Simple Summary
The healthy management of fishery resources requires the evaluation of a series of population attributes, such as mortality, fecundity, spawning biomass, recruitment and the age at which fish reach sexual maturity. All these attributes depend on the age of fish. Then, the adequate administration and management of these resources require estimators of fish age. Age is estimated by ring counts in the otoliths, which are hard and calcified structures responsible for the balance of fish; however, this is an expensive and time-consuming methodology. An alternative is the use of otolith weight due to its expected relationship with fish age. Yellowtail king fish is a valuable resource, which arrives at the Chilean northern coast in the summer (southern hemisphere). Many aspects of the biology of this fish, such as age and growth, remain unknown. In this study, we estimated the age and growth using otolith weight, which was measured from fish with a known age, and we calculated parameters explaining growth using four different models. The best model was the seasonalized von Bertalanffy growth function, which takes into account that fish do not grow at the same rate year round.
Abstract
The age and growth of fish populations is a critical issue for stock assessment, population dynamics and fishery management. Spawner biomass, mortality, growth, recruitment and age at maturity can be misconstrued if the age estimator is inaccurate. Age can be estimated by annuli count, but this requires expertise and is expensive. Otolith weight (OW) is a good indicator of how long a fish has lived, because OW increases during an individual’s life. Seriola lalandi is a migratory fish and is an important resource for local fishermen in northern Chile. Aspects of its biology, i.e., age and growth, remain unknown, at least for the population annually arriving in northern Chile. Fish of a known age (n = 105; from 5.5 to 25.7 cm in FL) from yellowtail aquaculture facilities at Universidad de Antofagasta allowed us to estimate the growth rate of OW, and fish obtained from local fishermen (n = 71; from 37.9 to 109 cm in FL) allowed us to estimate the age and growth of S. lalandi from the analysis of OW, without the need for calibration. The following four models were fitted with the known ages and fork lengths: the von Bertalanffy growth function, the Gompertz and logistic models and the seasonalized von Bertalanffy growth function. The latter model showed the best adjustment (according to the Akaike information criterion) with the following parameters: L∞, 98.58 cm.; K, 0.59; t0, 0.07; ts, 0.84; and C, 0.97.
1. Introduction
An accurate estimation of the age and growth of fish populations is a critical issue in stock assessment, population dynamics and successful fishery management [1,2]; specifically, spawner biomass, mortality, growth, recruitment and age at maturity in particular can be misconstrued if the age estimators are inaccurate [3,4].
Age structures in fish populations have traditionally been estimated by interpreting marks in bone structures such as scales and otoliths; the opercular bones of fish [4,5] have also been used, as has the analysis of annual rings in the otolith, one of the most common methodologies, which requires an initial preparation of the otolith (embedding, sectioning and polishing), and a microscopic examination and counts of the annuli [6]. This process is generally time consuming, and interpreting the annuli requires expertise; consequently, age determination from hard structures is very expensive [7] and is highly subject to the researcher’s skills [8]. Age estimates are typically accepted when readers attain some minimum coefficient of the variation benchmark, although this does not imply that the age is accurate [3]. Age validation methods increase both precision and the accuracy of age estimates [9] but require the use of either the mark-recapture method or the regular sampling of fish over relatively long time periods, which is not always possible [10].
Otoliths are biomineralized concretions of calcium carbonate and other minor elements that are metabolically inert. In teleosts, the inner ears are multi-sensory, stato-acoustic organs with basic vestibular and acoustic functions, and they are critical structures in the perception of angular and linear acceleration. Each inner ear is composed of three semicircular canals, three end organs and three otolith organs (sacculus, utriculus and lagena); three otoliths are located inside: sagitta, lapillus and asteriscus. The otoliths act as transducers of acoustic and vestibular signals to the fish nervous system [11]. The otolith shape (otolith contour) provides us with important information related to paleontological, ictiological and ecological sciences, especially in food web studies [12], and is an efficient tool for fish stock discrimination [13], but otolith chemistry has also indicated large-scale connectivity among fish populations [14]. Otolith growth is continuous over the fish life [15].
