Simple Summary
Gastric carcinoma (GC) continues to be one of the leading causes of death in humans and is the most common neoplasm in the stomachs of dogs. In both species, previous studies have demonstrated that the disease is heterogeneous, with genetic and environmental factors playing a quintessential role in disease pathogenesis. Compared to humans, the incidence of gastric carcinoma in dogs is low although, in a small number of breeds, a higher incidence has been reported. In dogs, the etiology and molecular pathways involved remain largely unknown. This retrospective study reviews current signalment data, evaluates the inflammatory component and association with Helicobacter spp. presence in various canine gastric carcinoma histological subtypes, and investigates potential molecular pathways involved in one of the largest study cohorts to date. The benefit of such a comparative study is to highlight the parallel histological features and molecular pathways between dogs and humans.
Abstract
Canine gastric carcinoma (CGC) affects both sexes in relatively equal proportions, with a mean age of nine years, and the highest frequency in Staffordshire bull terriers. The most common histological subtype in 149 CGC cases was the undifferentiated carcinoma. CGCs were associated with increased chronic inflammation parameters and a greater chronic inflammatory score when Helicobacter spp. were present. Understanding the molecular pathways of gastric carcinoma is challenging. All markers showed variable expression for each subtype. Expression of the cell cycle regulator 14-3-3σ was positive in undifferentiated, tubular and papillary carcinomas. This demonstrates that 14-3-3σ could serve as an immunohistochemical marker in routine diagnosis and that mucinous, papillary and signet-ring cell (SRC) carcinomas follow a 14-3-3σ independent pathway. p16, another cell cycle regulator, showed increased expression in mucinous and SRC carcinomas. Expression of the adhesion molecules E-cadherin and CD44 appear context-dependent, with switching within tumor emboli potentially playing an important role in tumor cell survival, during invasion and metastasis. Within neoplastic emboli, acinar structures lacked expression of all markers, suggesting an independent molecular pathway that requires further investigation. These findings demonstrate similarities and differences between dogs and humans, albeit further clinicopathological data and molecular analysis are required.
1. Introduction
Gastric carcinoma is a rare form of cancer in domestic animals, and in dogs accounts for <1% of all reported neoplasms [1], with adenocarcinoma the most frequent (50–90%) [1]. The median age of gastric carcinoma development is 10 years, with rough collies, Staffordshire bull terriers, chow-chows, Belgian shepherds, Norwegian Lundehunds, Cairn terriers and West Highland white terriers being the breeds most likely to be affected [2].
Compared to gastric carcinoma in humans, the incidence of canine gastric carcinoma (CGC) is relatively low; however, in recent years the disease has been more frequently diagnosed [3,4]. Considering the proposed causal effect of diet on gastric neoplasia in humans [5], this increased frequency in dogs over the last 30 years could be similarly attributed. Breed predisposition has also contributed [6], with those breeds at increased risk becoming more popular. In addition, increased longevity and advances in veterinary diagnostic techniques, such as gastroscopy, may have also contributed to increased diagnosis.
Clinical signs of CGC include vomiting that may progress to hematemesis, melaena, anemia, lethargy, ptyalism, polydipsia, abdominal distension, and abdominal discomfort. Prognosis is generally poor, with a median survival time of 35 days, and confirmed metastasis in about 70–90% of cases at the time of diagnosis or death [7]. Common sites of metastasis include the gastric lymph nodes, omentum, liver, duodenum, pancreas, spleen, esophagus, adrenal glands and lungs [2].
Classification of gastric carcinomas in dogs follows the World Health Organization (WHO) [8] scheme, adapted from humans, which is based on the predominant histological features and the main patterns of cells within the neoplasm: papillary, tubular, mucinous, signet-ring cell (SRC) and undifferentiated types [2]. An alternative scheme, again adapted from human medicine, the Lauren classification, divides tumors into intestinal—cohesive masses and tubular structures; diffuse—individual or scattered nests of neoplastic cells; and mixed—incorporating features of both intestinal and diffuse types [9]. Both schemes have been applied in previous studies [1,3].
The association between chronic inflammation, caused by a variety of factors (bacterial, viral, and parasitic infections, chemical irritants, and nondigestible particles), and carcinogenesis is now well established in humans and animals [10]. The risk of carcinogenesis is higher, the longer the inflammation persists [11]. In humans, it has been shown that several risk factors, such as Helicobacter pylori infection, diet, and smoking, are involved in the precancerous cascade of events that lead to gastric adenocarcinomas [12].
