Alterations in Skeletal Muscle mRNA Abundance in Response to Ethyl-Cellulose Rumen-Protected Methionine during the Periparturient Period in Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Housing and Care
2.2. Experimental Design and Diets
2.3. Sampling
2.4. RNA Extraction and Quality Assessment
2.5. cDNA Synthesis
2.6. Primer Design and Testing
2.7. Real-Time qPCR
2.8. Statistical Analysis
3. Results and Discussion
3.1. Percentage mRNA Abundance
3.2. Amino Acid Transport
3.3. Carnitine Transport and β-Oxidation
3.4. Vitamin Transport
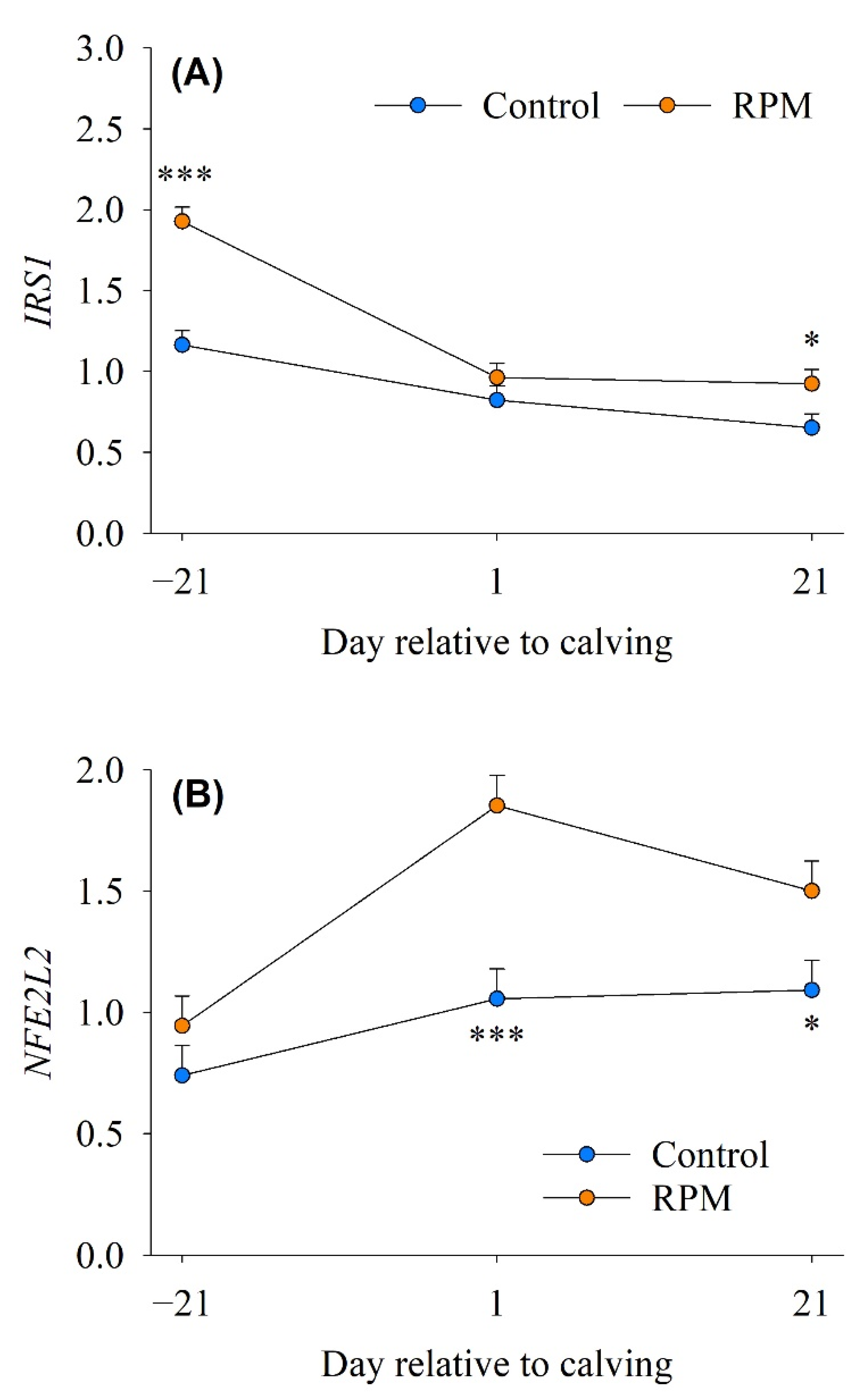
3.5. mTOR/Insulin Signaling
3.6. Antioxidant Response
3.7. CDP-Choline Pathway
3.8. Arginine Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NRC. National Research Council: Nutrient Requirements of Dairy Cattle; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Komaragiri, M.V.; Erdman, R.A. Factors affecting body tissue mobilization in early lactation dairy cows. 1. Effect of dietary protein on mobilization of body fat and protein. J. Dairy Sci. 1997, 80, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Komaragiri, M.V.; Casper, D.P.; Erdman, R.A. Factors affecting body tissue mobilization in early lactation dairy cows. 2. Effect of dietary fat on mobilization of body fat and protein. J. Dairy Sci. 1998, 81, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Grummer, R.R. Impact of changes in organic nutrient metabolism on feeding the transition dairy cow. J. Anim. Sci. 1995, 73, 2820–2833. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W.; Burhans, W.S.; Overton, T.R. Protein nutrition in late pregnancy, maternal protein reserves and lactation performance in dairy cows. Proc. Nutr. Soc. 2000, 59, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Batistel, F.; Arroyo, J.M.; Bellingeri, A.; Wang, L.; Saremi, B.; Parys, C.; Trevisi, E.; Cardoso, F.C.; Loor, J.J. Ethyl-cellulose rumen-protected methionine enhances performance during the periparturient period and early lactation in Holstein dairy cows. J. Dairy Sci. 2017, 100, 7455–7467. [Google Scholar] [CrossRef]
- Fehlberg, L.K.; Guadagnin, A.R.; Thomas, B.L.; Sugimoto, Y.; Shinzato, I.; Cardoso, F.C. Feeding rumen-protected lysine prepartum increases energy-corrected milk and milk component yields in Holstein cows during early lactation. J. Dairy Sci. 2020, 103, 11386–11400. [Google Scholar] [CrossRef]
- Tebbe, A.W.; Weiss, W.P. Concurrent and carryover effects of feeding blends of protein and amino acids in high-protein diets with different concentrations of forage fiber to fresh cows. 1. Production and blood metabolites. J. Dairy Sci. 2021, 104, 5583–5600. [Google Scholar] [CrossRef]