The relation between otolith weight (OW) and fish age is based on the growth of the otolith, with a linear relationship between both variables; otolith growth continues but in weight [15]. In addition, this relationship states that larger fish (for a given age class) generally have heavier otoliths than smaller fish of the same age class [3]. Consequently, the OW is a good indicator of how long a fish has lived. The direct relationship between fish otolith weight and age has been studied since 1990 [16,17,18], and this relationship has great potential for estimating the age and age structure in fish [18], simply because OW increases during the whole life of an individual, unlike fish length or otolith size [19]. It is a faster and inexpensive method and has been applied to a variety of fishes from different habitats and regions worldwide [20]. Otolith weight could be a valuable criterion as an age determination technique that is objective, economic and easy to perform, compared to traditional methods that have been defined ‘‘as much an art as a science” [21]. A meta-analysis [16] suggested that otolith weight is a good predictor for the age estimation of fish. Specifically, calibration would require precise and exact estimations of the age of only a relatively small number of fish that cover the whole range of otolith weights and ages present in the samples [22].
The use of OW requires a calibration stage, implying that age estimated based on annuli counting can be considered as the ‘‘true’’ age, and that age calculated using otolith weight can be considered as the ‘‘estimated’’ age [21]. This methodology has been widely used when OW is used as an estimator of age (e.g., among many others in the last decade, [5,6,10,21,23,24]). Calibration can be performed using other methodologies and not only with annuli counts; for instance, the persistence and progression of modes in otolith-weight frequency distributions suggest a direct relationship between otolith weight and fish age [22,25]. A different approach was developed [26] following changes in otolith-weight distribution of pilchard Sardinops sagax neopilchardus for a captive population and wild-caught fish, and the modes in the otolith-weight frequency distributions appeared to persist and progress in a fashion that was generally consistent with them representing different year classes. The relation between OW and fish age in lake trout Salvelinus namaycush was analyzed using a reference sample reared in a hatchery with a known age of 14–16 months [3]. Consequently, both approaches were independent of a “true age” assignation from annuli counts.
The relationship between the age and growth of members of the genus Seriola have been estimated from different localities [27,28,29,30,31,32,33,34]. The maximum age estimated for S. lalandi Valenciennes, 1833, ranged between 7 and 21 years. Otolith ring counts suggested a maximum age of 7 years for males and 8 years for females [34], whereas age estimated based on the analysis of scales varied between 9 and 12 years [28,30] and between 9 and 11 years when the marks in bones (vertebrae) were studied [30,33]. Finally, an approach based on data covering older fish representative of all ages suggested that S. lalandi can reach 21 years [32].
Seriola lalandi is a highly migratory pelagic fish that is widely distributed in temperate and subtropical waters around the world. Along the south-eastern Pacific Coast, it arrives annually in summer (between 20° S and 30° S) [35] and is an important resource for local fishermen in northern Chile. Many aspects of its biology, i.e., age and growth, remain unknown, at least for the population arriving annually in northern Chile. Universidad de Antofagasta has hatchery facilities for the experimental development of the yellowtail kingfish aquaculture. From the hatchery facilities, we obtained a sample of fish of a known age, which allowed us to estimate the growth rate of OW. Our goal was to estimate the age and growth of S. lalandi from the analysis of OW, without the need for calibration using annuli counts or another methodology.
2. Materials and Methods
In January–April 2018, we sampled a total of 71 specimens of S. lalandi from the fish market at Antofagasta, in northern Chile (23°20′ S). Additionally, we sampled 105 specimens of a known age from the aquaculture facilities at Universidad de Antofagasta. The fork length (FL) for each fish was measured to the nearest centimeter, and otoliths were extracted, cleaned and stored in tagged vials; broken otoliths were discarded. Then, the otoliths were weighed, without being dried in an oven, with an analytic balance (to the nearest 0.0001 gr). To test if the left and right otoliths differed significantly, a “t- test” for paired data was used [36] for 132 pairs of otoliths.
