The Influence of Fusarium Mycotoxins on the Liver of Gilts and Their Suckling Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
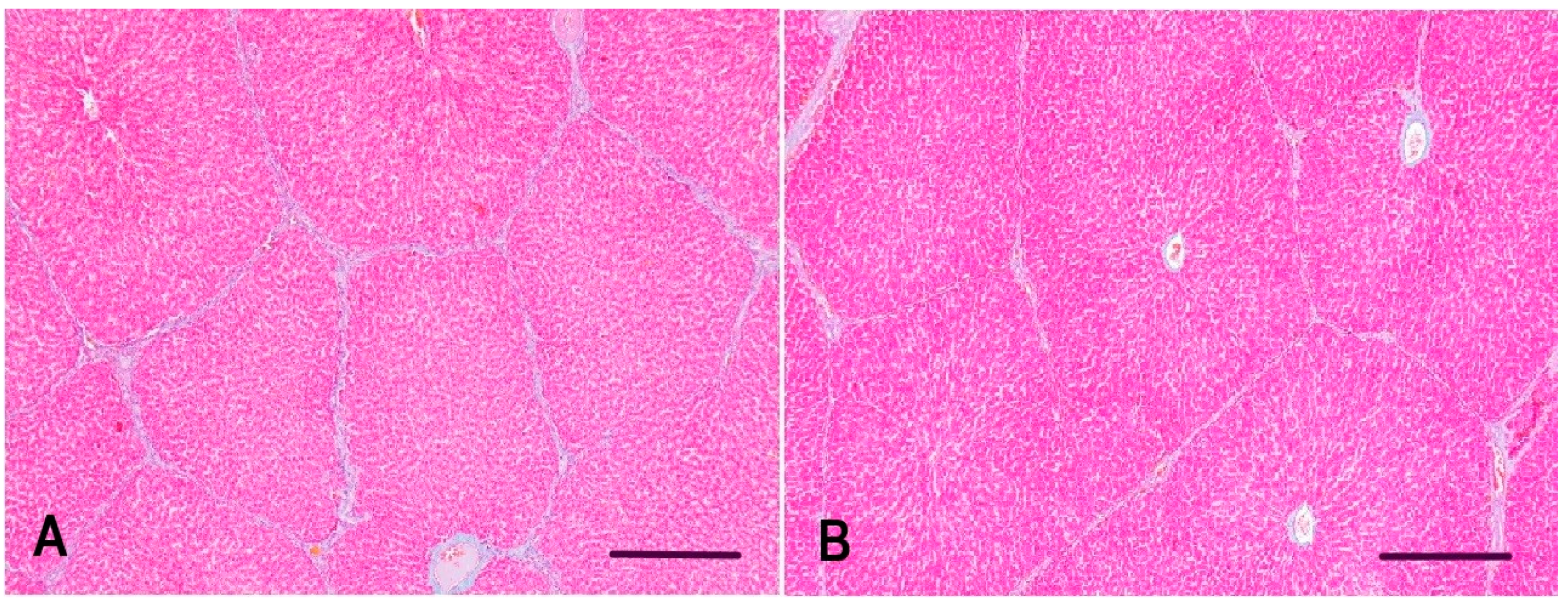
2.2. Histopathology of the Liver of Gilts and Their Suckling Piglets
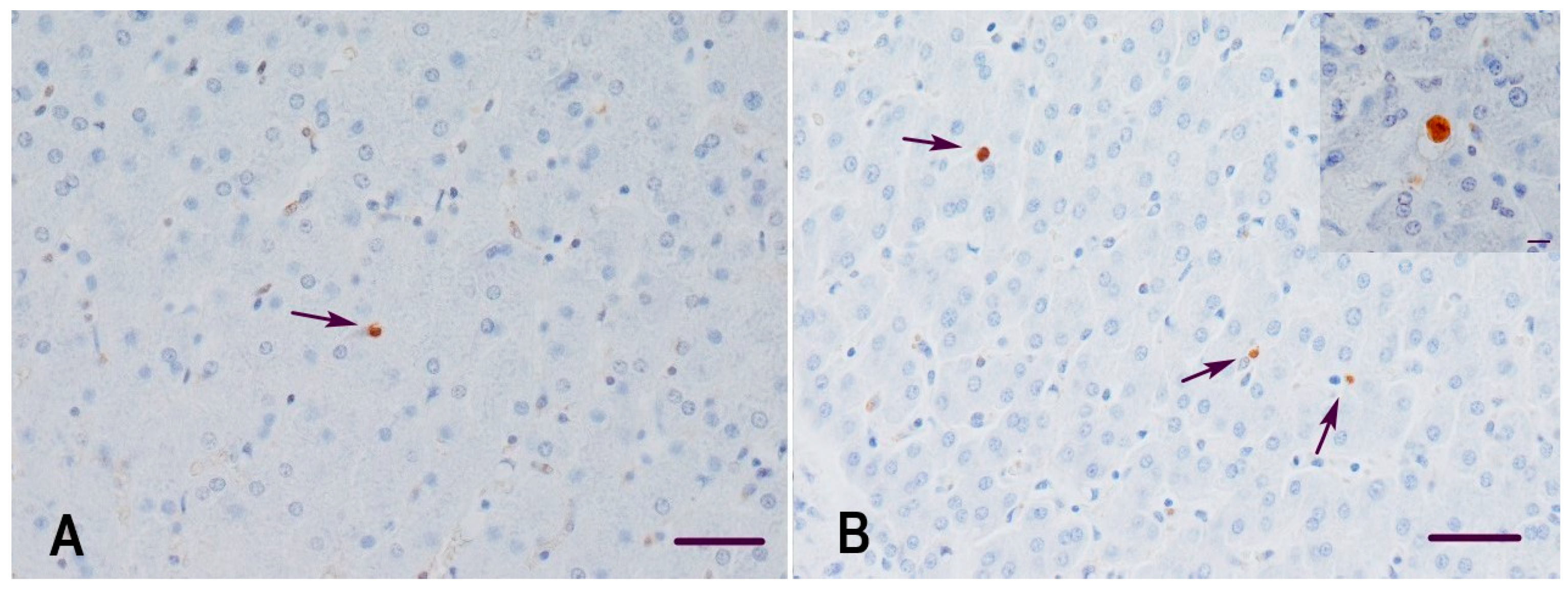
2.3. Detection of Apoptotic Cells in the Liver of Gilts and Their Suckling Piglets
2.4. Determining the Proliferation Index in the Liver of Gilts and Their Suckling Piglets
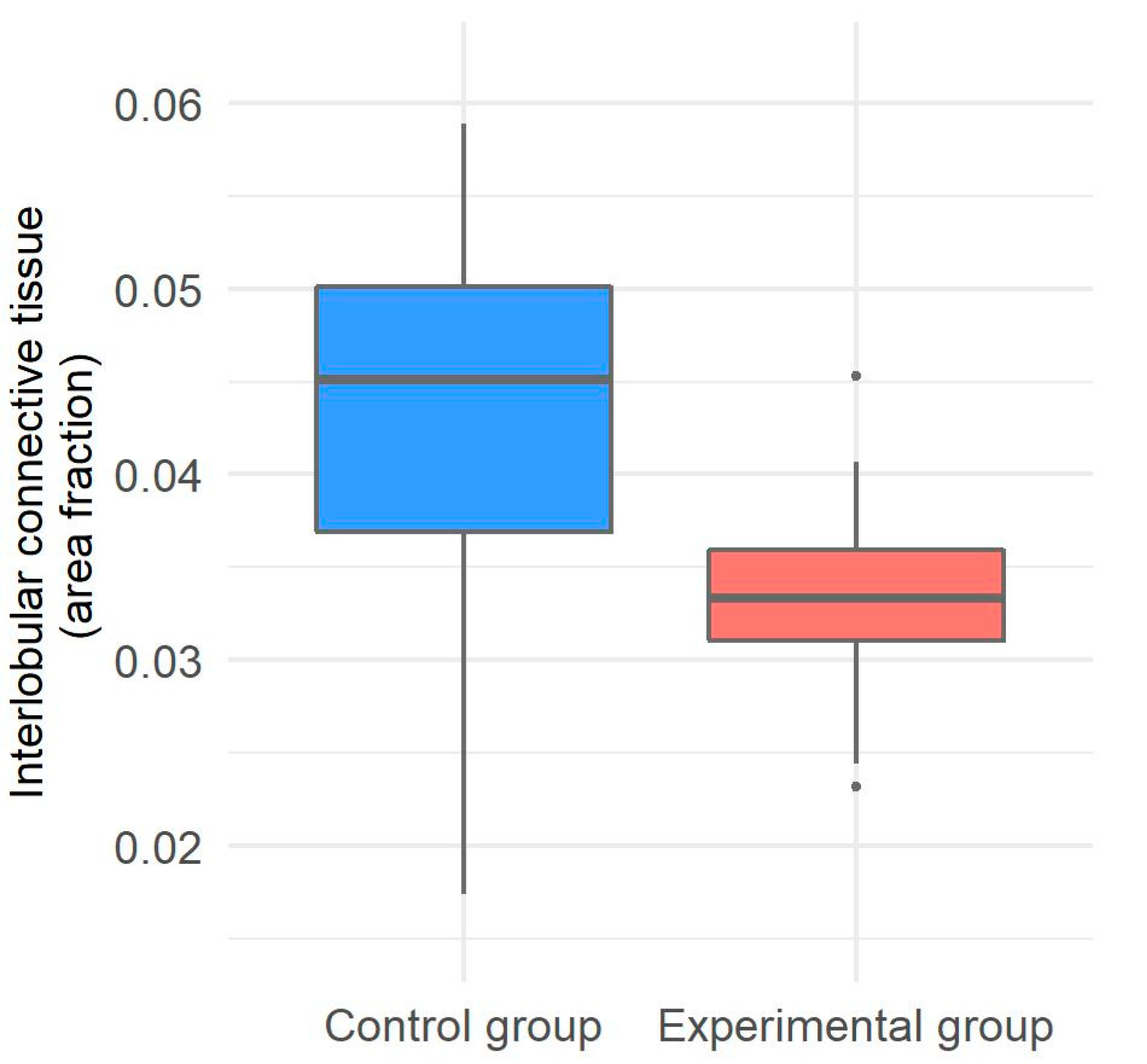
2.5. Morphometrical Evaluation of Interlobular Connective Tissue in the Liver of Gilts
2.6. Statistical Analysis
3. Results
3.1. Histopathology of the Liver of Gilts and Their Suckling Piglets
3.2. Apoptosis and Proliferation Index in the Liver of Gilts and Their Suckling Piglets
3.3. Interlobular Connective Tissue in the Liver of Gilts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Y.; Turner, P.C.; El-Nezami, H. Individual and combined cytotoxic effects of fusarium toxins (deoxynivalenol, nivalenol, zearalenone and fumonisins B1) on swine jejunal epithelial cells. Food Chem. Toxicol. 2013, 57, 276–283. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, J.P.F.; Placinta, C.M.; Macdonald, A.M.C. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 1999, 80, 183–205. [Google Scholar] [CrossRef]
- Bertero, A.; Moretti, A.; Spicer, L.J.; Caloni, F. Fusarium molds and mycotoxins: Potential species-specific effects. Toxins 2018, 10, 244. [Google Scholar] [CrossRef]
- D’Mello, J.P.F. Contaminants and toxins in animal feeds. In FAO Animal Production and Health Paper, Assessing Quality and Safety of Animal Feeds; Daya Publishing House: Rome, Italy, 2004; Volume 160, pp. 107–128. [Google Scholar]
- D’Mello, J.P.F.; Porter, J.K.; Macdonald, A.M.C.; Placinta, C.M. Fusarium mycotoxins. In Handbook of Plant and Fungal Toxicants; D’Mello, J.P.F., Ed.; CRC Press: Boca Raton, FL, USA, 1997; pp. 287–301. [Google Scholar]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging mycotoxins: Beyond traditionally determined food contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Advances in mycotoxin research: Public health perspectives. J. Food Sci. 2015, 80, T2970–T2983. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Placinta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Khoshal, A.K.; Novak, B.; Martin, P.G.P.; Jenkins, T.; Neves, M.; Schatzmayr, G.; Oswald, I.P.; Pinton, P. Co-occurrence of DON and emerging mycotoxins in worldwide finished pig feed and their combined toxicity in intestinal cells. Toxins 2019, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Loureiro-Bracarense, A.P.; Lucioli, J.; Pacheco, G.D.; Cossalter, A.M.; Moll, W.D.; Schatzmayr, G.; Oswald, I.P. Individual and combined effects of subclinical doses of deoxynivalenol and fumonisins in piglets. Mol. Nutr. Food Res. 2011, 55, 761–771. [Google Scholar] [CrossRef]
- Commission of the European Communities. Commission recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- European Commission. Commission Recommendation (EU) 2016/1319 of 29 July 2016 amending recommendation 2006/576/EC as regards deoxynivalenol, zearalenone and ochratoxin A in pet food. Off. J. Eur. Union 2016, L 208, 58–60. [Google Scholar]
- Escrivá, L.; Font, G.; Manyes, L. In Vivo toxicity studies of fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef]
- Schelstraete, W.; Devreese, M.; Croubels, S. Comparative toxicokinetics of fusarium mycotoxins in pigs and humans. Food Chem. Toxicol. 2020, 137, 111140. [Google Scholar] [CrossRef] [PubMed]
- Coffey, M.T.; Hagler, W.M.; Jones, E.E.; Cullen, J.M. Interactive effects of multiple mycotoxin contamination of swine diets. Biodeterior. Res. 1990, 3, 117–128. [Google Scholar] [CrossRef]
- Dänicke, S.; Brüssow, K.P.; Goyarts, T.; Valenta, H.; Ueberschär, K.H.; Tiemann, U. On the transfer of the fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) from the sow to the full-term piglet during the last third of gestation. Food Chem. Toxicol. 2007, 45, 1565–1574. [Google Scholar] [CrossRef]