- Sadri, H.; Ghaffari, M.H.; Schuh, K.; Dusel, G.; Koch, C.; Prehn, C.; Adamski, J.; Sauerwein, H. Metabolome profiling in skeletal muscle to characterize metabolic alterations in over-conditioned cows during the periparturient period. J. Dairy Sci. 2020, 103, 3730–3744. [Google Scholar] [CrossRef]
- Tebbe, A.W.; Hanson, J.; Weiss, W.P. Effects of metabolizable protein concentration, amino acid profile, and fiber source on the messenger RNA expression of skeletal muscle in peripartum dairy cows. J. Dairy Sci. 2021, 104, 7888–7901. [Google Scholar] [CrossRef]
- Toledo, M.Z.; Stangaferro, M.L.; Gennari, R.S.; Barletta, R.V.; Perez, M.M.; Wijma, R.; Sitko, E.M.; Granados, G.; Masello, M.; Van Amburgh, M.E.; et al. Effects of feeding rumen-protected methionine pre- and postpartum in multiparous Holstein cows: Lactation performance and plasma amino acid concentrations. J. Dairy Sci. 2021, 104, 7583–7603. [Google Scholar] [CrossRef]
- Osorio, J.S.; Ji, P.; Drackley, J.K.; Luchini, D.; Loor, J.J. Smartamine M and MetaSmart supplementation during the peripartal period alter hepatic expression of gene networks in 1-carbon metabolism, inflammation, oxidative stress, and the growth hormone-insulin-like growth factor 1 axis pathways. J. Dairy Sci. 2014, 97, 7451–7464. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.S.; Ji, P.; Drackley, J.K.; Luchini, D.; Loor, J.J. Supplemental Smartamine M or MetaSmart during the transition period benefits postpartal cow performance and blood neutrophil function. J. Dairy Sci. 2013, 96, 6248–6263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Vailati-Riboni, M.; Trevisi, E.; Drackley, J.K.; Luchini, D.N.; Loor, J.J. Better postpartal performance in dairy cows supplemented with rumen-protected methionine compared with choline during the peripartal period. J. Dairy Sci. 2016, 99, 8716–8732. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.G. Protected proteins and amino acids for ruminants. In Biotechnology in Animal Feeds and Animal Feeding; Wallace, R.J., Chesson, A., Eds.; VCH: New York, UK, 1995; pp. 115–141. [Google Scholar]
- Overton, T.R.; LaCount, D.W.; Cicela, T.M.; Clark, J.H. Evaluation of a ruminally protected methionine product for lactating dairy cows. J. Dairy Sci. 1996, 79, 631–638. [Google Scholar] [CrossRef]
- Coleman, D.N.; Vailati-Riboni, M.; Elolimy, A.A.; Cardoso, F.C.; Rodriguez-Zas, S.L.; Miura, M.; Pan, Y.-X.; Loor, J.J. Hepatic betaine-homocysteine methyltransferase and methionine synthase activity and intermediates of the methionine cycle are altered by choline supply during negative energy balance in Holstein cows. J. Dairy Sci. 2019, 102, 8305–8318. [Google Scholar] [CrossRef] [PubMed]
- Batistel, F.; Alharthi, A.S.; Wang, L.; Parys, C.; Pan, Y.-X.; Cardoso, F.C.; Loor, J.J. Placentome nutrient transporters and mammalian target of rapamycin signaling proteins are altered by the methionine supply during late gestation in dairy cows and are associated with newborn birth weight. J. Nutr. 2017, 147, 1640–1647. [Google Scholar] [CrossRef]