The pathogenesis of CGC remains elusive, albeit a high prevalence in certain breeds (e.g., Staffordshire bull terrier, Norwegian Lundehund and Belgian shepherd dog) suggests an underlying genetic etiology [6]. A clear role for Helicobacter spp., similar to H. pylori in humans, has not been reported in domestic species to date [13]. Whether other Helicobacter spp. are involved with carcinogenesis is unclear. H. pylori has occasionally been recognized in the canine stomach [2,12], however, the predominant species in dogs are H. felis, H. bizzozeronii and H. heilmannii [14,15]. A clear association between gastric inflammation and Helicobacter spp. presence has not been made in previous studies, and in addition, an association with gastric carcinoma has also not been investigated [16].
The role of cell cycle regulators and cell adhesion molecules in cancer is complex and paradoxical, varying by cell type and stage of tumorigenesis [17]. In this study, we aimed to examine the involvement of important cell cycle regulators and cell adhesion molecules, previously studied in human gastric carcinoma, in dogs.
E-cadherin is a calcium-dependent cell–cell adhesion molecule that preserves epithelial integrity and can act both as a tumor-suppressor and as an oncoprotein [18,19]. A recent large-scale study separating subtypes according to their growth pattern (polypoid or non-polypoid, i.e., signet cell, mucinous and undifferentiated carcinoma) showed that there is a complete loss of E-cadherin in non-polypoid and undifferentiated carcinomas, and reduced expression in polypoid, with no evidence of malignant alteration or invasion in canine gastrointestinal tumors [20].
CD44 is a cell surface receptor for hyaluronic acid and binds to collagen, fibronectin and chondroitin sulfate [21]. Its role in tumorigenesis and metastasis is thought to be through signaling pathways that regulate cell adhesion, migration, proliferation, differentiation and survival [22,23]. Histopathological studies of human gastric carcinoma have associated high CD44 expression with tumor invasion, lymph node metastasis and patient survival [24,25,26,27], although the expression of CD44 in CGC has so far not been investigated.
p16 protein inhibits cyclinD-CDK4/6, and previous studies of human gastric carcinoma have shown that loss of p16 expression has been associated with increased measures of malignancy and poor clinical outcome [28]. One previous study showed loss of expression of p16 in seventeen cases of CGC [29].
14-3-3σ is the focus of much research in human medicine, including gastric carcinomas [30,31]. 14-3-3σ protein regulates the G1/S and G2/M cell cycle checkpoints through sequestration of CDK4, CDK2, and CDK1, and thus prevents mitosis and allows DNA repair. It may act as a tumor suppressor [32] or it may serve as an oncoprotein. Previously, in veterinary species, 14-3-3σ has been reported as an oncoprotein in canine mammary and urinary bladder carcinomas [33,34]. The association and implication of 14-3-3σ with CGC will be examined later in this study.
The aims of this study were to provide an update on the signalment data and histopathological classification of a large case series of CGC, and to further investigate the potential association of chronic inflammation and the presence of Helicobacter spp. with cancer. Furthermore, using a subset of cases, the expression patterns of four proteins (E-cadherin, p16, 14-3-3σ and CD44) were studied to determine their potential involvement in CGC development.
2. Materials and Methods
2.1. Case Selection
The surgical biopsy databases at VPG Histology, United Kingdom, and the SIDAVE-University of Lleida, Spain were searched for cases of CGC from 2009 to 2019. All cases included relevant medical records (signalment, clinical history, gross description, microscopic description and original diagnosis), and tissues were received at the Royal Veterinary College (RVC) as formalin-fixed paraffin-embedded wax blocks. Individually identifiable owner information was redacted by both supplying institutions. Cases with insufficient tissue or where the diagnosis was not certain on review were excluded.
A control group of canine gastric biopsy samples was established from cases provided by VPG and from the RVC pathology archive. Control group samples were defined based on sample quality, absence of gastric carcinoma, and no previous history of gastric carcinoma. Controls were animals presenting with typical gastrointestinal clinical signs including vomiting, diarrhea and weight loss; however, no tumor was present on histopathological examination.