- Goyarts, T.; Dänicke, S.; Brüssow, K.P.; Valenta, H.; Ueberschär, K.H.; Tiemann, U. On the transfer of the fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) from sows to their fetuses during days 35–70 of gestation. Toxicol. Lett. 2007, 171, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Jakovac-Strajn, B.; Vengušt, A.; Pestevšek, U. Effects of a deoxynivalenol-contaminated diet on the reproductive performance and immunoglobulin concentrations in pigs. Vet. Rec. 2009, 165, 713–718. [Google Scholar] [PubMed]
- Malovrh, T.; Jakovac-Strajn, B. Feed contaminated with fusarium toxins alter lymphocyte proliferation and apoptosis in primiparous sows during the perinatal period. Food Chem. Toxicol. 2010, 48, 2907–2912. [Google Scholar] [CrossRef]
- Friend, D.W.; Thompson, B.K.; Trenholm, H.L.; Boermans, H.J.; Hartin, K.E.; Panich, P.L. Toxicity of T-2 toxin and its interaction with deoxynivalenol when fed to young pigs. Can. J. Anim. Sci. 1992, 72, 703–711. [Google Scholar] [CrossRef]
- Harvey, R.B.; Edrington, T.S.; Kubena, L.F.; Elissalde, M.H.; Casper, H.H.; Rottinghaus, G.E.; Turk, J.R. Effects of dietary fumonisin B1-containing culture material, deoxynivalenol-contaminated wheat, or their combination on growing barrows. Am. J. Vet. Res. 1996, 57, 1790–1794. [Google Scholar]
- Tiemann, U.; Brüssow, K.P.; Jonas, L.; Pöhland, R.; Schneider, F.; Dänicke, S. Effects of diets with cereal grains contaminated by graded levels of two fusarium toxins on selected immunological and histological measurements in the spleen of gilts. J. Anim. Sci. 2006, 84, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Brüssow, K.P.; Küchenmeister, U.; Jonas, L.; Kohlschein, P.; Pöhland, R.; Dänicke, S. Influence of diets with cereal grains contaminated by graded levels of two fusarium toxins on selected enzymatic and histological parameters of liver in gilts. Food Chem. Toxicol. 2006, 44, 1228–1235. [Google Scholar] [CrossRef]
- Chen, F.; Ma, Y.; Xue, C.; Ma, J.; Xie, Q.; Wang, G.; Bi, Y.; Cao, Y. The combination of deoxynivalenol and zearalenone at permitted feed concentrations causes serious physiological effects in young pigs. J. Vet. Sci. 2008, 9, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Brüssow, K.P.; Küchenmeister, U.; Jonas, L.; Pöhland, R.; Reischauer, A.; Jäger, K.; Dänicke, S. Changes in the spleen and liver of pregnant sows and full-term piglets after feeding diets naturally contaminated with deoxynivalenol and zearalenone. Vet. J. 2008, 176, 188–196. [Google Scholar] [CrossRef]