- Hu, L.; Chen, Y.; Cortes, I.M.; Coleman, D.N.; Dai, H.; Liang, Y.; Parys, C.; Fernandez, C.; Wang, M.; Loor, J.J. Supply of methionine and arginine alters phosphorylation of mechanistic target of rapamycin (mTOR), circadian clock proteins, and α-s1-casein abundance in bovine mammary epithelial cells. Food Funct. 2020, 11, 883–894. [Google Scholar] [CrossRef]
- Naeem, A.; Drackley, J.K.; Stamey, J.; Loor, J.J. Role of metabolic and cellular proliferation genes in ruminal development in response to enhanced plane of nutrition in neonatal Holstein calves. J. Dairy Sci. 2012, 95, 1807–1820. [Google Scholar] [CrossRef]
- Han, L.Q.; Zhou, Z.; Ma, Y.; Batistel, F.; Osorio, J.S.; Loor, J.J. Phosphorylation of nuclear factor erythroid 2-like 2 (NFE2L2) in mammary tissue of Holstein cows during the periparturient period is associated with mRNA abundance of antioxidant gene networks. J. Dairy Sci. 2018, 101, 6511–6522. [Google Scholar] [CrossRef]
- Zhou, Z.; Garrow, T.A.; Dong, X.; Luchini, D.N.; Loor, J.J. Hepatic activity and transcription of betaine-homocysteine methyltransferase, methionine synthase, and cystathionine synthase in periparturient dairy cows are altered to different extents by supply of methionine and choline. J. Nutr. 2017, 147, 11–19. [Google Scholar] [CrossRef]
- Dai, H.; Coleman, D.N.; Hu, L.; Martinez-Cortés, I.; Wang, M.; Parys, C.; Shen, X.; Loor, J.J. Methionine and arginine supplementation alter inflammatory and oxidative stress responses during lipopolysaccharide challenge in bovine mammary epithelial cells in vitro. J. Dairy Sci. 2020, 103, 676–689. [Google Scholar] [CrossRef]
- Ding, L.Y.; Chen, L.M.; Wang, M.Z.; Zhang, J.; Loor, J.J.; Zhou, G.; Zhang, X.; Wang, H.R. Inhibition of arginase via jugular infusion of N(ω)-hydroxy-nor-l-arginine inhibits casein synthesis in lactating dairy cows. J. Dairy Sci. 2018, 101, 3514–3523. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Loor, J.J. Identification of reference genes for quantitative real-time PCR in the bovine mammary gland during the lactation cycle. Physiol. Genom. 2007, 29, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kuhla, B.; Nürnberg, G.; Albrecht, D.; Görs, S.; Hammon, H.M.; Metges, C.C. Involvement of skeletal muscle protein, glycogen, and fat metabolism in the adaptation on early lactation of dairy cows. J. Proteome Res. 2011, 10, 4252–4262. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Cao, Y.; Cai, C.; Li, S.; Yu, C.; Yao, J. Regulation of Nutritional Metabolism in Transition Dairy Cows: Energy Homeostasis and Health in Response to Post-Ruminal Choline and Methionine. PLoS ONE 2016, 11, e0160659. [Google Scholar] [CrossRef]