Due to the expected linear relationship between the age and weight of the otolith [20], to estimate age from OW, the following expression was used:
where ti is the estimated age (years) of the ith fish, OWi is the weight of the otolith of the ith fish (in mg) and OWmgr is the mean growth rate of the otolith (mg/year). Due to the absence of growth differences between sexes for this species [34,37], age was estimated for the whole sample. OWmgr was estimated as the slope of the regression between OW and the known age of captive fish from aquaculture facilities.
Four growth models were adjusted with the known age and FL as previously suggested [38,39].
Von Bertalanffy growth function (vBGF):
where Lt is the fork length at age t, L∞ is the asymptotic fork length, K is the growth coefficient (1/time) and t0 is the theoretical age when the length is zero (time).
Gompertz growth function (GZ):
where Lt and L∞ are as in the von Bertalanffy growth function, g is the instantaneous rate of growth when t = t0 and t0 is the age at which the absolute rate of increase in length begins to decrease.
Logistic growth function (LG):
where Lt and L∞ are as in the vBGF, g is the instantaneous rate of growth when the length tends to 0 and t0 is the age at which the absolute rate of increase in length begins to decrease.
Seasonalized von Bertalanffy growth function (SvBGF):
where L∞, K and t0 are as in the vBGF, C is the amplitude of the growth oscillation and ts is the time between t = 0 and the start of a sinusoid growth oscillation.
The growth models were fitted using the FSA package with R statistical language [40,41].
The best model explaining the growth of S. lalandi was selected according to the Akaike information criterion [42].
3. Results
Specimens from the aquaculture facilities at Universidad de Antofagasta ranged from 5.5 to 25.7 cm in FL and had known ages from 99 to 329 days (from 0.27 to 0.9 years) (Supplementary Material Table S1).
Specimens caught by local fishermen ranged from 37.9 to 109 cm in FL. Otoliths from 71 specimens where used. For both wild and captive fish, the mean weights of the left and right otoliths did not show significant differences (two-tailed tests = 1.978; p = 0.958; df = 131) for the whole sample.
The slope of the regression between OW and FL for captive fish, that is, otolith growth rate, was 3.044 mg/year (n = 105; SE = 0.231; r2 = 0.629). Using Eq. 1, the estimated age of wild fish ranged from 0.8 to 5.7 years.
The estimated growth parameters obtained from the non-linear fit are given in Table 1. According to the Akaike information criterion, the model that best fit the growth of S. lalandi from the south-eastern Pacific was the seasonalized von Bertalanffy growth function (Figure 1).

Table 1.
Values for the growth parameter from the four fitted models. vBGF, von Bertalanffy growth function; GZ, Gompertz model; SvBGF, seasonalized vBGF and LG, logistic model; SE, standard error; AIC, Akaike information criterion.
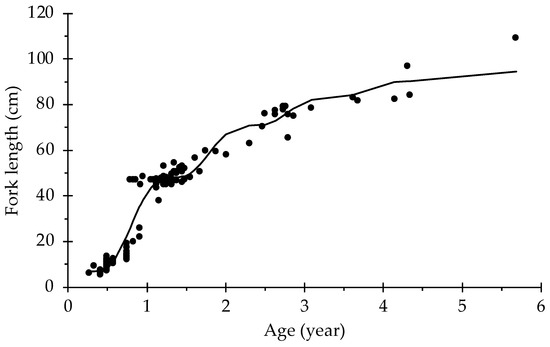
Figure 1.
Seasonalized von Bertalanffy growth function for Seriola lalandi, estimated by the captive and natural populations.
4. Discussion
Age assignments are among the most important biological measures in fishery management [9]; spawner biomass, mortality, growth, recruitment and age at maturity in particular can be misconstrued if age estimators are inaccurate [3,4]. Consequently, biological reference points, which are used in inferring stock status in fisheries and are the targets or thresholds in fishery management, can be affected by an accurate estimate of age and, particularly, individual growth rates [39].