- Tiemann, U.; Brüssow, K.P.; Dannenberger, D.; Jonas, L.; Pöhland, R.; Jäger, K.; Dänicke, S.; Hagemann, E. The effect of feeding a diet naturally contaminated with deoxynivalenol (DON) and zearalenone (ZON) on the spleen and liver of sow and fetus from day 35 to 70 of gestation. Toxicol. Lett. 2008, 179, 113–117. [Google Scholar] [CrossRef]
- Goyarts, T.; Brüssow, K.P.; Valenta, H.; Tiemann, U.; Jäger, K.; Dänicke, S. On the effects of the fusarium toxin deoxynivalenol (DON) administered per Os or intraperitoneal infusion to sows during days 63 to 70 of gestation. Mycotoxin Res. 2010, 26, 119–131. [Google Scholar] [CrossRef]
- Chaytor, A.C.; See, M.T.; Hansen, J.A.; de Souza, A.L.; Middleton, T.F.; Kim, S.W. Effects of chronic exposure of diets with reduced concentrations of aflatoxin and deoxynivalenol on growth and immune status of pigs. J. Anim. Sci. 2011, 89, 124–135. [Google Scholar] [CrossRef]
- Gerez, J.R.; Pinton, P.; Callu, P.; Grosjean, F.; Oswald, I.P.; Bracarense, A.P. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. Pathol. 2015, 67, 89–98. [Google Scholar] [CrossRef]
- Skiepko, N.; Przybylska-Gornowicz, B.; Gajęcka, M.; Gajęcki, M.; Lewczuk, B. Effects of deoxynivalenol and zearalenone on the histology and ultrastructure of pig liver. Toxins 2020, 12, 463. [Google Scholar] [CrossRef]
- Ujčič-Vrhovnik, I.; Švara, T.; Malovrh, T.; Jakovac-Strajn, B. The effects of feed naturally contaminated with fusarium mycotoxins on the thymus in suckling piglets. Acta Vet. Hung. 2020, 68, 186–192. [Google Scholar] [CrossRef]
- Alm, H.; Brüssow, K.P.; Torner, H.; Vanselow, J.; Tomek, W.; Dänicke, S.; Tiemann, U. Influence of fusarium-toxin contaminated feed on initial quality and meiotic competence of gilt oocytes. Reprod. Toxicol. 2006, 22, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Bracarense, A.P.; Lucioli, J.; Grenier, B.; Drociunas Pacheco, G.; Moll, W.D.; Schatzmayr, G.; Oswald, I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012, 107, 1776–1786. [Google Scholar] [CrossRef]