2.2. Histopathological Evaluation
Histological sections, cut at 4 μm and stained with hematoxylin and eosin, were produced from the provided paraffin blocks and were evaluated microscopically by three veterinary pathologists (A.H., A.S.-B. and S.L.P.). Each case was confirmed as gastric carcinoma and further classified according to the WHO standard into five categories: tubular, papillary, mucinous, SRC, or undifferentiated carcinoma [35,36]. The most frequent histological pattern served as the main classification criterion for each case.
Mucosal inflammation present within histological sections was assessed using modified World Small Animal Veterinary Association standards [8,37,38]. For the purposes of this study, only those parameters consistent with chronic inflammation (intraepithelial lymphocytes, lamina propria lymphocytes and plasma cells, and gastric lympho-follicular hyperplasia) were scored as normal—0, mild—1, moderate—2, and marked—3, following modified WSAVA criteria [37]. A total chronic inflammation score (TCIS, ranging from 0 to 9) was recorded as the sum of the three parameters. Regions of surface ulceration were avoided when assessing chronic inflammatory parameters.
Sections were stained with Warthin–Starry for Helicobacter spp. identification. Quantification of Helicobacter spp. was performed based on the presence or absence of bacteria in the mucosa using the following system: 0—no helicobacter found in the sample; 1—low numbers of helicobacter found (<15 bacteria per field of highest helicobacter density); and 2—high numbers of helicobacter found (>15 bacteria per field).
2.3. Immunohistochemistry
Immunohistochemical labeling for E-cadherin, CD44, p16 and 14-3-3σ was performed on 4-μm-thick sections mounted on positively charged slides (SuperFrost Plus; Menzel Gläser, Braunschweig, Germany). Antigen retrieval, labeling, and counterstaining were performed on a Bond-Max Autostainer (Leica Biosystems, Newcastle-upon-Tyne, UK) using the Bond Polymer Refine detection system (Leica Biosystems). Primary antibodies and retrieval conditions were as follows: p16 (PA0016, 1:100,Novocastra, Newcastle Upon Tyne, UK); pH 6.0 buffer (ER1, Leica Biosystems) for 20 min, 14-3-3σ (SC-100638, 1:40, Santa Cruz Biotechnology, Heidelberg, Germany); pH 6.0 buffer (ER1) for 20 min, E-cadherin (NCH-38, 1:100, Agilent, Stockport, UK); pH 6.0 buffer (ER1) for 20 min, and CD44 (ab157107, 1:50 Abcam, Cambridge, UK); pH 9.0 buffer (ER2, Leica Biosystems) for 20 min. Internal positive tissue controls for E-cadherin and CD44 were available on each section.
2.4. Evaluation of E-Cadherin, CD44, p16 and 14-3-3σ Immunolabeling
Analysis of immunolabeled samples was performed by 3 veterinary pathologists (A.H., A.S.-B. and S.L.P.), discrepancies were discussed by use of a multi-headed microscope, and a consensus was reached. In all cases, areas of ulceration and/or necrosis were avoided for interpretation.
The distribution and intensity of E-cadherin and CD44 labeling were analyzed for each case using a similar semiquantitative scoring system. The percentages of tumor cells, neoplastic acini and intravascular tumor emboli that expressed CD44 and E-cadherin were assessed. Labeling for CD44 was scored as follows: 0, no labeling; 1, 1–9% positive tumor cells; 2, 10–49% cells; and 3, 50–99% cells. Labeling for E-cadherin was scored as follows: 0, no labeling; 1, 1–9% positive tumor cells; 2, 10–49% cells; 3, 50–79% cells; and 4, 80–100% cells. The intensity of E-cadherin and CD44 labeling (0—negative, 1—mild, 2—moderate, and 3—strong) and whether labeling was membranous and/or cytoplasmic was also recorded [39,40,41]. A total immunohistochemical score (TIS) [39] for E-cadherin (ranging from 0 to 12) and TIS CD44 (ranging from 0 to 9) was calculated as the product of the distribution and intensity scores. Normal gastric surface epithelium served as a positive internal control for E-cadherin and lymphoid follicles, satellite glial cells and macrophages for CD44.
