Bobolink (Dolichonyx oryzivorus) Declines Follow Bison (Bison bison) Reintroduction on Private Conservation Grasslands
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
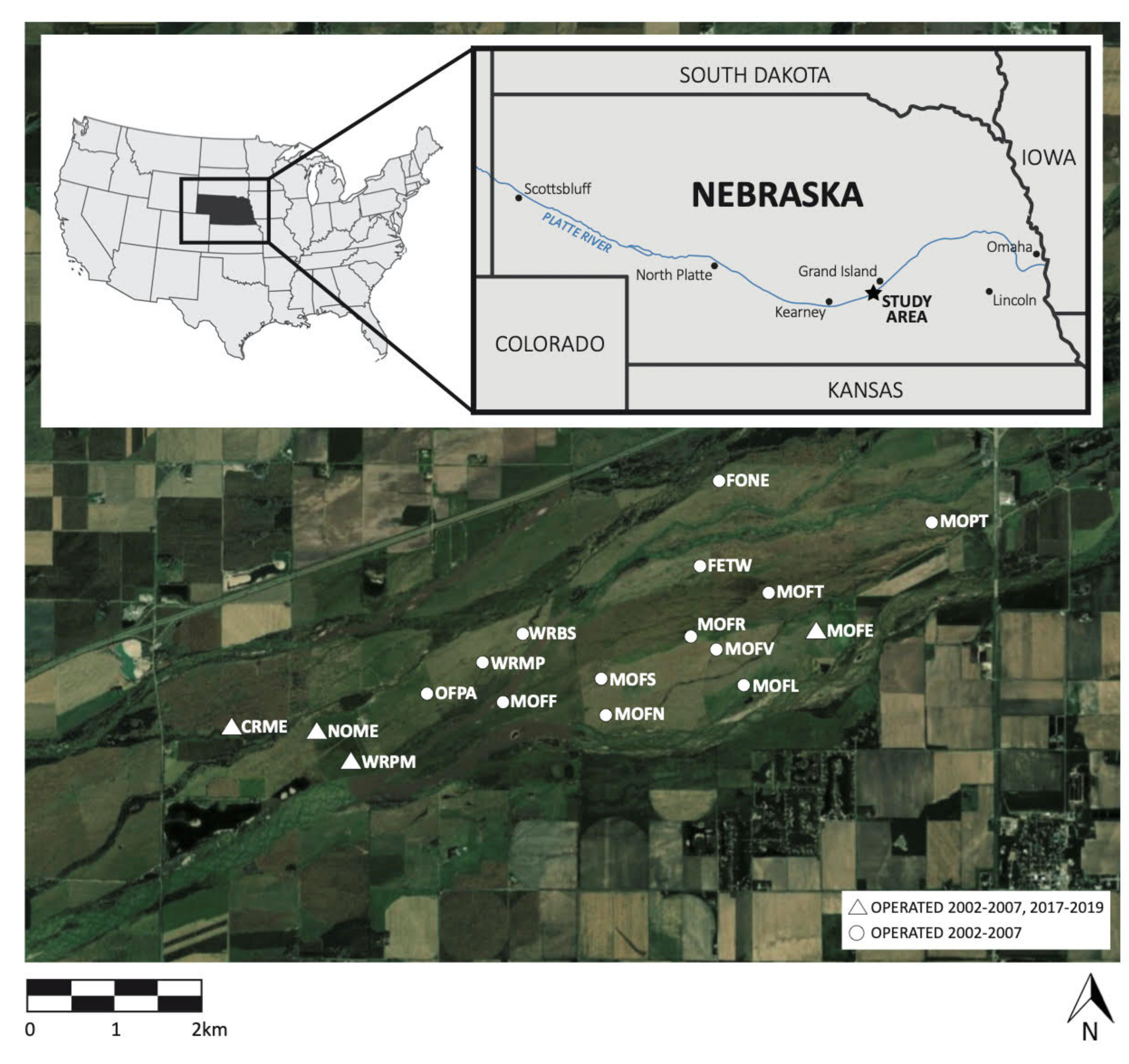
2.1. Study Area
2.2. Bird Sampling
2.3. Analytical Approach
2.4. Statistical Analyses
2.5. Land Management Parameters
2.6. Climate Parameters
3. Results
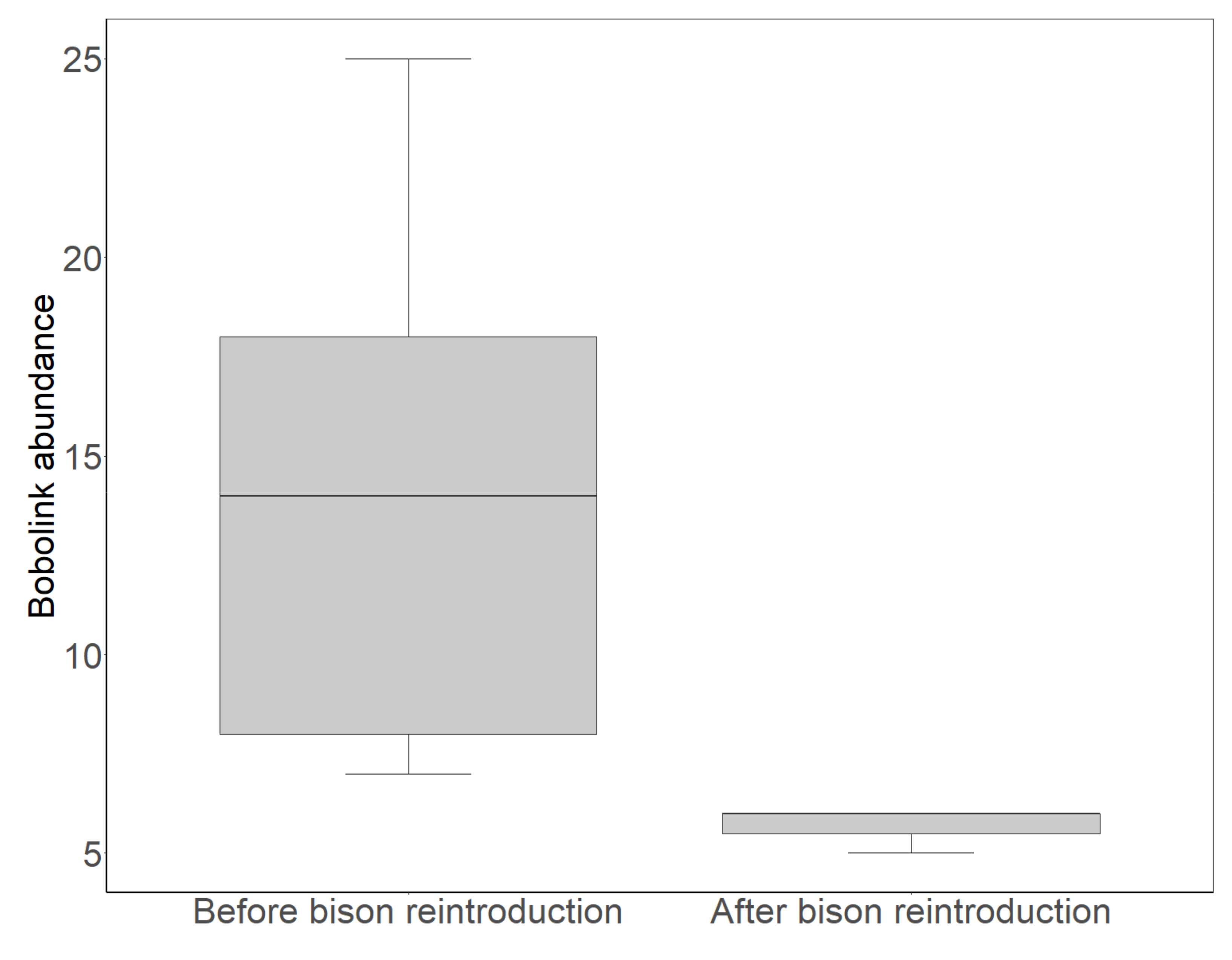
3.1. Bobolink Abundance and Productivity before and after Bison Reintroduction
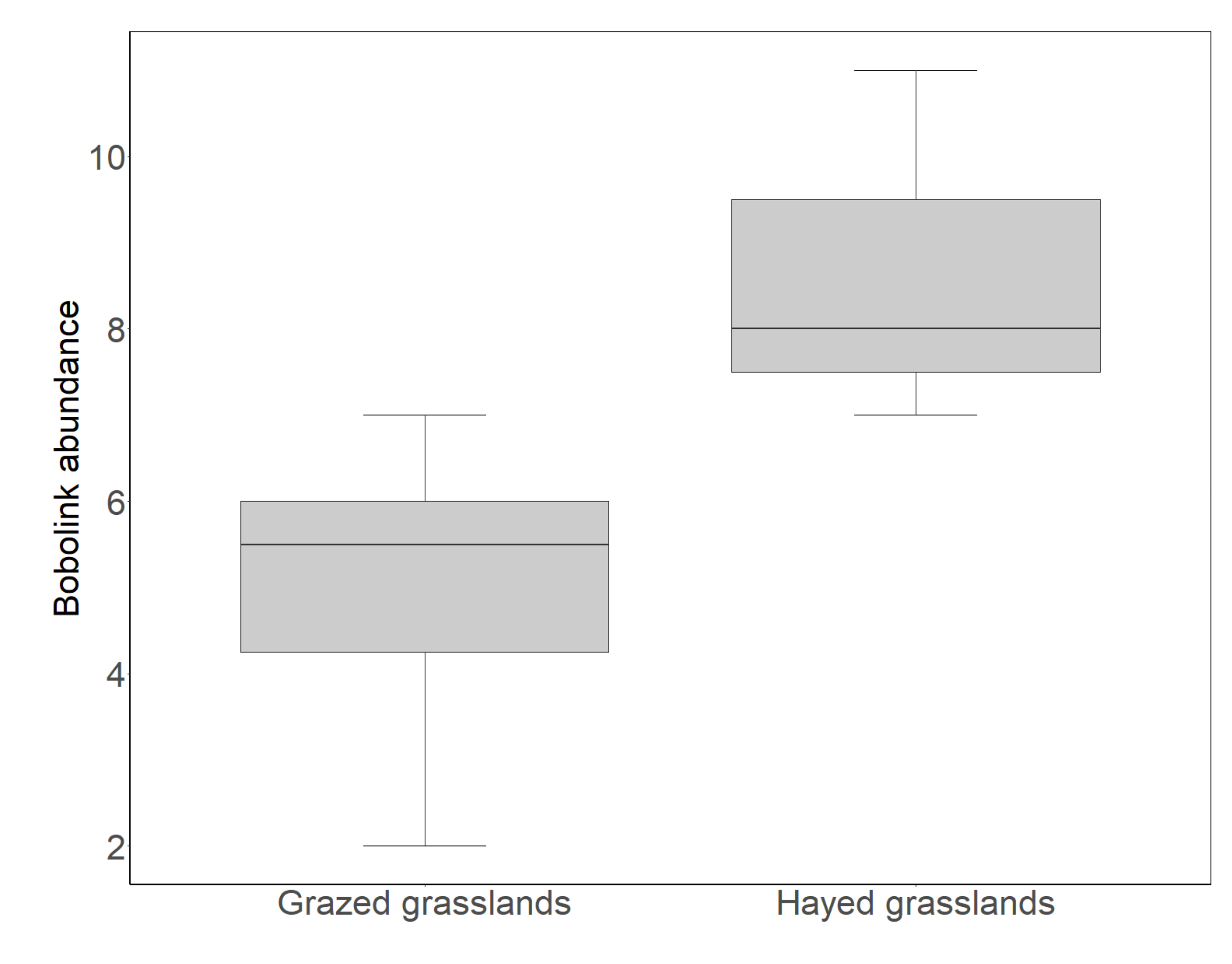
3.2. Bobolink Abundance in Grazed Grasslands Compared to Hayed Grasslands
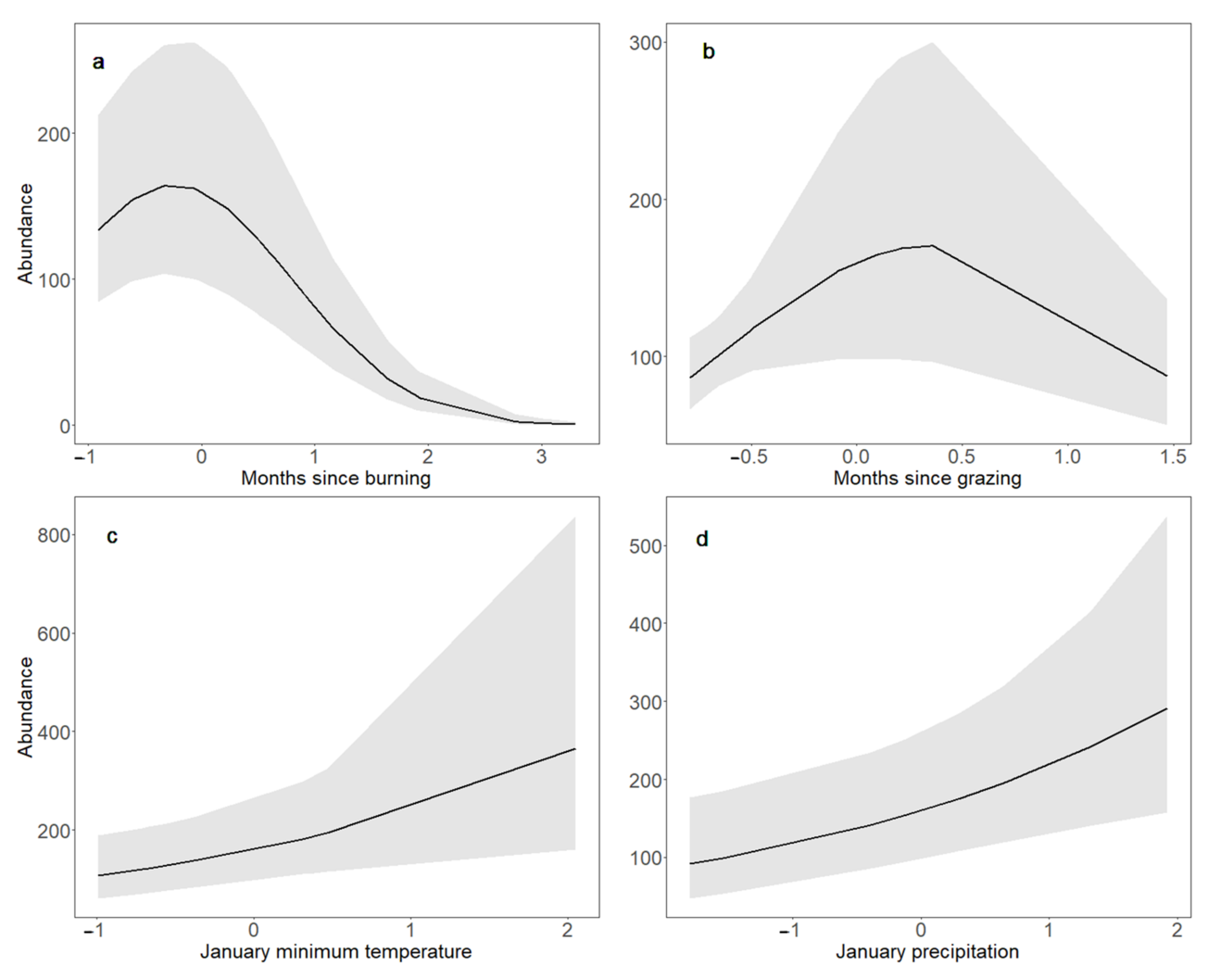
3.3. Bobolink Abundance and Productivity in Response to Changes in Land Management and Climate Parameters and Their Interactions
4. Discussion
4.1. Bobolink Abundance and Productivity Exhibited Steep Declines after Bison Reintroduction