- Reddy, K.E.; Jeong, J.Y.; Lee, Y.; Lee, H.J.; Kim, M.S.; Kim, D.W.; Jung, H.J.; Choe, C.; Oh, Y.K.; Lee, S.D. Deoxynivalenol- and zearalenone-contaminated feeds alter gene expression profiles in the livers of piglets. Asian-Australas J. Anim. Sci. 2018, 31, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Friend, D.W.; Thompson, B.K.; Trenholm, H.L.; Hartin, K.E.; Prelusky, D.B. Effects of feeding deoxynivalenol (DON)-contaminated wheat diets to pregnant and lactating gilts and on their progeny. Can. J. Anim. Sci. 1986, 66, 229–236. [Google Scholar] [CrossRef]
- Wippermann, W.; Heckmann, A.; Jäger, K.; Dänicke, S.; Schoon, H.A. Exposure of pregnant sows to deoxynivalenol during 35–70 days of gestation does not affect pathomorphological and immunohistochemical properties of fetal organs. Mycotoxin Res. 2018, 34, 99–106. [Google Scholar] [CrossRef]
- Mikami, O.; Yamaguchi, H.; Murata, H.; Nakajima, Y.; Miyazaki, S. Induction of apoptotic lesions in liver and lymphoid tissues and modulation of cytokine mRNA expression by acute exposure to deoxynivalenol in piglets. J. Vet. Sci. 2010, 11, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Stanek, C.; Reinhardt, N.; Diesing, A.K.; Nossol, C.; Kahlert, S.; Panther, P.; Kluess, J.; Rothkötter, H.J.; Kuester, D.; Brosig, B.; et al. A chronic oral exposure of pigs with deoxynivalenol partially prevents the acute effects of lipopolysaccharides on hepatic histopathology and blood clinical chemistry. Toxicol. Lett. 2012, 215, 193–200. [Google Scholar] [CrossRef]
- Renner, L.; Kahlert, S.; Tesch, T.; Bannert, E.; Frahm, J.; Barta-Böszörményi, A.; Kluess, J.; Kersten, S.; Schönfeld, P.; Rothkötter, H.J.; et al. Chronic DON exposure and acute LPS challenge: Effects on porcine liver morphology and function. Mycotoxin Res. 2017, 33, 207–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 26 February 2020).