For p16, neoplastic cells with cytoplasmic and/or nuclear antigen expression were interpreted as immunopositive. An immunohistochemical score was assigned based on the intensity of staining (0—none, 1—weak, 2—moderate, and 3—strong) and percentage of positive tumor cells (0, 0% cells; 1, <25% cells; 2, 25–50% cells; 3, 51–75% cells; 4, >75% cells) [42]. The product of the intensity and distribution scores gave a TIS p16 ranging from 0 to 12. As a positive control, normal skin was used where epithelial cells within the basal layer of the epidermis exhibited weak immunoreactivity.
14-3-3σ labeling was assessed using a previously published semiquantitative scoring system [43]. The scores for the percentage (1, ≤10% cells; 2, 11–50% cells; and 3, >50% cells) and staining intensity (0, negative; 1, weak; 2, moderate; and 3, intense) of positive cells were recorded and a TIS 14-3-3σ (ranging from 0 to 9) was calculated as the product of these two parameters for each of the studied cases. Normal canine urinary bladder, which showed cytoplasmic immunolabeling of the urothelium, was used as a positive control.
2.5. Statistical Analysis
Results were analyzed for any significant relationship between the following: signalment (i.e., age, sex and breed); biopsy type (endoscopic vs. full-thickness biopsies); and histopathological features (tumor classification, chronic inflammation score, helicobacter identification, quantification, and localization). Statistical comparisons were performed using GraphPad Prism version 8.0 for Windows (GraphPad Software, San Diego, CA, USA). Bar graphs were constructed to depict the mean and standard error of the parameter assessed for each group. To test for the significance of a relationship between two categorical variables, a Fisher’s test was used to analyze the significance of contingency tables such as endoscopic vs. full-thickness biopsies for finding helicobacter. An unpaired t-test was used to compare the mean inflammation scores of different groups. For continuous data, the Student’s t-test, analysis of variance (ANOVA), or Mann–Whitney U analysis test was used, depending on whether the data were normally distributed and whether two, or more than two groups, were compared. The significance level for all statistical tests was set at p < 0.05. The expression of p16, 14-3-3σ, E-cadherin and CD44 were compared with histological subtype and features of malignancy.
3. Results
3.1. Case Details and Signalment
One hundred and eighty-two (182) cases of CGC were identified from the archives of VPG Histology and the University of Lleida and submitted to the Department of Pathobiology and Population Sciences at the Royal Veterinary College. Following the initial histopathological review, 149 were suitable for inclusion in the study based on sample quality, presence of gastric carcinoma, and amount of tumor present in the sample. Cases included 85 (57.0%) endoscopically obtained (mucosa only) samples, and 64 (44.0%) full-thickness biopsies collected by surgical excision. Of the 149 dogs, 69 (46.3%) were female, 75 (50.3%) male and 5 (3.4%) of unreported sex. Neuter status was known in 118 cases, with 88 (74.6%) neutered. Twenty-nine non-tumor control cases were included, 24 (82.8%) endoscopic and 5 (3.4%) full-thickness biopsies. The control group comprised 15 males (51.7%), 13 females (44.8%), and one of unreported sex (3.4%). For the carcinoma group the mean and median ages were 9.1 and 9 years, respectively (range 1–16 years), and for the control group were 7.5 and 7, respectively.
The Staffordshire bull terrier (22/149, 14.8%) was the breed most frequently affected by gastric carcinoma. Of the other non-cross breeds, Labrador retrievers (12, 8.1%), golden retrievers (10, 6.7%), Boxers (9, 6.0%), Border collies (8, 5.4%), rough collies (6, 4.0%), and Belgian shepherd dogs (6, 4.0%) were also commonly affected (Table S1). A similar range of breeds was seen in the control population, with Staffordshire bull terriers, Labrador retrievers, Border collies and Belgian shepherd dogs all represented. No statistical correlation with breed, sex and age was found with regards to each CGC subtype.
3.2. Histopathological Subtypes
Undifferentiated carcinoma was the most frequent carcinoma type overall, comprising 59/149 (39.6%) samples (Table 1). SRC carcinoma made up 47 (31.5%), tubular 32 (21.5%), and mucinous 10 (6.7%). The papillary type was rare, with only a single case (0.7%) (Figure 1) There were no significant associations identified between breed or sex and any of the five classifications of gastric carcinoma.

Table 1.
Canine gastric carcinoma subtype by sex.