4.2. Bobolink Abundance and Productivity Were Lower in Grazed Than Hayed Grasslands, but Increased after the Departure of Grazers
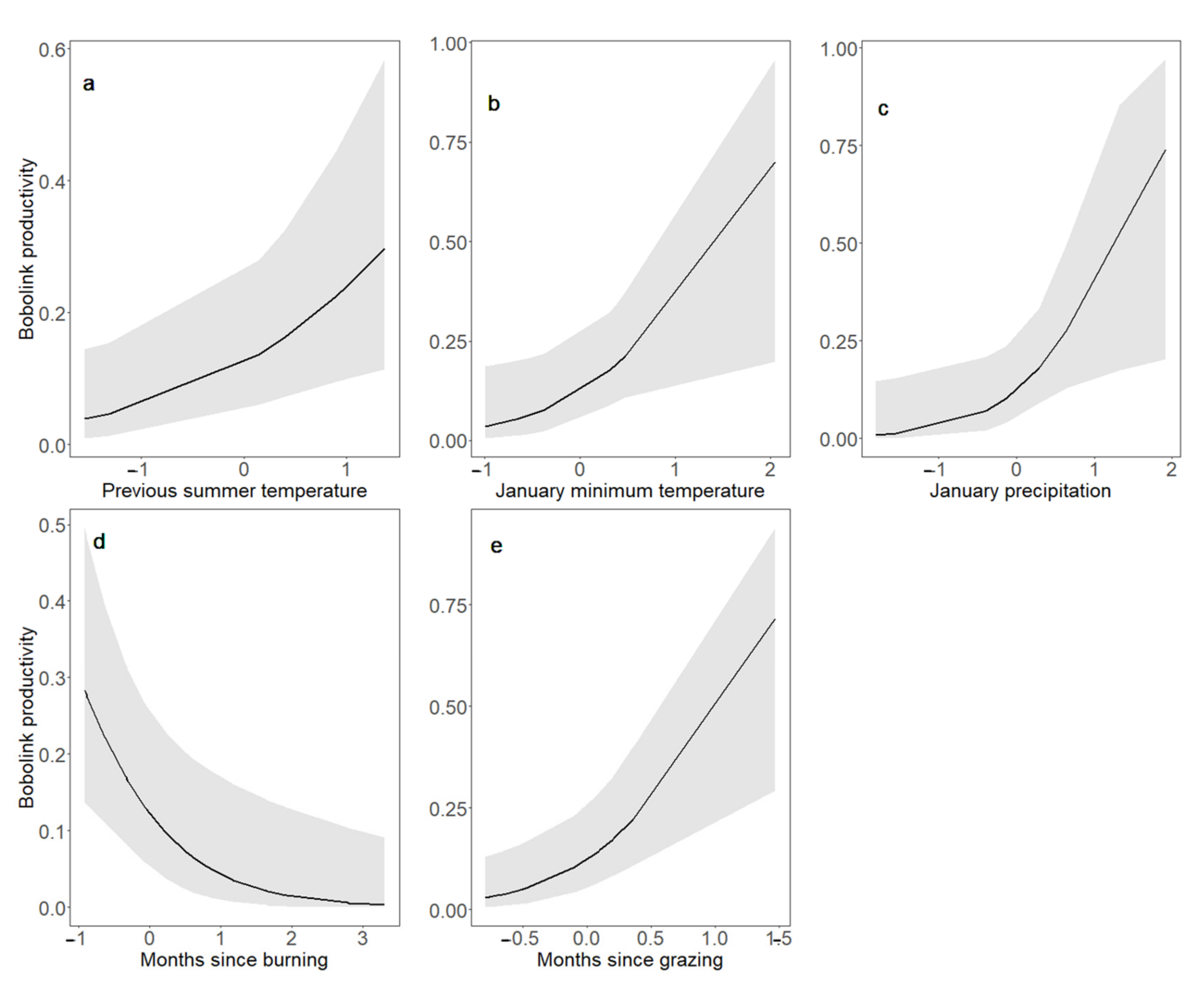
4.3. Bobolink Abundance and Productivity Increased following Warmer, Wetter Winters, and after Patch Burning; Bobolink Productivity Also Increased in Response to Warmer, Wetter Summers
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herkert, J.R. An analysis of midwestern breeding bird population trends: 1966–1993. Am. Midl. Nat. 1995, 134, 41–50. [Google Scholar] [CrossRef]
- Johnson, R.J.; Jedlicka, J.A.; Quinn, J.E.; Brandle, J.R. Global perspectives on birds in agricultural landscapes. In Integrating Agriculture, Conservation and Ecotourism: Examples from the Field; Campbell, W.B., López Ortíz, S., Eds.; Issues in Agroecology—Present Status and Future Prospectus 1; Springer: London, UK, 2011; pp. 55–140. [Google Scholar]
- Rosenberg, K.V.; Dokter, A.M.; Blancher, P.J.; Sauer, J.R.; Smith, A.C.; Smith, P.A.; Stanton, J.C.; Panjabi, A.; Helft, L.; Parr, M.; et al. Decline of the North American avifauna. Science 2019, 366, 120–124. [Google Scholar] [CrossRef]
- Ripple, W.J.; Beschta, R.L. Trophic cascades in Yellowstone: The first 15 years after wolf reintroduction. Biol. Conserv. 2012, 145, 205–213. [Google Scholar] [CrossRef]
- Bouley, P.; Paulo, A.; Angela, M.; Du Plessis, C.; Marneweck, D.G. The successful reintroduction of African wild dogs (Lycaon pictus) to Gorongosa National Park, Mozambique. PLoS ONE 2021, 16, e0249860. [Google Scholar] [CrossRef] [PubMed]
- Bison Conservation Initiative Fact Sheet. Available online: https://www.nps.gov/articles/bison-conservation-initiative-fact-sheet.htm (accessed on 22 July 2021).
