hodgkinson, S.; Boland, M. Shockwave processing of beef brisket in conjunction with sous vide cooking: Effects on protein structural characteristics and muscle microstructure. Food Chem. 2021, 343, 128500. [Google Scholar] [CrossRef]
- Meunier, B.; Normand, J.; Albouy-Kissi, B.; Micol, D.; El Jabri, M.; Bonnet, M. An open-access computer image analysis (CIA) method to predict meat and fat content from an android smartphone-derived picture of the bovine 5th-6th rib. Methods 2021, 186, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Ruedt, C.; Gibis, M.; Weiss, J. Influence of muscle type and microstructure on iridescence in cooked, cured pork meat products. J. Food Sci. 2021, 86, 3563–3573. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.Y.; Kridli, R.T.; Shaker, M.M.; Obeidat, M.D. Investigation of growth and carcass characteristics of pure and crossbred Awassi lambs. Small Rumin. Res. 2010, 94, 167–175. [Google Scholar] [CrossRef]
- Tabbaa, M.J.; Al-Atiyat, R.M.; Abdullah, B.M. Evaluate purebreeding and crossbreeding strategy for better carcass and non-carcass traits of Awassi, Chios and their reciprocal crossbred lambs. Jordan J. Agric. Sci. 2019, 15, 65–79. [Google Scholar]
- Henchion, M.M.; McCarthy, M.; Resconi, V.C. Beef quality attributes: A systematic review of consumer perspectives. Meat Sci. 2017, 128, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, C.E.; Cornforth, D.P.; Whittier, D. Consumer preferences for beef color and packaging did not affect eating satisfaction. Meat Sci. 2001, 57, 359–363. [Google Scholar] [CrossRef]
- Dilara Kecici, P.; Ziya Ograk, Y.; Yalcintan, H.; Ozturk, N.; Kocak, O.; Demir, K.; Yilmaz, A.; Ekiz, B. Effect of slaughter weight on slaughtering and carcass characteristics in lambs from thin-tailed Kivircik and fat-tailed Kangal Akkaraman breeds. Small Rumin. Res. 2021, 205, 106563. [Google Scholar] [CrossRef]
- Ates, S.; Keles, G.; Demirci, U.; Dogan, S.; Kirbas, M.; Filley, S.J.; Parker, N.B. The effects of feeding system and breed on the performance and meat quality of weaned lambs. Small Rumin. Res. 2020, 192, 106225. [Google Scholar] [CrossRef]
- Yousefi, A.R.; Kohram, H.; Zare Shahneh, A.; Nik-khah, A.; Campbell, A.W. Comparison of the meat quality and fatty acid composition of traditional fat-tailed (Chall) and tailed (Zel) Iranian sheep breeds. Meat Sci. 2012, 92, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Miguel, E.; Blázquez, B.; Ruiz de Huidobro, F. Liveweight and sex effects on instrumental meat quality of Rubia de El Molar autochthonous ovine breed. Animals 2021, 11, 1323. [Google Scholar] [CrossRef]
- Fusaro, I.; Cavallini, D.; Giammarco, M.; Serio, A.; Mammi, L.M.E.; de Matos Vettori, J.; Lanzoni, L.; Formigoni, A.; Vignola, G. Effect of diet and essential oils on the fatty acid composition, oxidative stability and microbiological profile of Marchigiana burgers. Antioxidants 2022, 11, 827. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).