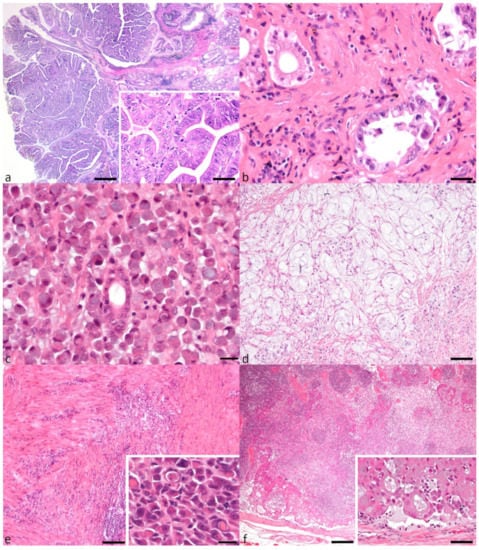
Figure 1.
Gastric carcinoma subtypes and local lymph node metastasis. (a) Papillary adenocarcinoma with characteristic fibrovascular stalks supporting neoplastic cells that form fingerlike projections (bar = 500 μm). Inset shows increased mitoses (bar = 20 μm). (b) Tubular adenocarcinoma with neoplastic tubules lined by pleomorphic cells (bar = 20 μm). (c) Signet-ring cell carcinoma with characteristic signet-ring cells diffusely replacing gastric mucosa (bar = 20 μm). (d) Mucinous adenocarcinoma with abundant lakes of mucin and occasional small numbers of signet-ring cells (bar = 50 μm). (e) Undifferentiated adenocarcinoma with neoplastic cells replacing and dissecting through muscularis layers (bar = 200 μm). Inset showing entotic cell-in-cell (CIC) patterns in an undifferentiated adenocarcinoma (bar = 20 μm). (f) Lymph node metastasis of a tubular adenocarcinoma with multifocal intravascular neoplastic emboli (bar = 200 μm). Inset shows a high-power magnification of neoplastic tubule formation within the neoplastic emboli (bar = 20 μm).
Of the 85 endoscopic samples, 36 (42.4%) were SRC carcinoma, 25 (29.4%) were undifferentiated, 19 (22.3%) tubular, and 5 (5.8%) mucinous. Of the 64 full-thickness samples, 34 (53.1%) were undifferentiated carcinoma, 13 (20.3%) tubular, 11 (17.2%) SRC, and 5 (7.7%) mucinous. The single papillary adenocarcinoma was recorded amongst the full-thickness samples (1.5%) (Figure 2). The prevalence of SRC among endoscopic biopsies was significantly higher than among full-thickness biopsies (p = 0.0002). Comparisons between other subtypes in endoscopic and full-thickness biopsies did not show any statistical significance.
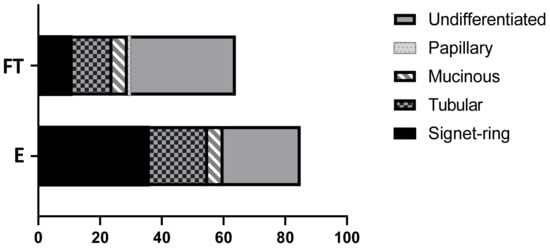
Figure 2.
WHO classification of 149 canine gastric carcinomas separated by biopsy technique. Full-thickness surgical (FT), endoscopic (E).
3.3. Inflammation and Presence of Helicobacter spp.
Mean total chronic inflammation score (TCIS) for the control group was 0.5/9 and for the carcinoma group was 2.8/9. Mean TCIS for the carcinoma group was significantly higher than that of the control group (p = 0.0001).
Of all subtypes, tubular adenocarcinoma had the highest TCIS, 3.4, followed by mucinous—3.1, SRC—2.6, and undifferentiated carcinoma—2.5. Papillary adenocarcinoma had the lowest TCIS of 2.0. All subtypes (except for papillary) had a consistently significantly greater TCIS when compared with the control group (p = 0.0001) (Figure 3).