As previously stated [40], the development of the standard von Bertalanffy growth function (vBGF) and the seasonalized SvBGF, as well as the logistic and Gompertz models, makes it possible to estimate population parameters such as asymptotic length (L∞) and growth coefficient (K), under the condition that size at the age is known.
Age structures in fish populations have traditionally been estimated by interpreting marks in hard structures, such as scales and otoliths, as well as the opercular bones of fish [4,5]. The age structure is often derived by interpreting annual rings in sectioned otoliths from several individuals, giving a relative proportion of the number of specimens in each age class; however, two main disadvantages of this methodology are evident: a consistent age reading requires a certain degree of skill, which mainly depends on the reader’s experience, and the method is time-consuming and thus expensive [17]. One way to mitigate these disadvantages is using OW as an estimator of fish age [16], although the calibration of age is required, such as the traditional otolith reading as a “true age”, whereas age from OW is considered as an “estimate age”. Alternatively, the modal progression of OW or otolith-weight distribution of the captive population and wild-caught fish or the reference sample of known-age fish reared in a hatchery can be used [3,17,18,22]. Unless an exact value for the fish age is available, any method based on OW requires an age estimate from otoliths (or another structure). We used the OW of S. lalandi to estimate size at the age under reared conditions; as a result, we knew the exact age (in days) of each fish studied, and calibration was not necessary. As previously stated [26], the ageing of S. lalandi requires a precise estimate of the first zone in order to validate estimates for all age classes. Similarly, and as previously described [37], the identification of the first ring of the otoliths of wild S. lalandi arriving in Chile was difficult, and a clear and accurate estimate of the first zone was not possible.
Growth parameters for S. lalandi were estimated in wild populations from Australia, New Zealand, Japan and South Africa using vertebrae (17th), length frequency distribution, tags and otoliths, and scale reads (Table 2). All those studies estimated the parameters of the von Bertalanffy growth function; our analysis is the only one that includes four different models for this species. The best model, according the Akaike information criterion, was the seasonalized von Bertalanffy growth function. As stated [40,43,44,45], the seasonalized von Bertalanffy growth function has been widely used to fit the growth of fish populations from temperate regions. Therefore, accounting for seasonality in growth is essential for understanding the ecology and management of fish.

Table 2.
Growth parameters for Seriola lalandi from different localities. L∞, asymptotic fork length; K, growth coefficient (1/time); t0, theoretical age when length is zero. Age = estimated age range; Loc. = locality; Ref = reference.
Our results suggest that previously, the growth parameters for this fish species were overestimated when seasonality was not considered.
5. Conclusions
Our results show that the growth of Seriola lalandi, calculated from the growth rate in the weight of the otoliths from a captive population of an exact known age, was best explained by the seasonalized von Bertalanffy growth function. This methodology avoided the need for a calibration factor estimated from the analysis of growth annuli; that is, the “true” age, as suggested [17], was not necessary, because in our study we knew the “real age”.
Supplementary Materials
The following supporting information can be download at: https://www.mdpi.com/article/10.3390/ani12131640/s1, Table S1: Data from captive fishes.
Author Contributions
Conceptualization, T.S.I.N., M.A. and M.E.O.; methodology, M.A. and M.E.O.; formal analysis, T.S.I.N. and M.A.; investigation, T.S.I.N., M.A. and M.E.O.; writing—original draft preparation, T.S.I.N.; writing—review and editing, M.A. and M.E.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research study received no external funding.
Institutional Review Board Statement
This study was approved by the Ethical Commission of Universidad de Antofagasta, Antofagasta, Chile. This study did not consider experiments with live animals. All fish were obtained from commercial catches or natural mortality from farmed conditions. This species is not subject to conservation measures. Commercial fishermen follow national regulations concerning these fisheries.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in Supplementary Material, Table S1: Data from captive fishes.