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The impact of fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Taszkun, I.; Tomaszewska, E.; Dobrowolski, P.; Żmuda, A.; Sitkowski, W.; Muszyński, S. Evaluation of collagen and elastin content in skin of multiparous minks receiving feed contaminated with deoxynivalenol (DON, vomitoxin) with or without bentonite supplementation. Animals 2019, 9, 1081. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Sagara, A.; Oshima, I.; Ono, Y.; Iwamoto, H.; Okano, K.; Miyachi, H.; Tabata, S. Immunohistochemical and scanning electron microscopic comparison of the collagen network constructions between pig, goat and chicken livers. Anim. Sci. J. 2009, 80, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Mikami, O.; Yamamoto, S.; Yamanaka, N.; Nakajima, Y. Porcine hepatocyte apoptosis and reduction of albumin secretion induced by deoxynivalenol. Toxicology 2004, 204, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H. Deoxynivalenol and nivalenol toxicities in cultured cells: A review of comparative studies. Food Saf. 2018, 6, 51–57. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Histopathological Change | Group | N | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|---|---|
| Fibrosis | control | 10 | 0 | 0 | 0 | 0 | 1 |
| experimental | 10 | 0 | 0 | 0 | 0 | 0 | |
| Sinusoidal leukocytosis with inflammatory infiltrates of hepatic lobules * | control | 10 | 0 | 0 | 0 | 0 | 0 |
| experimental | 10 | 0 | 1 | 1 | 1 | 1 | |
| Portal tract inflammatory infiltrates | control | 10 | 0 | 1 | 1 | 1 | 2 |
| experimental | 10 | 0 | 1 | 1 | 1 | 2 | |
| Hepatocytes with vacuolaror or granular cytoplasm | control | 10 | 0 | 0 | 0 | 0 | 0 |
| experimental | 10 | 0 | 0 | 0 | 0 | 4 | |
| Hepatocellular necrosis and apoptosis * | control | 10 | 0 | 0 | 0 | 0.75 | 1 |
| experimental | 10 | 0 | 1 | 1 | 1 | 1 | |
| Hepatocellular megalocytosis | control | 10 | 0 | 0 | 0 | 0 | 1 |
| experimental | 10 | 0 | 0 | 0 | 0 | 1 | |
| Hepatocellular megakaryosis | control | 10 | 0 | 0 | 0 | 0 | 1 |
| experimental | 10 | 0 | 0 | 0 | 0 | 1 |
| Histopathological Change | Group | N | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|---|---|
| Hepatocytes with vacuolar or granular cytoplasm | control | 9 | 0 | 4 | 8 | 8 | 12 |