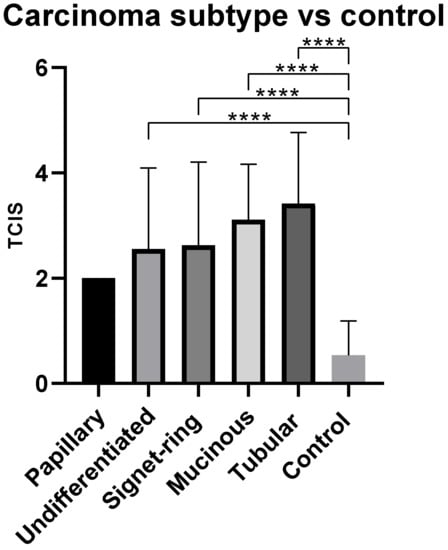
Figure 3.
Mean total chronic inflammation score (TCIS) by carcinoma histological subtype. **** p < 0.0001.
Fifty out of 149 (33.5%) gastric carcinoma samples had observable Helicobacter spp., of which 19 (38%) had large numbers present. The TCIS of Helicobacter spp. positive samples was 3.2, versus 2.6 for Helicobacter spp. negative samples. These two means were found to be significantly different (p = 0.039) (Figure 4).
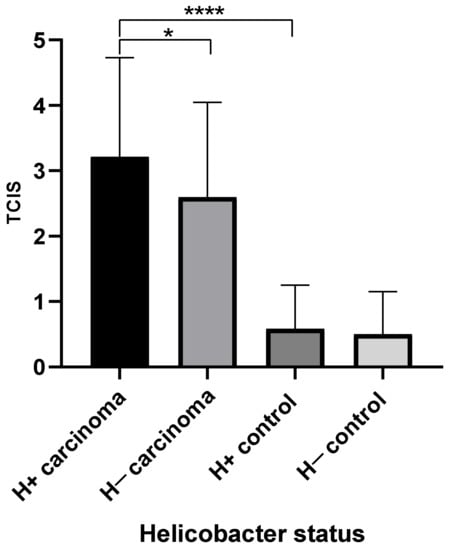
Figure 4.
Mean total chronic inflammation score (TCIS) by helicobacter status for carcinoma and control groups. H+, helicobacter positive samples, H−, helicobacter negative samples.
Thirteen out of 29 (44.8%) of the control samples had observable Helicobacter spp., but only 2 (15.4%) had large numbers present. The TCIS of Helicobacter spp. positive samples was 0.58, and helicobacter negative samples was 0.50. These two means were not significantly different.
3.4. Immunohistochemical Assessment of E-Cadherin, CD44, 14-3-3σ and p16
Twenty-two cases of gastric carcinoma, randomly selected from each histopathological subtype, were available for immunohistochemical assessment as follows: SRC (8, 36.4%), undifferentiated (7, 31.8%), tubular (5, 22.7%), papillary (1, 4.5%), and mucinous (1, 4.5%).
3.5. E-Cadherin Expression
In normal gastric surface and glandular epithelium, E-cadherin labeling was strong (3/3 intensity score) and membranous (Figure 5a). Seventeen (of 22) tumors demonstrated positive immunolabeling for E-cadherin, with both membranous and cytoplasmic (17/22, 77.3%) and nuclear (2/22, 9.1%) labeling, and with an intensity greatest in intravascular emboli, where present. Five tumors did not show immunopositivity for E-cadherin (2 undifferentiated, 1 SRC, 1 mucinous and 1 tubular carcinoma). Where dysplastic epithelium was present, E-cadherin expression decreased in intensity and distribution (compared with normal), and labeling became progressively cytoplasmic (from membranous) (Figure 5b). The TIS E-cadherin for each tumor subtype was as follows: papillary—6.0; undifferentiated—4.6; SRC—4.3; tubular—3.2; and mucinous—0 (Table 2). Aberrantly increased expression and TIS of E-cadherin in intralymphatic/intravascular emboli, compared with both normal epithelium and immunopositive neoplastic cells, was observed in seven tumors (7/17; 3/7 undifferentiated, 1/8 signet-ring cell and 3/5 tubular carcinoma). E-cadherin was not expressed by neoplastic emboli composed of well-differentiated acinar structures (Figure 5c).