- Redford, K.H.; Aune, K.; Plumb, G. Hope is a bison. Conserv. Biol. 2016, 30, 689–691. [Google Scholar] [CrossRef]
- Sanderson, E.W.; Redford, K.H.; Weber, B.; Aune, K.; Baldes, D.; Berger, J.; Carter, D.; Curtin, C.; Derr, J.; Dobrott, S.; et al. The ecological future of the North American bison: Conceiving long-term, large-scale conservation of wildlife. Conserv. Biol. 2008, 22, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, K.; Pejchar, L.; Garvoille, R. Ecological and social consequences of bison reintroduction in Colorado. Conserv. Sci. Pract. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Powell, A.F.L.A. Effects of prescribed burns and bison (Bos bison) grazing on breeding bird abundances in tallgrass prairie. Auk 2006, 123, 183–197. [Google Scholar] [CrossRef]
- Kays, R. Mammals of North America, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2009. [Google Scholar]
- Hale, S.L.; Koprowski, J.L. Ecosystem-level effects of keystone species reintroduction: A literature review. Restor. Ecol. 2018, 26, 439–445. [Google Scholar] [CrossRef]
- King, K.C.; Caven, A.J.; Leung, K.G.; Ranglack, D.H.; Arcilla, N. High society: Behavioral patterns as a feedback loop to social structure in Plains bison (Bison bison bison). Mammal. Res. 2019, 64, 365–376. [Google Scholar] [CrossRef]
- Freese, C.H.; Aune, K.E.; Boyd, D.P.; Derr, J.N.; Forrest, S.C.; Gates, C.C.; Gogan, P.J.P.; Grassel, S.M.; Halbert, N.D.; Kunkel, K.; et al. Second chance for the Plains bison. Biol. Conserv. 2007, 136, 175–184. [Google Scholar] [CrossRef]
- Ranglack, D.H.; Durham, S.; du Toit, J.T. Competition on the range: Science vs. perception in a bison–cattle conflict in the western USA. J. Appl. Ecol. 2015, 52, 467–474. [Google Scholar] [CrossRef]
- Ramankutty, N.; Evan, A.T.; Monfreda, C.; Foley, J.A. Farming the planet: 1. Geographic distribution of global agricultural lands in the year 2000. Global Biogeochem. Cycles 2008, 22, GB1003. [Google Scholar] [CrossRef]
- Dixon, A.P.; Faber-Langendoen, D.; Josse, C.; Morrison, J.; Loucks, C.J. Distribution mapping of world grassland types. J. Biogeogr. 2014, 41, 2003–2019. [Google Scholar] [CrossRef]
- White, R.P.; Murray, S.; Rohweder, M. Grassland Ecosystems. In Pilot Analysis of Global Ecosystems; White, R.P., Murray, S., Rohweder, M., Eds.; World Resources Institute: Washington, DC, USA, 2000. [Google Scholar]
- Blancher, P. Importance of North. America’s Grasslands to Birds; Commission for Environmental Cooperation and Bird Studies: Montreal, QC, Canada, 2003. [Google Scholar]
- Commission for Environmental Cooperation (CEC); The Nature Conservancy (TNC). North American Central Grassland Priority Conservation Areas: Technical Report and Documentation; Karl, J.W., Hoth, J., Eds.; Commission for Environmental Cooperation (CEC): Montreal, QC, Canada; The Nature Conservancy (TNC): Montreal, QC, Canada, 2005. [Google Scholar]
- Hoekstra, J.M.; Boucher, T.M.; Ricketts, T.H.; Roberts, C. Confronting a biome crisis: Global disparities of habitat loss and protection. Ecol. Lett. 2005, 8, 23–29. [Google Scholar] [CrossRef]
- Gruntorad, M.P.; Graham, K.A.; Arcilla, N.; Chizinski, C.J. Is hay for the birds? Investigating landowner willingness to time hay harvests for grassland bird conservation. Animals 2021, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Crosby, A.D.; Elmore, R.D.; Leslie, D.M., Jr.; Will, R.E. Looking beyond rare species as umbrella species: Northern Bobwhites (Colinus virginianus) and conservation of grassland and shrubland birds. Biol. Conserv. 2015, 186, 233–240. [Google Scholar] [CrossRef]
- Zipkin, E.F.; Royle, J.A.; Dawson, D.K.; Bates, S. Multi-species occurrence models to evaluate the effects of conservation and management actions. Biol. Conserv. 2010, 143, 479–484. [Google Scholar] [CrossRef]
- Koper, N.; Schmiegelow, F.K.A. Does management for duck productivity affect songbird nesting success? J. Wildl. Manag. 2007, 71, 2249–2257. [Google Scholar] [CrossRef]
- Moore, C.T.; Plummer, W.T.; Conroy, M.J. Forest management under uncertainty for multiple bird population objectives. In USDA Forest Service General Technical Report PSW-GTR.; Ralph, C.J., Rich, T.D., Eds.; USDA Forest Service Pacific Southwest Research Station: Albany, CA, USA, 2005; pp. 373–380. [Google Scholar]
- Dechant, J.A.; Sondreal, M.L.; Johnson, D.H.; Igl, L.D.; Goldade, C.M.; Zimmerman, A.L.; Euliss, B.R. Effects of Management Practices on Grassland Birds: Bobolink; Northern Prairie Wildlife Research Center: Jamestown, ND, USA, 2001. [Google Scholar]
- Vickery, P.D.; Herkert, J.R. (Eds.) Ecology and conservation of grassland birds in the Western Hemisphere. In Studies in Avian Biology; Cooper Ornithological Society: Waco, TX, USA, 1999; Volume 19. [Google Scholar]
- Conrey, R.Y.; Skagen, S.K.; Yackel Adams, A.A.; Panjabi, A.O. Extremes of heat, drought and precipitation depress reproductive performance in shortgrass prairie passerines. Ibis 2016, 158, 614–629. [Google Scholar] [CrossRef]
- Fuhlendorf, S.D.; Allred, B.W. Hamilton, R.G. Bison as Keystone Herbivores on the Great Plains: Can Cattle Serve as Proxy for Evolutionary Grazing Patterns? American Bison Society Working Paper 4; American Bison Society: Stillwater, OK, USA, 2010; Available online: www.americanbisonsocietyonline.org (accessed on 22 July 2021).