Acknowledgments
Our thanks to Rodolfo Wilson, Universidad de Antofagasta, yellowtail kingfish facilities, for providing otoliths from fish naturally dead under culture conditions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ihssen, P.E.; Booke, H.E.; Casselman, J.M.; Mcglade, J.M.; Payne, N.R. Stock identification: Materials and methods. Can. J. Fish. Aquat. Sci. 1981, 38, 1838–1855. [Google Scholar] [CrossRef]
- Lombardi-Carlson, L.A.; Andrews, A.H. Age estimation and lead-radium dating of golden tilefish, Lopholatilus chamaeleonticeps. Environ. Biol. Fish. 2015, 98, 1787–1801. [Google Scholar] [CrossRef]
- Hanson, S.D.; Stafford, C.P. Modeling Otolith Weight using Fish Age and Length: Applications to Age Determination. Trans. Am. Fish. Soc. 2017, 146, 778–790. [Google Scholar] [CrossRef]
- Radford, D.S.; Lackmann, A.R.; Moody-Carpender, C.J.; Colombo, R.E. Comparison of Four Hard Structures Including Otoliths for Estimating Age in Blue Suckers. Trans. Am. Fish. Soc. 2021, 150, 514–527. [Google Scholar] [CrossRef]
- Lepak, M.J.; Cathcart, N.C.; Hooten, B.M. Otolith Weight as a Predictor of Age in Kokanee Salmon from Four Colorado Reservoirs. Can. J. Fish. Aquat. Sci. 2012, 69, 1569–1575. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Nazir, A.; Banday, U.Z. Utility of otolith weight to estimate age of Labeo bata (Actinopterygii: Cypriniformes: Cyprinidae) inhabiting the Ganga River. Acta Ichthyol. Piscat. 2018, 48, 257–260. [Google Scholar] [CrossRef] [Green Version]
- Francis, R.C.; Campana, S.E. Inferring age from otolith measurements: A review and a new approach. Can. J. Fish. Aquat. Sc. 2004, 61, 1269–1284. [Google Scholar] [CrossRef]
- Wilson, C.D.; Boehlert, G.W. The effects of different otolith aging techniques on estimates of growth and mortality for the splitnose rockfish, Sebastes diploproa, and canary rockfish, S. pinniger. Calif. Fish Game 1990, 76, 146–160. [Google Scholar]
- Campana, S.E. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 2001, 59, 197–242. [Google Scholar] [CrossRef]
- Britton, J.R.; Blackburn, R. Application and utility of using otolith weights in the ageing of three flatfish species. Fish. Res. 2014, 154, 147–151. [Google Scholar] [CrossRef]
- D‘Iglio, C.; Natale, S.; Albano, M.; Savoca, S.; Famuari, S.; Gervasi, C.; Lanteri, G.; Panarello, G.; Spano, N.; Capillo, G. Otolith analysis higlight morpho-functional differences of three species of Mullet (Mugilidae) from transitional waters. Sustainability 2022, 14, 398. [Google Scholar] [CrossRef]
- Parisi-Baradad, V.; Manjabacas, A.; Lombarte, A.; Olivella, R.; Chic, O.; Piera, J.; Garcia-Ladona, E. Identification of teleost fishes using an otolith online database-AFOROV. Fis. Res. 2010, 105, 13–20. [Google Scholar] [CrossRef]
- Mahé, K.; Evano, H.; Mille, T.; Muths, D.; Bourjea, J. Otolith shape as a valuable tool to evaluate the stock structure of swordfish in the Indian Ocean. Afr. J. Mar. Sci. 2016, 38, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Ashford, J.; Serra, R.; Saavedra, J.; Letelier, J. Otolith chemistry indicates large-scale connectivity in Chilean jack mackerel (Trachurus murphyi), a highly mobile species in the Southern Pacific Ocean. Fish. Res. 2011, 107, 291–299. [Google Scholar] [CrossRef]