- Zhou, Z.; Riboni, M.V.; Bulgari, O.; Trevisi, E.; Garrow, T.A.; Drackley, J.K.; Cardoso, P.; Luchini, D.N.; Loor, J.J. Physiological and molecular mechanisms associated with performance, immunometabolic status, and liver function in transition dairy cows fed rumen-protected methionine or choline. J. Anim. Sci. 2016, 94, 22–23. [Google Scholar] [CrossRef][Green Version]
- Zou, K.; Ouyang, Q.; Li, H.; Zheng, J. A global characterization of the translational and transcriptional programs induced by methionine restriction through ribosome profiling and RNA-seq. BMC Genom. 2017, 18, 189. [Google Scholar] [CrossRef]
- Lager, S.; Powell, T.L. Regulation of nutrient transport across the placenta. J. Pregnancy 2012, 2012, 179827. [Google Scholar] [CrossRef]
- Stephens, F.B.; Constantin-Teodosiu, D.; Greenhaff, P.L. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J. Physiol. 2007, 581, 431–444. [Google Scholar] [CrossRef]
- Kato, Y.; Sugiura, M.; Sugiura, T.; Wakayama, T.; Kubo, Y.; Kobayashi, D.; Sai, Y.; Tamai, I.; Iseki, S.; Tsuji, A. Organic cation/carnitine transporter OCTN2 (Slc22a5) is responsible for carnitine transport across apical membranes of small intestinal epithelial cells in mouse. Mol. Pharmacol. 2006, 70, 829–837. [Google Scholar] [CrossRef]
- Ringseis, R.; Keller, J.; Eder, K. Regulation of carnitine status in ruminants and efficacy of carnitine supplementation on performance and health aspects of ruminant livestock: A review. Arch. Anim. Nutr. 2018, 72, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Schäff, C.; Börner, S.; Hacke, S.; Kautzsch, U.; Sauerwein, H.; Spachmann, S.K.; Schweigel-Röntgen, M.; Hammon, H.M.; Kuhla, B. Increased muscle fatty acid oxidation in dairy cows with intensive body fat mobilization during early lactation. J. Dairy Sci. 2013, 96, 6449–6460. [Google Scholar] [CrossRef] [PubMed]
- Javed, K.; Fairweather, S.J. Amino acid transporters in the regulation of insulin secretion and signalling. Biochem. Soc. Trans. 2019, 47, 571–590. [Google Scholar] [CrossRef]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef]
- González, I.M.; Martin, P.M.; Burdsal, C.; Sloan, J.L.; Mager, S.; Harris, T.; Sutherland, A.E. Leucine and arginine regulate trophoblast motility through mTOR-dependent and independent pathways in the preimplantation mouse embryo. Dev. Biol. 2012, 361, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Drackley, J.K.; Stamey-Lanier, J.A.; Keisler, D.; Loor, J.J. Effects of level of nutrient intake and age on mammalian target of rapamycin, insulin, and insulin-like growth factor-1 gene network expression in skeletal muscle of young Holstein calves. J. Dairy Sci. 2014, 97, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Batistel, F.; Ma, Y.; Alharthi, A.S.M.; Parys, C.; Loor, J.J. Methionine supply alters mammary gland antioxidant gene networks via phosphorylation of nuclear factor erythroid 2-like 2 (NFE2L2) protein in dairy cows during the periparturient period. J. Dairy Sci. 2018, 101, 8505–8512. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef]