- Kohl, M.T.; Krausman, P.R.; Kunkel, K.; Williams, D.M. Bison versus cattle: Are they ecologically synonymous? Rangel. Ecol. Manag. 2013, 66, 721–731. [Google Scholar] [CrossRef]
- Hartnett, D.C.; Steuter, A.A.; Hickman, K.R. Comparative ecology of native and introduced ungulates. In Ecology and Conservation of Great Plains Vertebrates; Knopf, F.L., Samson, F.B., Eds.; Springer: New York, NY, USA, 1997; pp. 72–101. [Google Scholar]
- Bollongino, R.; Burger, J.; Powell, A.; Mashkour, M.; Vigne, J.D.; Thomas, M.G. Modern taurine cattle descended from small number of near-eastern founders. Mol. Biol. Evol. 2012, 29, 2101–2104. [Google Scholar] [CrossRef]
- U.S. National Park Service. Grand Canyon National Park Bison Removal FAQs. Available online: https://www.nps.gov/grca/learn/nature/bison-reduction-faqs.htm (accessed on 10 August 2021).
- Geremia, C.; Wallen, R.; McGarvey, L.; Perez, R.; Blanton, D. Bison Conservation Update; National Park Service, Yellowstone Center for Resources, Wildlife and Aquatic Resources Branch, Bison Program: Yellowstone National Park, WY, USA, 2020. Available online: https://www.nps.gov/yell/learn/management/upload/BISON-Conservation-Update-2020.pdf (accessed on 10 August 2021).
- Herakovich, H.; Whelan, C.J.; Barber, N.A.; Jones, H.P. Impacts of a recent bison reintroduction on grassland bird nests and potential mechanisms for these effects. Nat. Areas J. 2021, 41, 93–103. [Google Scholar] [CrossRef]
- DeSante, D.F.; Kaschube, D.R.; Saracco, J.F. Vital Rates of North American Landbirds; The Institute for Bird Populations, Point Reyes Station, CA, USA. 2015. Available online: http://www.VitalRatesOfNorthAmericanLandbirds.org (accessed on 22 July 2021).
- Renfrew, R.; Strong, A.M.; Perlut, N.G.; Martin, S.G.; Gavin, T.A. Bobolink (Dolichonyx oryzivorus), version 1.0. In Birds of the World; Rodewald, P.G., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2021. [Google Scholar]
- Sauer, J.R.; Pardieck, K.L.; Ziolkowski, D.J., Jr.; Smith, A.C.; Hudson, M.-A.R.; Rodriguez, V.; Berlanga, H.; Niven, D.K.; Link, W.A. The first 50 years of the North American Breeding Bird Survey. Condor 2017, 119, 576–593. [Google Scholar] [CrossRef]
- Rosenberg, K.V.; Kennedy, J.A.; Dettmers, R.; Ford, R.P.; Reynolds, D.; Alexander, J.D.; Beardmore, C.J.; Blancher, P.J.; Bogart, R.E.; Butcher, G.S.; et al. Partners in Flight Landbird Conservation Plan: 2016 Revision for Canada and Continental United States; Cornell Lab of Ornithology: Ithaca, NY, USA, 2016. [Google Scholar]
- Herkert, J.R. Bobolink (Dolichonyx oryzivorus) population decline in agricultural landscapes in the Midwestern USA. Biol. Conserv. 1997, 80, 107–112. [Google Scholar] [CrossRef]
- Ethier, D.M.; Nudds, T.D. Complexity of factors affecting Bobolink population dynamics communicated with directed acyclic graphs. Wildl. Soc. Bull. 2017, 41, 4–16. [Google Scholar] [CrossRef]
- Renfrew, R.B.; Ribic, C.A. Influence of topography on density of grassland passerines in pastures. Am. Midl. Nat. 2002, 147, 315–325. [Google Scholar] [CrossRef]
- Campomizzi, A.J.; Lebrun-Southcott, Z.M.; Van Vliet, L.D.; Morris, G.A. Rotational grazing of beef cattle to support Bobolink breeding success. Avian Conserv. Ecol. 2019, 14, 13. [Google Scholar] [CrossRef]
- Huber, G.E.; Steuter, A.A. Vegetation profile and grassland bird response to spring burning. Prairie Nat. 1984, 16, 55–61. [Google Scholar]
- National Audubon Societies. Important Bird Areas, Nebraska: Crane Trust Wild Rose Ranch/Mormon Island Properties. Available online: https://www.audubon.org/important-bird-areas/state/nebraska (accessed on 22 July 2021).
- Williams, G.P. The Case of the Shrinking Channels: The North Platte and Platte Rivers in Nebraska. In U.S. Geological Survey Circular; Government Printing Office: Washington, DC, USA, 1978. [Google Scholar] [CrossRef]
- Anderson, R.C. Evolution and origin of the Central Grassland of North America: Climate, fire, and mammalian grazers. J. Torrey Bot. Soc. 2006, 133, 626–647. [Google Scholar] [CrossRef]
- Salter, J. Bison Calving Season. Available online: https://cranetrust.org/news-events/the-prairie-pulse.html/article/2019/04/19/bison-calving-season (accessed on 22 July 2021).
