Numerical Simulation of Airborne Disease Spread in Cage-Free Hen Housing with Multiple Ventilation Options
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
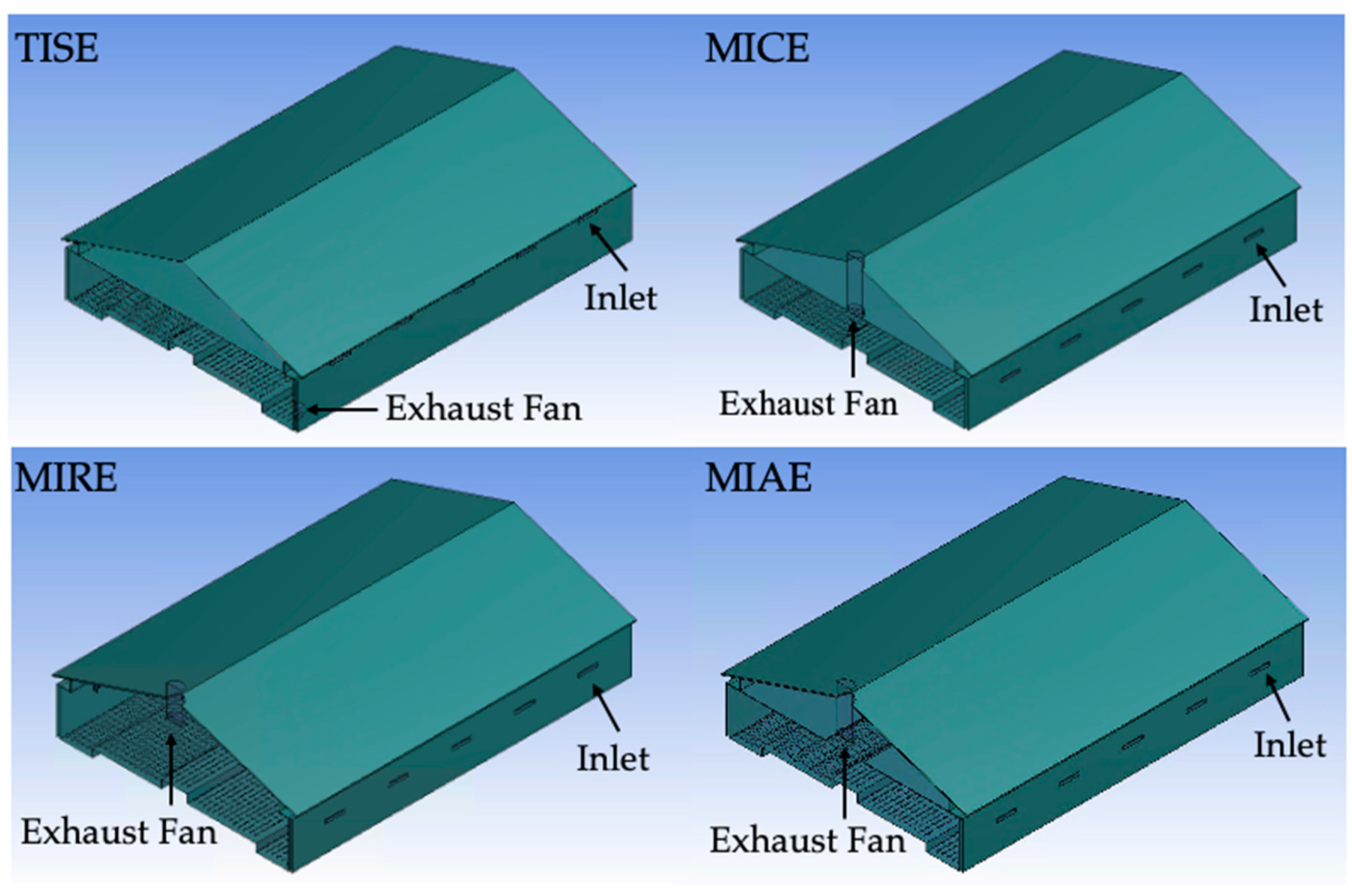
2.1. Floor-Raised Hen House and Ventilation Schemes
2.2. Procedure for CFD Modeling
2.2.1. Preconditions and Assumptions
2.2.2. Computational Domain
2.2.3. Modeling Individual Hens
2.2.4. Boundary Conditions
2.2.5. Modeling Airborne Disease Dispersal
2.2.6. Meshing and Solver Settings
2.3. Post-Processing of Simulation Data
2.3.1. Data Visualization
2.3.2. Statistical Analysis
3. Results
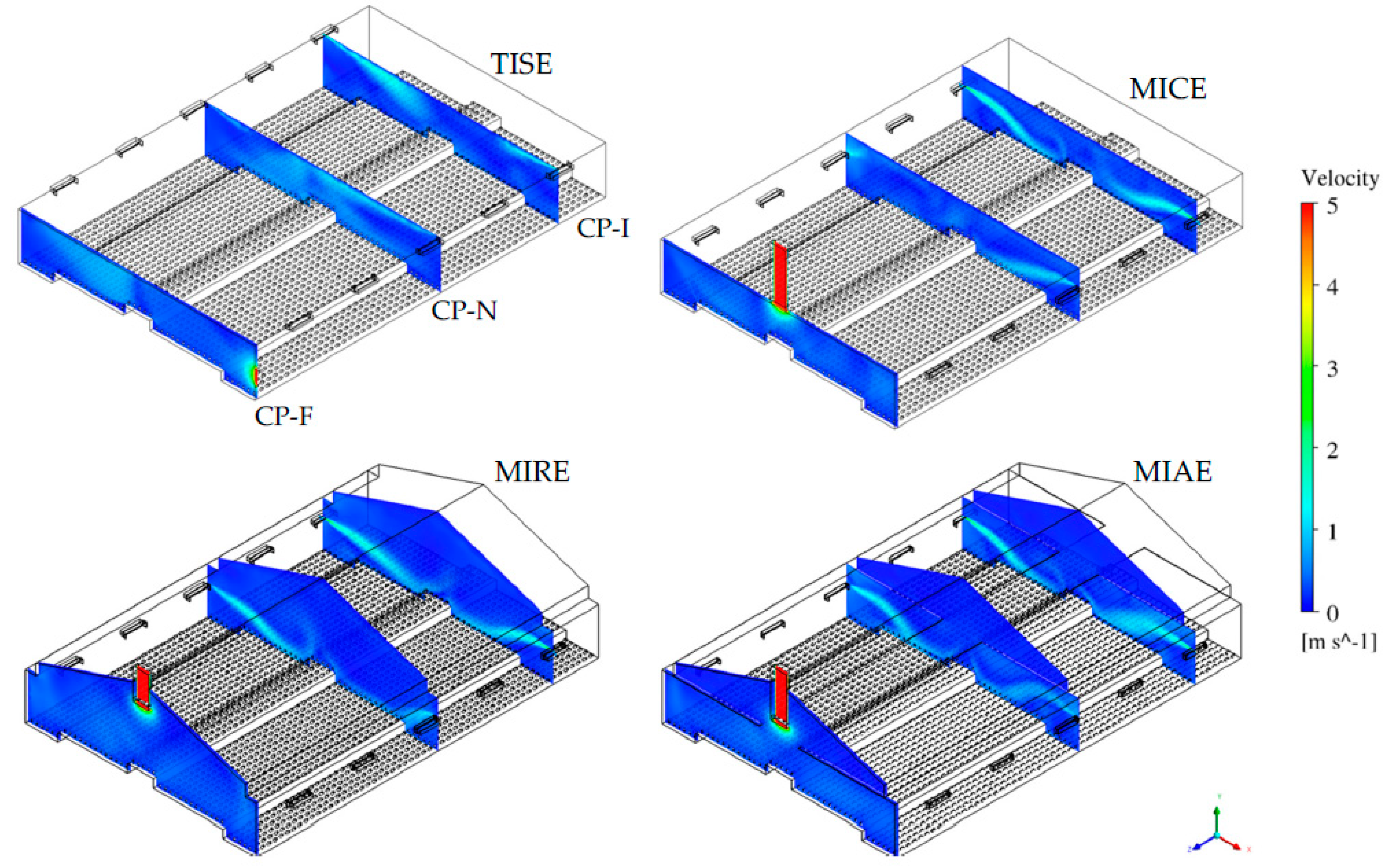
3.1. Simulated Indoor Airflow within Four Ventilation Schemes
3.2. Simulated Dispersal of Airborne Viruses
3.2.1. Overall Indoor Dispersal of Airborne Viruses within Four Models
3.2.2. Transmission of Viruses at Inlets
3.2.3. Disease Spread at Cross-Sectional Reference Planes
3.3. Virus Dispersion at Bird Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mongeau, E.; Risser, K. A Look at Cage-Free Housing Solutions & Practices for a Changing Market. Available online: https://www.val-co.com/modern-hen-look-cage-free-housing-solutions (accessed on 10 April 2018).