- Elolimy, A.A.; Liang, Y.; Lopes, M.G.; Loor, J.J. Antioxidant networks and the microbiome as components of efficiency in dairy cattle. Livest. Sci. 2021, 251, 104656. [Google Scholar] [CrossRef]
- Kitaoka, Y. The role of Nrf2 in skeletal muscle on exercise capacity. Antioxidants 2021, 10, 1712. [Google Scholar] [CrossRef] [PubMed]
- Fagone, P.; Jackowski, S. Phosphatidylcholine and the CDP-choline cycle. Biochim. Biophys. Acta 2013, 1831, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F.; Zhou, Z.; Batistel, F.; Martinez-Cortés, I.; Pate, R.T.; Luchini, D.L.; Loor, J.J. Methionine and choline supply alter transmethylation, transsulfuration, and cytidine 5′-diphosphocholine pathways to different extents in isolated primary liver cells from dairy cows. J. Dairy Sci. 2018, 101, 11384–11395. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Nakamura, K.; Takeda, S.; Yoshizawa, I.; Yoshida, F.; Ohshima, N.; Izumi, T.; Klein, J.D.; Kumrungsee, T.; Sands, J.M.; et al. GDE5 inhibition accumulates intracellular glycerophosphocholine and suppresses adipogenesis at a mitotic clonal expansion stage. Am. J. Physiol. Cell Physiol. 2019, 316, C162–C174. [Google Scholar] [CrossRef]
- Newsom, S.A.; Brozinick, J.T.; Kiseljak-Vassiliades, K.; Strauss, A.N.; Bacon, S.D.; Kerege, A.A.; Bui, H.H.; Sanders, P.; Siddall, P.; Wei, T.; et al. Skeletal muscle phosphatidylcholine and phosphatidylethanolamine are related to insulin sensitivity and respond to acute exercise in humans. J. Appl. Physiol. 2016, 120, 1355–1363. [Google Scholar] [CrossRef]
- Wang, R.; Jiao, H.; Zhao, J.; Wang, X.; Lin, H. L-Arginine enhances protein synthesis by phosphorylating mTOR (Thr 2446) in a nitric oxide-dependent manner in C2C12 cells. Oxid. Med. Cell. Longev. 2018, 2018, 7569127. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Satterfield, M.C.; Gilbreath, K.R.; Posey, E.A.; Sun, Y. L-Arginine nutrition and metabolism in ruminants. Adv. Exp. Med. Biol. 2022, 1354, 177–206. [Google Scholar] [CrossRef]
- Ding, L.Y.; Wang, Y.F.; Shen, Y.Z.; Zhou, G.; Wu, T.Y.; Zhang, X.; Wang, M.Z.; Loor, J.J.; Zhang, J. Effects of intravenous arginine infusion on inflammation and metabolic indices of dairy cows in early lactation. Animal 2020, 14, 346–352. [Google Scholar] [CrossRef]
- Saladin, R.; De Vos, P.; Guerre-Millot, M.; Leturque, A.; Girard, J.; Staels, B.; Auwerx, J. Transient increase in obese gene expression after food intake or insulin administration. Nature 1995, 377, 527–528. [Google Scholar] [CrossRef]



| Item | Close-Up Diet | Fresh Diet |
|---|---|---|