- Smith, T.; Caven, A. Crane Trust 2019 Habitat Management Plan; Crane Trust: Wood River, NE, USA, 2019. [Google Scholar]
- DeSante, D.F. Monitoring avian productivity and survivorship (MAPS): A sharp, rather than blunt, tool for monitoring and assessing landbird populations. In Wildlife 2001: Populations; McCullough, D.C., Barrett, R.H., Eds.; Elsevier Applied Science: London, UK, 1992; pp. 511–521. [Google Scholar]
- DeSante, D.F.; Burton, K.M.; Velez, P.; Froehlich, D.; Kaschube, D. MAPS Manual: 2015 Protocol; The Institute for Bird Populations: Point Reyes Station, CA, USA, 2015. [Google Scholar]
- Pyle, P. Identification Guide to North American Birds; Slate Creek Press: Bolinas, CA, USA, 1997. [Google Scholar]
- Williams, B.K.; Nichols, J.D.; Conroy, M.J. Analysis and Management of Animal Populations: Modeling, Estimation, and Decision Making; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Manly, B.F.J.; McDonald, T.L.; Amstrup, S.C.; Regehr, E.V. Improving size estimates of open animal populations by incorporating information on age. BioScience 2003, 53, 666–669. [Google Scholar] [CrossRef]
- Amstrup, S.C.; McDonald, T.L.; Manly, B.F.J. Handbook of Capture-Recapture Analysis; Princeton University Press: Princeton, NJ, USA, 2005. [Google Scholar]
- Thomson, D.L.; Conroy, M.J.; Anderson, D.R.; Burnham, K.P.; Cooch, E.G.; Francis, C.M.; Lebreton, J.-D.; Lindberg, M.S.; Morgan, B.J.T.; Otis, D.L.; et al. Standardising terminology and notation for the analysis of demographic processes in marked populations. Environ. Ecol. Stat. 2009, 3, 1099–1106. [Google Scholar] [CrossRef]
- Gopalaswamy, A.M.; Royle, J.A.; Hines, J.E.; Singh, P.; Jathanna, D.; Kumar, N.S.; Karanth, K.U. Program SPACECAP: Software for estimating animal density using spatially explicit capture-recapture models. Methods Ecol. Evol. 2012, 3, 1067–1072. [Google Scholar] [CrossRef]
- Barker, R.J.; Burnham, K.P.; White, G.C. Encounter history modeling of joint mark-recapture, tag-resighting and tag-recovery data under temporary emigration. Stat. Sin. 2004, 14, 1037–1055. [Google Scholar]
- Remsen, J.V., Jr.; Good, D.A. Misuse of data from mist-net captures to assess relative abundance in bird populations. Auk 1996, 113, 381–398. [Google Scholar] [CrossRef]
- Martin, T.E.; Nightingale, J.; Baddams, J.; Monkhouse, J.; Kaban, A.; Sastranegara, H.; Mulyani, Y.; Blackburn, G.A.; Simcox, W. Variability in the effectiveness of two ornithological survey methods between tropical forest ecosystems. PLoS ONE 2017, 12, e0169786. [Google Scholar] [CrossRef]
- Foster, K.R.; Godwin, C.M.; Pyle, P.; Saracco, J.F. Reclamation and habitat-disturbance effects on landbird abundance and productivity indices in the oil sands region of northeastern Alberta, Canada. Restor. Ecol. 2016, 25, 532–538. [Google Scholar] [CrossRef]
- Glass, A.J.; Caven, A.J.; Kim, D.; Sutton, M.O.; Arcilla, N. Climate change and land management implications for a declining Neotropical migratory songbird breeding in the North American Great Plains. Avian Conserv. Ecol. 2020, 15, 4. [Google Scholar] [CrossRef]
- Rosamond, K.M.; Goded, S.; Soultan, A.; Kaplan, R.H.; Glass, A.; Kim, D.H.; Arcilla, N. Not singing in the rain: Linking migratory songbird declines with increasing precipitation and brood parasitism vulnerability. Front. Ecol. Evol. 2020, 8, 536769. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Revelle, W.R. Psych: Procedures for Personality and Psychological Research; Northwestern University: Evanston, IL, USA, 2018. [Google Scholar]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Gust, L.; D’journo, X.B. The use of correlation functions in thoracic surgery research. J. Thorac. Dis. 2015, 7, E11–E15. [Google Scholar] [CrossRef]
- Ratner, B. The correlation coefficient: Its values range between +1/−1, or do they? J. Target. Meas. Anal. Mark. 2009, 17, 139–142. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Jamil, T.; Ozinga, W.A.; Kleyer, M.; ter Braak, C.J.F. Selecting traits that explain species–environment relationships: A generalized linear mixed model approach. J. Veg. Sci. 2013, 24, 988–1000. [Google Scholar] [CrossRef]
- Young, M.; Ierodiaconou, D.; Womersley, T. Forests of the sea: Predictive habitat modelling to assess the abundance of canopy forming kelp forests on temperate reefs. Remote Sens. Environ. 2015, 170, 178–187. [Google Scholar] [CrossRef]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R.; Springer: Berlin, Germany, 2009. [Google Scholar]
- Maydeu-Olivares, A.; García-Forero, C. Goodness-of-Fit Testing. Int. Encycl. Educ. 2010, 7, 190–196. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R. J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Collins, S.L. Disturbance frequency and community stability in native tallgrass prairie. Am. Nat. 2000, 155, 311–325. [Google Scholar] [CrossRef]
- Hamilton, R.G. Restoring heterogeneity on the Tallgrass Prairie Preserve: Applying the fire-grazing interaction model. In Proceedings of the 23rd Tall Timbers Fire Ecology Conference: Fire in Grassland and Shrubland Ecosystems, Tallahasee, FL, USA, 1 January 2007; Masters, R.E., Galley, K.E.M., Eds.; Timbers Research Station: Tallahassee, FL, USA, 2007; pp. 163–169. [Google Scholar]
- Johnson, T.N.; Kennedy, P.L.; DelCurto, T.; Taylor, R.V. Bird community responses to cattle stocking rates in a Pacific Northwest bunchgrass prairie. Agric. Ecosyst. Environ. 2011, 144, 338–346. [Google Scholar] [CrossRef]
- Google Earth Pro V 6.2.2.6613. Available online: https://www.google.com/earth/versions/#earth-pro (accessed on 23 July 2021).
- Climate at a Glance. Available online: https://www.ncdc.noaa.gov/cag/ (accessed on 22 July 2021).
- Bateman, B.L.; Abell-Davis, S.E.; Johnson, C.N. Climate-driven variation in food availability between the core and range edge of the endangered Northern Bettong (Bettongia tropica). Aust. J. Zool. 2011, 59, 177–185. [Google Scholar] [CrossRef]
- Bateman, B.L.; Pidgeon, A.M.; Radeloff, V.C.; Flather, C.H.; VanDerWal, J.; Akçakaya, H.R.; Thogmartin, W.E.; Albright, T.P.; Vavrus, S.J.; Heglund, P.J. Potential breeding distributions of US birds predicted with both short-term variability and long-term average climate data. Ecol. Appl. 2016, 26, 2720–2731. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Barve, N.; Lira-Noriega, A.; Maher, S.P.; Nakazawa, Y.; Papeş, M.; Soberón, J.; Sukumaran, J.; Peterson, A.T. Dominant climate influences on North American bird distributions. Glob. Ecol. Biogeogr. 2011, 20, 114–118. [Google Scholar] [CrossRef]