- Chen, L. Cage-Free-Hen Housing Ventilation Options to Improve Air Quality and Bird Welfare. Ph.D. Thesis, The Pennsylvania State University, University Park, PA, USA, 19 August 2019. [Google Scholar]
- Chen, L.; Fabian-Wheeler, E.E.; Cimbala, J.M.; Hofstetter, D.; Patterson, P. Computational fluid dynamics modeling of ventilation and hen environment in cage-free egg facility. Animals 2020, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fabian-Wheeler, E.E.; Cimbala, J.M.; Hofstetter, D.; Patterson, P. Computational fluid dynamics analysis of alternative ventilation schemes in cage-free poultry housing. Animals 2021, 11, 2352. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.N.; Elkin, E. Egg Empires Are Coming as Bird Flu, Cage-Free Rules Drive Up Farm Costs. Available online: https://www.bloomberg.com/news/articles/2022-03-30/egg-empires-are-coming-as-bird-flu-cage-free-rules-drive-up-farm-costs (accessed on 11 April 2022).
- Lu, H.; Castro, A.E. Evaluation of the infectivity, length of infection, and immune response of a low-pathogenicity H7N2 avian influenza virus in specific-pathogen-free chickens. Avian Dis. 2004, 48, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mora, A.F.; Larios, A.D.; Rousseau, A.N.; Godbout, S.; Morin, C.; Palacios, J.H.; Grenier, M.; Fournel, S. Assessing environmental control strategies in cage-free egg production systems: Effect on spatial occupancy and natural behaviors. Animals 2021, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- USDA-APHIS. 2022 Confirmations of Highly Pathogenic Avian Influenza in Commercial and Backyard Flocks. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/avian/avian-influenza/hpai-2022 (accessed on 11 April 2022).
- Schnirring, L. High-Path Avian Flu Strikes Texas Flock, Expands in Midwest. Available online: https://www.cidrap.umn.edu/news-perspective/2022/04/high-path-avian-flu-strikes-texas-flock-expands-midwest (accessed on 11 April 2022).
- Dargatz, D.; Beam, A.; Wainwright, S.; McCluskey, B. Case series of turkey farms from the H5N2 highly pathogenic avian influenza outbreak in the United States during 2015. Avian Dis. 2016, 60, 467–472. [Google Scholar] [CrossRef] [PubMed]
- USDA-APHIS Epidemiologic and Other Analyses of HPAI-Affected Poultry Flocks: 15 July 2015 Report. Available online: https://www.aphis.usda.gov/animal_health/animal_dis_spec/%0Dpoultry/downloads/Epidemiologic-Analysis-July-15-2015.pdf (accessed on 20 March 2022).