- Beamish, R.J. Differences in the age of Pacific hake (Merluccius productus) using whole otoliths and sections of otoliths. J. Fish. Res. Board Can. 1979, 36, 141–151. [Google Scholar] [CrossRef]
- Pawson, M.G. Using otolith weight to age fish. J. Fish Biol. 1990, 36, 521–531. [Google Scholar] [CrossRef]
- Fletcher, W.J. A test of the relationship between otolith weight and age for the pilchard Sardinops neopilchardus. Can. J. Fish. Aquat. Sc. 1991, 48, 35–38. [Google Scholar] [CrossRef]
- Worthington, D.G.; Fowler, A.J.; Doherty, P.J. Determining the most efficient method of age determination for estimating the age structure of a fish population. Can. J. Fish. Aquat. Sc. 1995, 52, 2320–2326. [Google Scholar] [CrossRef]
- Fowler, A.J.; Doherty, P.J. Validation of annual growth increments in the otoliths of two species of Damselfish from the southern Great Barrier Reef. Can. J. Fish. Aquat. Sci. 1992, 43, 1057–1068. [Google Scholar] [CrossRef]
- Pacheco, C.; Bustamante, C.; Araya, M. Mass-effect: Understanding the relationship between age and otolith weight in fishes. Fish Fish. 2021, 22, 623–633. [Google Scholar] [CrossRef]
- Cardinale, M.; Arrhenius, F.; Johnsson, B. Potential use of otolith weight for the determination of age-structure of Baltic cod (Gadus morhua) and plaice (Pleuronectes platessa). Fish. Res. 2000, 45, 239–252. [Google Scholar] [CrossRef]
- Araya, M.; Cubillos, L.A.; Guzmán, M.; Peñailillo, J.; Sepúlveda, A. Evidence of a relationship between age and otolith weight in the Chilean jack mackerel, Trachurus symmetricus murphyi (Nichols). Fish. Res. 2001, 51, 17–26. [Google Scholar] [CrossRef]
- Ghanbarzadeh, M.; Soofiani, N.M.; Keivany, Y.; Taghavi-Motlagh, S.A. Use of otolith length and weight in age estimations of the kingsoldier bream, Argyrops spinifer, in the Persian Gulf. Iran J. Ichthyol. 2014, 1, 1–6. [Google Scholar]
- Nazir, A.; Khan, M.A. Using otolith weight to predict the age of different stocks of Sperata aor (Siluriformes: Bagridae) from the River Ganga. Rev. Biol. Trop. 2019, 67, 534–540. [Google Scholar] [CrossRef]
- Pino, C.A.; Cubillos, L.A.; Araya, M.; Sepulveda, A. Otolith weight as an estimator of age in the Patagonian grenadier, Macruronus magellanicus, in central-south Chile. Fish. Res. 2004, 66, 145–156. [Google Scholar] [CrossRef]
- Fletcher, W.J. Application of the otolith weight—Age relationship for the pilchard, Sardinops sagax neopilchardus. Can. J. Fish. Aquat. Sci. 1995, 52, 657–664. [Google Scholar] [CrossRef]
- Mitani, F.; Sato, T. Studies on the growth and age of the yellowtail, Seriola quinqueradiata T. & S.; found in Japan and the adjacent region–II. Estimation of age and growth from the opercula bone. Bull. Japan Soc. Sci. Fish. 1959, 24, 803–808. (In Japanese) [Google Scholar]
- Baxter, J.L. A study of the yellowtail Seriola dorsalis (GILL). State of Californea Department of fish and Game. Fish. Bull. 1960, 110, 1–96. [Google Scholar]
- Nishioka, J.; Inoue, H.; Kawagishi, M.; Iizuka, S.; Sinoda, M. On results of measurement of vertebral centrum by means of replica method. Bull. Kyoto Inst. Ocean. Fish. Sci. 1985, 9, 5–10. [Google Scholar]
- Gillanders, B.M.; Ferrell, D.J.; Andrew, N.L. Ageing methods for yellowtail kingfish, Seriola lalandi, and results from age and size based growth models. Fish. Bull. 1999, 97, 812–827. [Google Scholar]