- Illán, J.G.; Thomas, C.D.; Jones, J.A.; Wong, W.K.; Shirley, S.M.; Betts, M.G. Precipitation and winter temperature predict long-term range-scale abundance changes in Western North American birds. Glob. Chang. Biol. 2014, 20, 3351–3364. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.A.; Barbet-Massin, M.; Lopes, L.E.; Jiguet, F. Predicted climate-driven bird distribution changes and forecasted conservation conflicts in a neotropical savanna. Conserv. Biol. 2009, 23, 1558–1567. [Google Scholar] [CrossRef]
- O’Connor, R.J.; Jones, M.T.; Boone, R.B.; Lauber, T.B. Linking continental climate, land use, and land patterns with grassland bird distribution across the conterminous United States. Stud. Avian Biol. 1999, 19, 45–59. [Google Scholar]
- Brown, C.R.; Brown, M.B. Ectoparasitism shortens the breeding season in a colonial bird. R. Soc. Open Sci. 2015, 2, 140508. [Google Scholar] [CrossRef]
- Niemuth, N.D.; Solberg, J.W.; Shaffer, T.L. Influence of moisture on density and distribution of grassland birds in North Dakota. Condor 2008, 110, 211–222. [Google Scholar] [CrossRef][Green Version]
- Kim, D.H.; Newton, W.E.; Lingle, G.R.; Chavez-Ramirez, F. Influence of grazing and available moisture on breeding densities of grassland birds in the Central Platte River Valley, Nebraska. Wilson J. Ornithol. 2008, 120, 820–829. [Google Scholar] [CrossRef]
- Grinde, A.R.; Niemi, G.J.; Sturtevant, B.R.; Panci, H.; Thogmartin, W.; Wolter, P. Importance of scale, land cover, and weather on the abundance of bird species in a managed forest. For. Ecol. Manag. 2017, 405, 295–308. [Google Scholar] [CrossRef]
- Ruff, Z.J. Breeding Ecology of the Mountain Plover (Charadrius montanus) in Phillips County, Montana. Master’s Thesis, Iowa State University, Ames, IA, USA, 2016. [Google Scholar]
- Bock, C.E.; Saab, V.A.; Rich, T.D.; Dobkin, D.S. Effects of livestock grazing on Neotropical migratory landbirds in western North America. In Status and Management of Neotropical Migratory Landbirds; Finch, D.M., Stangel, P.W., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1993; pp. 296–309. [Google Scholar]
- Pietz, P.J.; Granfors, D.A. Identifying predators and fates of grassland passerine nests using miniature video cameras. J. Wildl. Manag. 2000, 64, 71–87. [Google Scholar] [CrossRef]
- Zöckler, C.; Miles, L.; Fish, L.; Wolf, A.; Rees, G.; Danks, F. Potential impact of climate change and reindeer density on tundra indicator species in the Barents Sea region. Clim. Change 2008, 87, 119–130. [Google Scholar] [CrossRef]
- Metera, E.; Sakowski, T.; Słoniewski, K.; Romanowicz, B. Grazing as a tool to maintain biodiversity of grassland—A review. Anim. Sci. Pap. Rep. 2010, 28, 315–334. [Google Scholar]
- Kotsonas, E.G.; Bakaloudis, D.E.; Vlachos, C.G.; Abraham, E.M.; Goutner, V. Effect of transhumant livestock grazing on pseudo-alpine grassland bird communities. Birds 2021, 2, 2. [Google Scholar] [CrossRef]
- Fondell, T.F.; Ball, I.J. Density and success of bird nests relative to grazing on western Montana grasslands. Biol. Conserv. 2004, 117, 203–213. [Google Scholar] [CrossRef]
- Ahlering, M.A.; Merkord, C.L. Cattle grazing and grassland birds in the northern tallgrass prairie. J. Wildl. Manag. 2016, 80, 643–654. [Google Scholar] [CrossRef]
- Perlut, N.G.; Strong, A.M. Grassland birds and rotational-grazing in the northeast: Breeding ecology, survival and management opportunities. J. Wildl. Manag. 2011, 75, 715–720. [Google Scholar] [CrossRef]
- Jonas, J.L.; Joern, A. Grasshopper (Orthoptera: Acrididae) communities respond to fire, bison grazing and weather in North American tallgrass prairie: A long-term study. Oecologia 2007, 153, 699–711. [Google Scholar] [CrossRef]
- Weiss, N.; Zucchi, H.; Hochkirch, A. The effects of grassland management and aspect on Orthoptera diversity and abundance: Site conditions are as important as management. Biodivers. Conserv. 2013, 22, 2167–2178. [Google Scholar] [CrossRef]
- Chisté, M.N.; Mody, K.; Gossner, M.M.; Simons, N.K.; Köhler, G.; Weisser, W.W.; Blüthgen, N. Losers, winners, and opportunists: How grassland land-use intensity affects orthopteran communities. Ecosphere 2016, 7, e01545. [Google Scholar] [CrossRef]
- Messmer, T.A. Influence of Grazing Treatments on Nongame Birds and Vegetation Structure in South Central North Dakota. Ph.D. Thesis, North Dakota State University, Fargo, ND, USA, 1990. [Google Scholar]
- Johnson, R.G.; Temple, S.A. Nest predation and brood parasitism of tallgrass prairie birds. J. Wildl. Manag. 1990, 54, 106–111. [Google Scholar] [CrossRef]
- Risser, P.G.; Birney, E.C.; Blocker, H.D.; May, S.W.; Parton, W.J.; Wiens, J.A. The True Prairie Ecosystem; Hutchinson Ross Publishing Company: Stroudsburg, PA, USA, 1981. [Google Scholar]
- Temple, S.A.; Fevold, B.M.; Paine, L.K.; Undersander, D.J.; Sample, D.W. Nesting birds and grazing cattle: Accommodating both on midwestern pastures. Stud. Avian Biol. 1999, 19, 196–202. [Google Scholar]
- Renfrew, R.B.; Ribic, C.A. Grassland birds associated with agricultural riparian practices in southwestern Wisconsin. J. Range Manag. 2001, 54, 546–552. [Google Scholar] [CrossRef]
- Kantrud, H.A. Grazing intensity effects on the breeding avifauna of North Dakota native grasslands. Can. Field-Nat. 1981, 95, 404–417. [Google Scholar]
- Byers, C.M.; Ribic, C.A.; Sample, D.W.; Dadisman, J.D.; Guttery, M.R. Grassland bird productivity in warm season grass fields in southwest Wisconsin. Am. Midl. Nat. 2017, 178, 47–63. [Google Scholar] [CrossRef]
- Diemer, K.M.; Nocera, J.J. Associations of Bobolink territory size with habitat quality. Ann. Zool. Fenn. 2014, 5, 515–525. [Google Scholar] [CrossRef]
- Wittenberger, J.F. Vegetation structure, food supply, and polygyny in Bobolinks (Dolichonyx oryzivorus). Ecology 1980, 61, 140–150. [Google Scholar] [CrossRef]
- Hovick, T.J.; Elmore, R.D.; Fuhlendorf, S.D.; Engle, D.M.; Hamilton, R.G. Spatial heterogeneity increases diversity and stability in grassland bird communities. Ecol. Appl. 2015, 25, 662–672. [Google Scholar] [CrossRef]
- Bolger, D.T.; Patten, M.A.; Bostock, D.C. Avian reproductive failure in response to an extreme climatic event. Oecologia 2005, 142, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Barnett, K.L.; Facey, S.L. Grasslands, invertebrates, and precipitation: A review of the effects of climate change. Front. Plant. Sci. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- George, T.L.; Fowler, A.C.; Knight, R.L.; McEwen, L.C. Impacts of a severe drought on grassland birds in western North Dakota. Ecol. Appl. 1992, 2, 275–284. [Google Scholar] [CrossRef]
- Lipsey, M.K.; Naugle, D.E. Precipitation and soil productivity explain effects of grazing on grassland songbirds. Rangel. Ecol. Manag. 2017, 70, 331–340. [Google Scholar] [CrossRef]