- Li, Y.; Nielsen, P.V. Commemorating 20 years of Indoor Air: CFD and ventilation research. Indoor Air 2011, 21, 442–453. [Google Scholar] [CrossRef]
- Çengel, Y.A.; Cimbala, J.M. Fluid Mechanics: Fundamentals and Applications, 3rd ed.; McGraw-Hill: New York, NY, USA, 2014; ISBN 978-0-07-338032-2. [Google Scholar]
- Lee, I.-B.; Bitog, J.P.P.; Hong, S.-W.; Seo, I.-H.; Kwon, K.-S.; Bartzanas, T.; Kacira, M. The past, present and future of CFD for agro-environmental applications. Comput. Electron. Agric. 2013, 93, 168–183. [Google Scholar] [CrossRef]
- Mistriotis, A.; De Jong, T.; Wagemans, M.J.M.; Bot, G.P. Computational Fluid Dynamics (CFD) as a tool for the analysis of ventilation and indoor microclimate in agricultural buildings. Neth. J. Agric. Sci. 1997, 45, 81–96. [Google Scholar] [CrossRef]
- Norton, T.; Sun, D.-W.; Grant, J.; Fallon, R.; Dodd, V. Applications of computational fluid dynamics (CFD) in the modelling and design of ventilation systems in the agricultural industry: A review. Bioresour. Technol. 2007, 98, 2386–2414. [Google Scholar] [CrossRef]
- Nielsen, P.V. Fifty years of CFD for room air distribution. Build. Environ. 2015, 91, 78–90. [Google Scholar] [CrossRef]
- Küçüktopcu, E.; Cemek, B.; Simsek, H.; Ni, J.-Q. Computational Fluid Dynamics Modeling of a Broiler House Microclimate in Summer and Winter. Animals 2022, 12, 867. [Google Scholar] [CrossRef]
- Pawar, S.R.; Cimbala, J.M.; Wheeler, E.F.; Lindberg, D.V. Analysis of poultry house ventilation using computational fluid dynamics. Trans. ASABE 2007, 50, 1373–1382. [Google Scholar] [CrossRef]
- Tong, X.; Hong, S.W.; Zhao, L. Using CFD simulations to develop an upward airflow displacement ventilation system for manure-belt layer houses to improve the indoor environment. Biosyst. Eng. 2019, 178, 294–308. [Google Scholar] [CrossRef]
- Seo, I.-H.; Lee, I.-B.; Moon, O.-K.; Jung, N.-S.; Lee, H.-J.; Hong, S.-W.; Kwon, K.-S.; Bitog, J.P. Prediction of the spread of highly pathogenic avian influenza using a multifactor network: Part 1—Development and application of computational fluid dynamics simulations of airborne dispersion. Biosyst. Eng. 2014, 121, 160–176. [Google Scholar] [CrossRef]
- Lee, H.; Suh, K.; Jung, N.; Lee, I.; Seo, I.; Moon, O.; Lee, J. Prediction of the spread of highly pathogenic avian influenza using a multifactor network: Part 2—Comprehensive network analysis with direct/indirect infection route. Biosyst. Eng. 2014, 118, 115–127. [Google Scholar] [CrossRef]
- ANSYS. User Guide; Release 12; ANSYS: Lebanon, NH, USA, 2009. [Google Scholar]
- Launder, B.E.; Spalding, D.B. The numerical computation of turbulent flows. Comput. Methods Appl. Mech. Eng. 1974, 3, 269–289. [Google Scholar] [CrossRef]
- Worley, M.S.; Manbeck, H.B. Modeling particle transport and air flow in ceiling inlet ventilation systems. Trans. Am. Soc. Agric. Eng. 1995, 38, 231–239. [Google Scholar] [CrossRef]
- Rong, L.; Nielsen, P.V.; Bjerg, B.; Zhang, G. Summary of best guidelines and validation of CFD modeling in livestock buildings to ensure prediction quality. Comput. Electron. Agric. 2016, 121, 180–190. [Google Scholar] [CrossRef]