| experimental | 9 | 0 | 0 | 4 | 4 | 8 | |
| Hepatocellular megakaryosis | control | 9 | 0 | 0 | 0 | 0 | 1 |
| experimental | 9 | 0 | 0 | 0 | 0 | 1 | |
| Hepatocellular megakaryosis | control | 9 | 0 | 0 | 0 | 1 | 1 |
| experimental | 9 | 0 | 0 | 0 | 1 | 1 |
| Group | N | Min | Q1 | Median | Q3 | Max | |
|---|---|---|---|---|---|---|---|
| Apoptotic hepatocytes * | control | 10 | 0 | 0 | 0 | 1 | 2 |
| experimental | 9 | 0 | 1 | 1 | 2 | 5 | |
| Apoptotic cells in hepatic sinusoids * | control | 10 | 0 | 2.5 | 5.5 | 7.5 | 13 |
| experimental | 9 | 11 | 13 | 16 | 19 | 35 | |
| Proliferation index of hepatocytes | control | 10 | 0 | 0 | 0 | 0.08 | 0.2 |
| experimental | 10 | 0 | 0 | 0 | 0 | 0.2 |
| Group | N | Min | Q1 | Median | Q3 | Max | |
|---|---|---|---|---|---|---|---|
| Apoptotic hepatocytes | control | 9 | 0 | 0 | 1 | 1 | 2 |
| experimental | 9 | 0 | 0 | 0 | 0 | 1 | |
| Apoptotic cells in hepatic sinusoids | control | 9 | 1 | 5 | 5 | 8 | 22 |
| experimental | 9 | 1 | 4 | 6 | 6 | 20 | |
| Proliferation index of hepatocytes | control | 9 | 0.2 | 0.3 | 0.3 | 0.6 | 1.2 |
| experimental | 9 | 0 | 0.1 | 0.1 | 0.3 | 5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolenšek, T.; Švara, T.; Knific, T.; Gombač, M.; Luzar, B.; Jakovac-Strajn, B. The Influence of Fusarium Mycotoxins on the Liver of Gilts and Their Suckling Piglets. Animals 2021, 11, 2534. https://doi.org/10.3390/ani11092534
Dolenšek T, Švara T, Knific T, Gombač M, Luzar B, Jakovac-Strajn B. The Influence of Fusarium Mycotoxins on the Liver of Gilts and Their Suckling Piglets. Animals. 2021; 11(9):2534. https://doi.org/10.3390/ani11092534
Chicago/Turabian StyleDolenšek, Tamara, Tanja Švara, Tanja Knific, Mitja Gombač, Boštjan Luzar, and Breda Jakovac-Strajn. 2021. "The Influence of Fusarium Mycotoxins on the Liver of Gilts and Their Suckling Piglets" Animals 11, no. 9: 2534. https://doi.org/10.3390/ani11092534
APA StyleDolenšek, T., Švara, T., Knific, T., Gombač, M., Luzar, B., & Jakovac-Strajn, B. (2021). The Influence of Fusarium Mycotoxins on the Liver of Gilts and Their Suckling Piglets. Animals, 11(9), 2534. https://doi.org/10.3390/ani11092534