- Boyce, A.J.; Shamon, H.; Kunkel, K.E.; McShea, W.J. Grassland bird diversity and abundance in the presence of native and non-native grazers. Avian Conserv. Ecol. 2021, 16, 13. [Google Scholar] [CrossRef]
- Bollinger, E.K. Conservation of grassland birds in agricultural areas. In Challenges in the Conservation of Biological Resources; Decker, D.J., Krasny, M.E., Goff, G.R., Smith, C.R., Gross, D.W., Eds.; Westview Press: Boulder, CO, USA, 1991; pp. 279–287. [Google Scholar]
- Johnson, D.H. Effects of fire on bird populations in mixed-grass prairie. In Ecology and Conservation of Great Plains Vertebrates; Knopf, F.L., Samson, F.B., Eds.; Mountain Prairie Information Node: Bozeman, MT, USA, 1997; Chapter 8; pp. 181–206. [Google Scholar]
- Caven, A.J.; Brinley Buckley, E.M.; King, K.C.; Wiese, J.D.; Baasch, D.M.; Wright, G.D.; Harner, M.J.; Pearse, A.T.; Rabbe, M.; Varner, D.M.; et al. Temporospatial shifts in Sandhill Crane staging in the Central Platte River Valley in response to climatic variation and habitat change. Monogr. W. N. Am. Nat. 2019, 11, 33–76. [Google Scholar] [CrossRef]
- Gorzo, J.M.; Pidgeon, A.M.; Thogmartin, W.E.; Allstadt, A.J.; Radeloff, V.C.; Heglund, P.J.; Vavrus, S.J. Using the North American Breeding Bird Survey to assess broad-scale response of the continent’s most imperiled avian community, grassland birds, to weather variability. Condor 2016, 118, 502–512. [Google Scholar] [CrossRef]
| Variable | Type | Definition |
|---|---|---|
| Months since grazing | Continuous | Months since the site was last grazed |
| Months since haying | Continuous | Months since the site was last hayed |
| Months since burning | Continuous | Months since the site was last burned |
| Grazing intensity | Continuous | Grazing effort (AUM/ha) of the site at the time the sample was taken |
| Grassland type | Categorical | Remnant: grasslands that were never tilled for agriculture; restored: grasslands replanted after being previously used for agriculture |
| Tree coverage | Continuous | Percent tree cover within a 200 m radius of a site |
| Variable | Type | Definition |
|---|---|---|
| July maximum temperature | Continuous | Average daily maximum temperature (°C) in July |
| January minimum temperature | Continuous | Average daily minimum temperature (°C) in January |
| May precipitation | Continuous | Total precipitation (mm) in May |
| January precipitation | Continuous | Total precipitation (mm) in January |
| August PDSI | Categorical | Breeding season (May–July) drought intensity |
| Previous August PDSI | Categorical | Previous breeding season (May–July) drought intensity |
| August–April PDSI | Categorical | Nonbreeding season (August–April) drought intensity |
| Previous breeding season temperature | Continuous | Average daily temperature (°C) in the previous breeding season |
| Model Type | Formula | Family | ΔAIC |
|---|---|---|---|
| Bobolink adult abundance | Adult abundance ~ Period + Grazing intensity + Months since grazing + Months since grazing2 + Months since haying + Months since burning + Months since burning2 + Percent trees + January minimum temperature + January precipitation + July maximum temperature + May precipitation + August PDSI + August-April PDSI + (1|site) | Negative binomial corrected for overdispersion | 0.0 |
| Adult abundance ~ Period + Grazing intensity + Grazing intensity2 + Months since grazing + Months since grazing2 + Months since haying + Months since haying2 + Months since burning + Months since burning2 + Percent trees + January minimum temperature + January precipitation + July maximum temperature + July maximum temperature2 + May precipitation + August PDSI + August PDSI2 + August-April PDSI + (1|site) | Negative binomial corrected for overdispersion | 3.0 | |
| Adult abundance ~ Period + Grazing intensity + Months since grazing + Months since haying + Months since burning+ Percent trees + January minimum temperature + January precipitation + July maximum temperature + May precipitation +August PDSI + August-April PDSI + (1|site) | Negative binomial corrected for overdispersion | 31.6 | |
| Adult abundance ~ Period + Grazing intensity + Months since grazing + Months since haying + Months since burning+ Percent trees + January minimum temperature + January precipitation + July maximum temperature + May precipitation +August PDSI + August-April PDSI + (1|site) | Quasipoisson | 4367.9 | |
| Bobolink productivity | Bobolink productivity ~ Period + landuse + Percent trees + Previous breeding season temperature + January minimum temperature + January precipitation + July maximum temperature + Months since haying x August PDSI + Months since burning x Months since grazing + August PDSI x Grazing intensity + August PDSI x Months since grazing + (1|site) | Binomial | 0 |
| Bobolink productivity ~ Period + landuse + Grazing intensity + Months since grazing + Months since haying + Months since burning + Percent trees + Previous breeding season temperature + January minimum temperature + January precipitation + July maximum temperature + August–April PDSI + August PDSI + (1|site) | Binomial | 11.3 | |
| Bobolink productivity ~ Period + landuse + Grazing intensity + Grazing intensity2 + Months since grazing + Months since grazing2 + Months since haying + Months since haying2 + Months since burning + Months since burning2 + Percent trees + Percent trees2 + January minimum temperature + January precipitation + July maximum temperature + July maximum temperature2 + August–April PDSI + August PDSI + August PDSI2 + (1|site) | Binomial | 21.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaplan, R.H.; Rosamond, K.M.; Goded, S.; Soultan, A.; Glass, A.; Kim, D.H.; Arcilla, N. Bobolink (Dolichonyx oryzivorus) Declines Follow Bison (Bison bison) Reintroduction on Private Conservation Grasslands. Animals 2021, 11, 2661. https://doi.org/10.3390/ani11092661
Kaplan RH, Rosamond KM, Goded S, Soultan A, Glass A, Kim DH, Arcilla N. Bobolink (Dolichonyx oryzivorus) Declines Follow Bison (Bison bison) Reintroduction on Private Conservation Grasslands. Animals. 2021; 11(9):2661. https://doi.org/10.3390/ani11092661
Chicago/Turabian StyleKaplan, Rachel H., Kristen M. Rosamond, Sandra Goded, Alaaeldin Soultan, Alex Glass, Daniel H. Kim, and Nico Arcilla. 2021. "Bobolink (Dolichonyx oryzivorus) Declines Follow Bison (Bison bison) Reintroduction on Private Conservation Grasslands" Animals 11, no. 9: 2661. https://doi.org/10.3390/ani11092661
APA StyleKaplan, R. H., Rosamond, K. M., Goded, S., Soultan, A., Glass, A., Kim, D. H., & Arcilla, N. (2021). Bobolink (Dolichonyx oryzivorus) Declines Follow Bison (Bison bison) Reintroduction on Private Conservation Grasslands. Animals, 11(9), 2661. https://doi.org/10.3390/ani11092661