| Feed ingredient (% DM) | ||
| Alfalfa haylage | 6.55 | 7.81 |
| Corn silage | 26.6 | 31.0 |
| Wheat straw | 26.5 | 3.25 |
| Corn grain (ground, dry) | 12.6 | 22.2 |
| Cottonseed | - | 2.17 |
| Molasses (beet sugar) | 4.03 | 5.50 |
| Soybean hulls | 3.46 | 4.25 |
| Soybean meal (48 % CP) | 7.83 | 10.1 |
| Expeller soybean meal 1 | 5.80 | 5.16 |
| Protein supplement 2 | 0.78 | 1.81 |
| Urea | 0.59 | 0.39 |
| Soychlor 3 | 1.23 | |
| Saturated fat supplement 4 | - | 2.25 |
| Limestone | - | 1.41 |
| Salt | - | 0.02 |
| Dicalcium phosphate | 0.52 | 1.17 |
| Magnesium oxide | - | 0.08 |
| Magnesium sulfate | 2.08 | 0.02 |
| Sodium bicarbonate | - | 0.84 |
| Mineral vitamin mix 5 | 0.17 | 0.17 |
| Vitamin A (30,000 kIU/kg) | 0.03 | 0.02 |
| Vitamin D (5000 kIU/kg) | 0.03 | - |
| Vitamin E (44,000 kIU/kg) | 0.60 | - |
| Biotin 6 | 0.70 | 0.42 |
| Ethyl-cellulose RPM 7 | 0.09 | 0.10 |
| Chemical composition | ||
| CP, % | 15.7 | 17.7 |
| NDF, % | 40.7 | 29.3 |
| ADF, % | 27.4 | 19.6 |
| EE, % | 2.33 | 5.11 |
| Mcal NEL/kg | 1.47 | 1.67 |
| Gene | Description | Diet | SEM | p-Value | ||
|---|---|---|---|---|---|---|
| Control | RPM | Diet | Diet × Time | |||
| Amino acid transport | ||||||
| SLC1A5 | Neutral amino acid transporter | 1.24 | 1.44 | 0.25 | 0.346 | 0.339 |
| SLC3A2 | Heavy-chain amino acid transporter | 1.18 | 1.52 | 0.13 | 0.064 | 0.601 |
| SLC7A5 | Branched-chain and aromatic amino acid transporter | 0.91 | 0.99 | 0.45 | 0.863 | 0.330 |
| SLC7A8 | Branched-chain and aromatic amino acid transporter | 0.86 | 1.60 | 0.45 | 0.047 | 0.035 |
| SLC38A1 | Neutral amino acid transporter | 0.96 | 1.41 | 0.29 | 0.132 | 0.293 |
| SLC38A2 | Neutral amino acid transporter | 1.06 | 1.30 | 0.14 | 0.098 | 0.074 |
| SLC38A6 | Sodium-dependent amino acid transporter | 1.26 | 1.52 | 0.29 | 0.118 | 0.067 |
| SLC38A7 | Glutamate and serine transporter, Gln, His, Ser, Ala, Asn | 1.15 | 1.32 | 0.22 | 0.339 | 0.268 |
| SLC43A2 | L-amino acid transporter-3 (Leu, Phe, Val, Met) | 1.02 | 1.61 | 0.23 | 0.003 | 0.006 |
| SLC25A29 | Mitochondrial transporter of basic AA (Arg and Lys) | 1.95 | 3.87 | 0.84 | 0.016 | 0.681 |
| SCL38A9 | Gln, Leu, and Arg transporter | 0.95 | 1.37 | 0.22 | 0.017 | 0.447 |
| Carnitine transport and β-oxidation | ||||||
| SLC22A5 | Sodium-dependent high-affinity carnitine transporter | 0.85 | 1.04 | 0.08 | 0.030 | 0.022 |
| CPT1A | Carnitine palmitoyltransferase 1A | 1.02 | 1.38 | 0.29 | 0.068 | 0.382 |
| ACADVL | Acyl-CoA dehydrogenase very long chain | 0.98 | 1.36 | 0.17 | 0.013 | 0.191 |
| Vitamin transport | ||||||
| SLC5A6 | Multivitamin transporter | 1.14 | 1.86 | 0.38 | 0.006 | 0.267 |
| SLC19A2 | Thiamin transporter | 1.20 | 1.70 | 0.19 | 0.013 | 0.076 |
| SLC44A1 | Choline transporter | 1.05 | 1.25 | 0.16 | 0.082 | 0.174 |
| mTOR/insulin signaling | ||||||