- Chen, L.; Fabian-Wheeler, E.E.; Hofstetter, D. Ventilation options modeling for laying hens in cage-free environment: Three-dimensional case. In Proceedings of the ASABE 2018 Annual International Meeting, Detroit, MI, USA, 29 July–1 August 2018. [Google Scholar]
- Fabian-Wheeler, E.; Chen, L.; Hofstetter, D.; Patterson, P.; Cimbala, J. Modeling hen house ventilation options for cage-free environment- two-dimensional case. In Proceedings of the 10th International Livestock Environment Symposium (ILES X), Omaha, NE, USA, 25–27 September 2018. [Google Scholar]
- Chen, L.; Fabian-Wheeler, E.E. Computational fluid dynamics analysis of indoor air conditions in commercial floor-raised cage-free hen house. In Proceedings of the 5th International Conference of the International Commission of Agricultural and Biosystems Engineering (CIGR), Québec City, QC, Canada, 11–14 May 2021. [Google Scholar]
- Chen, L.; Fabian-Wheeler, E.E. A CFD study on ventilation options for cage-free hen houses in North America. In Proceedings of the International Symposium on Animal Environment and Welfare, Chongqing, China, 21–23 October 2021. [Google Scholar]
- Chen, L.; Fabian-Wheeler, E.E. Investigations on alternative ventilation schemes for floor-raised hen facility. In Proceedings of the 5th International Conference of the International Commission of Agricultural and Biosystems Engineering (CIGR), Québec City, QC, Canada, 11–14 May 2021. [Google Scholar]
- Inlets for Mechanical Ventilation Systems in Animal Housing. Available online: https://extension.psu.edu/inlets-for-mechanical-ventilation-systems-in-animal-housing (accessed on 26 March 2019).
- Walsberg, B.Y.G.E.; King, J.R. The relationship of the external surface area of birds to skin surface area and body mass. J. Exp. Biol. 1978, 76, 185–189. [Google Scholar] [CrossRef]
- Mutaf, S.; Şeber Kahraman, N.; Firat, M.Z. Surface wetting and its effect on body and surface temperatures of domestic laying hens at different thermal conditions. Poult. Sci. 2008, 87, 2441–2450. [Google Scholar] [CrossRef]
- Heymsfield, C. Computational Fluid Dynamics Model for Air Velocity through a Poultry Transport Trailer in a Holding Shed. Master’s Thesis, University of Arkansas, Fayetteville, AK, USA, 12 May 2018. [Google Scholar]
- Roache, P.J. Perspective: A method for uniform reporting of grid refinement studies. J. Fluids Eng. Trans. ASME. 1994, 116, 405–413. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002. [Google Scholar]
- Kutner, M.H.; Nachtsheim, C.J.; Neter, J.; Li, W. Applied Linear Statistical Models, 5th ed.; McGraw-Hill: Boston, MA, USA, 2004. [Google Scholar]
- Evaluating Air Quality in Livestock Housing Environments. Available online: https://extension.psu.edu/evaluating-air-quality-in-livestock-housing-environments (accessed on 26 March 2019).
- Selecting Rated Ventilation Fans. Available online: https://extension.psu.edu/selecting-rated-ventilation-fans (accessed on 26 March 2019).