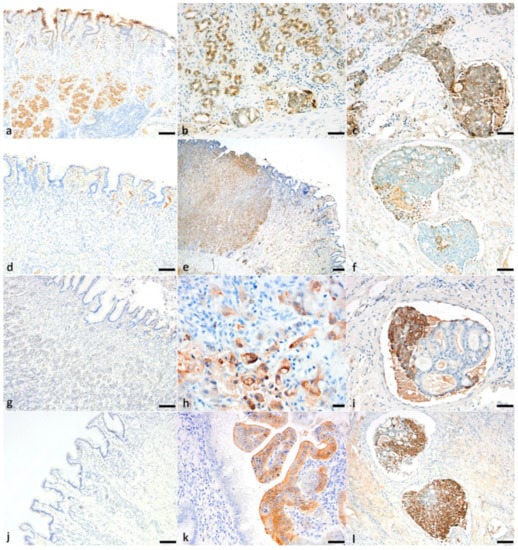
Figure 5.
Canine gastric carcinoma immunohistochemistry for E-cadherin, CD44, 14-3-3σ, p16. (a) Expression of E-cadherin in normal gastric surface and glandular epithelium (bar = 100 μm). (b) Reduced E-cadherin expression (progressively cytoplasmic from membranous) in neoplastic tubules in a tubular adenocarcinoma (bar = 50 μm). (c) Poorly differentiated neoplastic cells in emboli showing cytoplasmic and membranous labeling for E-cadherin (bar = 50 μm). (d) Expression of CD44 in lymphocytes, macrophages and dendritic cells of normal gastric mucosa. (bar = 100 μm) (e) Strong membranous labeling of CD44 in sheets and chains of poorly differentiated neoplastic cells in an undifferentiated gastric carcinoma (bar = 200 μm). (f) Loss of membranous and cytoplasmic labeling of CD44 in tubules and strong membranous and cytoplasmic labeling in poorly differentiated cells of a neoplastic embolus (bar = 100 μm). (g) Normal epithelium showing no expression of 14-3-3σ (bar = 100 μm). (h) Strong cytoplasmic with occasional nuclear labeling of 14-3-3σ in nests of poorly differentiated neoplastic cells in an undifferentiated gastric carcinoma (bar = 20 μm). (i) Loss of cytoplasmic labeling of 14-3-3σ in tubules and cytoplasmic labeling in poorly differentiated cells of a neoplastic embolus (bar = 50 μm). (j) Normal epithelium showing no expression of p16 (bar = 100 μm). (k) Strong cytoplasmic labeling of p16 in neoplastic epithelium (bar = 50 μm). (l) Loss of cytoplasmic labeling for p16 in tubules and strong nuclear and cytoplasmic labeling in poorly differentiated cells of a neoplastic embolus (bar = 50 μm).

Table 2.
Mean total immunohistochemical score by gastric carcinoma subtype. The same score for neoplastic vascular emboli, where present, is given in parentheses.
3.6. CD44 Expression
In the normal canine stomach, CD44 was expressed on the membrane of lymphocytes, macrophages, dendritic cells, and satellite glial cells (Figure 5d). Gastric epithelium, stromal tissue, smooth muscle fibers and matrix fibroblasts were negative. Positive immunolabeling (neo-expression) occurred in all gastric carcinoma cases. CD44 expression was cytoplasmic and membranous (5/22, 22.7%) or only membranous (9/22, 40.9%), with an intensity greatest in mucinous tumors (mean intensity score 2.5). Enhanced membranous expression was observed in those neoplastic epithelial cells that showed the greatest features of malignancy (pleomorphism and invasion), with a higher mean score in papillary, undifferentiated and tubular carcinomas (6, 4.7 and 4, respectively) (Figure 5e). Intravascular neoplastic emboli, present in 4/7 undifferentiated, 4/5 tubular and 3/7 SRC carcinomas, showed increased TIS compared to immunopositive neoplastic cells. Neoplastic cells in emboli exhibited membranous, or both cytoplasmic and membranous expression, in poorly differentiated neoplastic cells. Interestingly, there were neoplastic emboli that had solid nests and well-differentiated acinar arrangements, and, in these emboli, only the solid nests were strongly positive, while the well-differentiated embolic acini were negative (Figure 5f).