- Thompson, B.; Beasley, M.; Wilson, C. Age distribution and growth of greater amberjack, Seriola dumerili from the north-central Gulf of Mexico. Fish. Bull. 1999, 97, 362–371. [Google Scholar]
- Stewart, J.; Ferrell, D.; van der Walt, B. Sizes and ages in commercial landings with estimates of growth, mortality and yield per recruit of yellowtail kingfish (Seriola lalandi) from New South Wales, Australia. Mar. Freshw. Res. 2004, 55, 489–497. [Google Scholar] [CrossRef]
- Shiraishi, T.; Ohshimo, S.; Yukami, R. Age, growth and reproductive characterisitics of gold striped amberjack Seriola lalandi in the waters off western Kyushu, Japan. N. Z. J. Mar. Freshwater Res. 2010, 44, 117–127. [Google Scholar] [CrossRef]
- Dunn, K. The Diet, Reproductive Biology, Age and Growth of Yellowtail, Seriola lalandi, in South Africa. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2014; 106p. Available online: https://open.uct.ac.za/handle/11427/6254 (accessed on 9 March 2020).
- Sepulveda, F.A.; González, M.T. Spatio-temporal patterns of genetic variations in populations of yellowtail kingfish Seriola lalandi from the south-eastern Pacific Ocean and potential implications for its fishery management. J. Fish Biol. 2017, 90, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Zar, H. Biostatistical Analysis, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2010; 944p. [Google Scholar]
- McKenzie, J.S.; Watson, M.; Francis, T.; Malcolm, Ó.M.; Poortenaar, C.C.; Holdsworth, J. Age, Growth, Maturity and Natural Mortality of New Zealand Kingfish (Seriola lalandi lalandi). New Zealand Fisheries Assessment Report 2014/03. 38p. Available online: https://docs.niwa.co.nz/library/public/FAR-2014-03.pdf (accessed on 4 January 2022).
- Ricker, W.E. Growth rates and models. In Fish Physiology, III, Bioenergetics and Growth; Hoar, W.S., Randall, D.J., Brett, J.R., Eds.; Academic Press: New York, NY, USA, 1979; pp. 677–743. [Google Scholar]
- Somers, L.F. On a seasonally oscillating growth function. Fishbyte 1988, 6, 8–11. [Google Scholar]
- Ogle, D.H. Introductory Fisheries Analyses with R; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016; 327p. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 4 January 2022).
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer-Verlag: New York, NY, USA, 2002; 488p. [Google Scholar]
- Liu, Y.; Zhang, C.; Xu, B.; Xue, Y.; Ren, Y.; Chen, Y. Accounting for Seasonal Growth in Per-Recruit Analyses: A Case Study of Four Commercial Fish in Coastal China Seas. Front. Mar. Sci. 2021, 8, 567240. [Google Scholar] [CrossRef]
- Osei, I.K.; Yankson, K.; Obodai, E.A.; Okyere, I. Implications of overlooked seasonal growth dynamics in tropical fisheries assessment: A test case of an oyster (Crassostrea tulipa) fishery in the Densu Delta, Ghana. Fish. Res. 2021, 244, 106118. [Google Scholar] [CrossRef]
- Holdsworth, J.C.; McKenzie, J.R.; Walsh, C.; Bian, R.; Maolagáin, C.O. Catch-at-Age of Yellowtail Kingfish (Seriola lalandi) Caught by New Zealand Recreational Fishers 2014–15. New Zealand Fisheries Assessment Report 2016/45. Available online: https://www.mpi.govt.nz/dmsdocument/14725/direct (accessed on 4 January 2022).
- Schnute, J. A versatile growth model with statistically stable parameters. Can. J. Fish. Aquat. Sci. 1981, 38, 1128–1140. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).