- Perén, J.I.; van Hooff, T.; Leite, B.C.C.; Blocken, B. CFD simulation of wind-driven upward cross ventilation and its enhancement in long buildings: Impact of single-span versus double-span leeward sawtooth roof and opening ratio. Build. Environ. 2016, 96, 142–156. [Google Scholar] [CrossRef] [Green Version]





| Plane | Factor | df | Sum of Squares | Mean Square | F-Value | Pr (>F) |
|---|---|---|---|---|---|---|
| CP-I | Model | 3 | 0.4 | 0.1 | 168,988 | <2.2 × 10−16 |
| Zone | 4 | 0.4 | 0.09 | 107,878 | <2.2 × 10−16 | |
| Model × Zone | 12 | 0.08 | 0.006 | 7772 | <2.2 × 10−16 | |
| Residuals | 35,320 | 0.03 | 10−6 | |||
| CP-N | Model | 3 | 0.06 | 0.02 | 28,584 | <2.2 × 10−16 |
| Zone | 4 | 0.3 | 0.08 | 112,142 | <2.2 × 10−16 | |
| Model × Zone | 12 | 0.03 | 0.003 | 3704 | <2.2 × 10−16 | |
| Residuals | 34,966 | 0.03 | 10−6 | |||
| CP-F | Model | 3 | 0.05 | 0.02 | 22,953 | <2.2 × 10−16 |
| Zone | 4 | 0.4 | 0.1 | 130,070 | <2.2 × 10−16 | |
| Model × Zone | 12 | 0.1 | 0.008 | 11,152 | <2.2 × 10−16 | |
| Residuals | 34,158 | 0.03 | 10−6 |
| Plane | Zone | TISE | MICE | MIRE | MIAE |
|---|---|---|---|---|---|
| CP-I | Zone-1 | 0.0182 a | 0.0122 b | 0.0211 | 0.0171 c |
| Zone-2 | 0.0194 | 0.0145 | 0.0223 | 0.0182 a | |
| Zone-3 | 0.0203 | 0.0068 d | 0.0182 a | 0.0105 | |
| Zone-4 | 0.0171 c | 0.0058 | 0.0137 | 0.0097 | |
| Zone-5 | 0.0123 b | 0.0067 d | 0.0156 | 0.0110 | |
| CP-N | Zone-1 | 0.0177 a | 0.0156 | 0.0198 | 0.0178 a |
| Zone-2 | 0.0184 b | 0.0168 | 0.0197 | 0.0207 | |
| Zone-3 | 0.0185 b | 0.0124 c | 0.0152 | 0.0176 a | |
| Zone-4 | 0.0133 | 0.0098 | 0.0136 | 0.0112 | |
| Zone-5 | 0.0123 c | 0.0108 | 0.0149 | 0.0118 | |
| CP-F | Zone-1 | 0.0174 | 0.0220 | 0.0193 | 0.0158 |
| Zone-2 | 0.0168 a | 0.0228 | 0.0213 | 0.0169 a | |
| Zone-3 | 0.0155 b | 0.0138 | 0.0146 | 0.0116 | |
| Zone-4 | 0.0148 | 0.0112 c | 0.0104 | 0.0108 | |
| Zone-5 | 0.0156 b | 0.0130 | 0.0117 | 0.0113 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Fabian-Wheeler, E.E.; Cimbala, J.M.; Hofstetter, D.; Patterson, P. Numerical Simulation of Airborne Disease Spread in Cage-Free Hen Housing with Multiple Ventilation Options. Animals 2022, 12, 1516. https://doi.org/10.3390/ani12121516
Chen L, Fabian-Wheeler EE, Cimbala JM, Hofstetter D, Patterson P. Numerical Simulation of Airborne Disease Spread in Cage-Free Hen Housing with Multiple Ventilation Options. Animals. 2022; 12(12):1516. https://doi.org/10.3390/ani12121516
Chicago/Turabian StyleChen, Long, Eileen E. Fabian-Wheeler, John M. Cimbala, Daniel Hofstetter, and Paul Patterson. 2022. "Numerical Simulation of Airborne Disease Spread in Cage-Free Hen Housing with Multiple Ventilation Options" Animals 12, no. 12: 1516. https://doi.org/10.3390/ani12121516
APA StyleChen, L., Fabian-Wheeler, E. E., Cimbala, J. M., Hofstetter, D., & Patterson, P. (2022). Numerical Simulation of Airborne Disease Spread in Cage-Free Hen Housing with Multiple Ventilation Options. Animals, 12(12), 1516. https://doi.org/10.3390/ani12121516