| AKT1 | AKT serine/threonine kinase 1 | 0.90 | 1.23 | 0.16 | 0.006 | 0.438 |
| mTOR | Mechanistic target of rapamycin kinase | 1.02 | 1.26 | 0.15 | 0.033 | 0.623 |
| IRS1 | Insulin receptor substrate 1 | 0.88 | 1.27 | 0.12 | <0.001 | 0.002 |
| Antioxidant response | ||||||
| NFE2L2 | NFE2-like bZIP transcription factor 2 | 0.96 | 1.43 | 0.17 | 0.003 | 0.010 |
| KEAP1 | Kelch-like ECH-associated protein 1 | 0.92 | 1.15 | 0.12 | 0.017 | 0.552 |
| CUL3 | Cullin 3 | 1.03 | 1.36 | 0.15 | 0.009 | 0.396 |
| CDP-Choline pathway | ||||||
| CHKA | Choline kinase alpha | 1.06 | 1.91 | 0.44 | 0.066 | 0.356 |
| CHKB | Choline kinase beta | 1.12 | 1.70 | 0.19 | 0.006 | 0.391 |
| PCYT1A | Phosphate cytidylyltransferase 1A, choline | 0.97 | 1.46 | 0.14 | 0.001 | 0.792 |
| PCYT1B | Phosphate cytidylyltransferase 1B, choline | 1.25 | 1.85 | 0.15 | 0.001 | 0.119 |
| CEPT1 | Choline/ethanolamine phosphotransferase 1 | 1.15 | 1.86 | 0.35 | 0.054 | 0.130 |
| Arginine metabolism | ||||||
| ODC1 | Ornithine decarboxylase 1 | 0.97 | 1.10 | 0.17 | 0.440 | 0.628 |
| SRM | Spermidine synthase | 1.12 | 1.34 | 0.10 | 0.031 | 0.556 |
| AMD1 | Adenosylmethionine decarboxylase 1 | 1.12 | 1.33 | 0.16 | 0.198 | 0.234 |
| ARG1 | Arginase 1 | 0.53 | 1.96 | 0.43 | 0.004 | 0.370 |
| SMS | Spermine synthase | 0.80 | 0.98 | 0.08 | 0.020 | 0.444 |
| NOS2 | Nitric oxide synthase 2 | 0.38 | 1.31 | 0.29 | 0.005 | 0.323 |
| NOS3 | Nitric oxide synthase 3 | 0.71 | 1.11 | 0.11 | <0.001 | 0.257 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanh, L.P.; Jiang, Q.; Wichasit, N.; Batistel, F.; Parys, C.; Guyader, J.; Loor, J.J. Alterations in Skeletal Muscle mRNA Abundance in Response to Ethyl-Cellulose Rumen-Protected Methionine during the Periparturient Period in Dairy Cows. Animals 2022, 12, 1641. https://doi.org/10.3390/ani12131641
Thanh LP, Jiang Q, Wichasit N, Batistel F, Parys C, Guyader J, Loor JJ. Alterations in Skeletal Muscle mRNA Abundance in Response to Ethyl-Cellulose Rumen-Protected Methionine during the Periparturient Period in Dairy Cows. Animals. 2022; 12(13):1641. https://doi.org/10.3390/ani12131641
Chicago/Turabian StyleThanh, Lam Phuoc, Qianming Jiang, Nithat Wichasit, Fernanda Batistel, Claudia Parys, Jessie Guyader, and Juan J. Loor. 2022. "Alterations in Skeletal Muscle mRNA Abundance in Response to Ethyl-Cellulose Rumen-Protected Methionine during the Periparturient Period in Dairy Cows" Animals 12, no. 13: 1641. https://doi.org/10.3390/ani12131641
APA StyleThanh, L. P., Jiang, Q., Wichasit, N., Batistel, F., Parys, C., Guyader, J., & Loor, J. J. (2022). Alterations in Skeletal Muscle mRNA Abundance in Response to Ethyl-Cellulose Rumen-Protected Methionine during the Periparturient Period in Dairy Cows. Animals, 12(13), 1641. https://doi.org/10.3390/ani12131641