3.7. 14-3-3σ Expression
In a histologically normal canine stomach, 14-3-3σ was not expressed in any cell types, including epithelium, stromal tissue or lymphocytes (Figure 5g). Positive immunolabeling (neo-expression) occurred in 10 of 22 (45.4%) gastric carcinomas, 7/7 undifferentiated, and 3/5 tubular. TIS 14-3-3σ for undifferentiated and tubular carcinomas was 3.4 and 2.4, respectively. SRC, papillary and mucinous carcinomas were all negative for 14-3-3σ. Cytoplasmic and/or nuclear neo-expression of 14-3-3σ was present, with an intensity greatest in undifferentiated tumors (intensity mean 2.1, and total score 3.4). One case of undifferentiated carcinoma showed both nuclear and cytoplasmic labeling in neoplastic cells (Figure 5h). Where neoplastic emboli were present, TIS was higher compared to immunopositive neoplastic cells, and 14-3-3σ labeling intensity was increased in poorly differentiated and pleomorphic cells. However, when neoplastic cells formed intravascular acinar structures, 14-3-3σ was not expressed (Figure 5i).
3.8. p16 Expression
In a histologically normal canine stomach, p16 was not expressed (Figure 5j), but positive cytoplasmic and/or nuclear labeling was noted in regions of dysplastic epithelium (Figure 5k). p16 expression occurred in 19/22 (86.4%) carcinomas; 6/7 undifferentiated, 5/5 tubular, 1/1 papillary, 6/8 SRC, and 1/1 mucinous. Positive immunolabeling of pleomorphic cells and dysplastic tubules was found in all tumors, with high-grade expression in mucinous tumors (signet-ring cells in SRC and mucinous carcinomas were strongly immunopositive for p16). p16 exhibited cytoplasmic (11/22, 50%) and both nuclear/cytoplasmic (8/22, 36.36%) expression, with intensity greatest in mucinous tumors (intensity mean 3 and total score 9).
Where neoplastic emboli were present, TIS was higher compared to immunopositive neoplastic cells. Within intravascular and intralymphatic emboli, p16 intensity and cytoplasmic and/or nuclear expression was increased in poorly differentiated and pleomorphic cells. However, when neoplastic cells formed intravascular acinar structures, p16 was not expressed (Figure 5l). Similar findings were noted in the lymph node metastasis of one sample. The mean TIS p16 for undifferentiated, tubular, papillary, SRC and mucinous carcinomas was 4.3, 6.8, 6, 6.9 and 9, respectively.
4. Discussion
In this canine gastric carcinoma (CGC) study, using the WHO scheme, we have demonstrated that the most frequent subtype is the undifferentiated carcinoma, and the most common breed affected is the Staffordshire bull terrier. There is no sex predisposition, and the mean age is nine years. Helicobacter spp. presence was associated with increased chronic inflammation parameters and a greater chronic inflammatory score. We found that all markers showed variable expression for each subtype. CD44 and 14-3-3σ have not been previously investigated in CGC. 14-3-3σ was positive in undifferentiated, tubular and papillary carcinomas, and p16 expression was increased in mucinous and SRC carcinomas. E-cadherin and CD44 were variably expressed in all subtypes and were associated with criteria of malignancy. Within neoplastic emboli, acinar structures lacked expression of all markers, suggesting an independent molecular pathway that requires further investigation.
Of the 149 dogs included in this study from the UK and Spain, the mean and median age of 9.1 and 9 years, respectively, at the time of CGC diagnosis is in broad agreement with previously reported studies [3,6,7]. In this study, CGC affected male and female dogs roughly equally, with a slight preponderance towards males, as is consistent with previous reports [3]. The most commonly affected breed was the Staffordshire bull terrier, likely reflecting both the general population in the United Kingdom (only 2/22 Staffordshire bull terriers were from Spain) and previous reports of breed predisposition [7]. Breed predisposition to CGC is also reported in less common breeds, including Belgian shepherd dogs and rough collies, which also appeared with an increased frequency in this study [3,6].
Using the WHO classification for domestic animals, cases were divided into five categories based on the predominant histological features and the principal cell type of the tumor. All histological subtypes of carcinoma (SRC, tubular, mucinous, papillary and undifferentiated) were recorded, although squamous cell carcinoma, another CGC subtype, was not present in this study [44]. Undifferentiated carcinoma was the most frequent subtype, followed by SRC, contrary to the previous studies reporting an increased frequency for the tubular subtype, similar to humans [45,46]. This could likely reflect a geographic variation in gastric adenocarcinoma incidence.
Signet-ring cell carcinomas were the most frequently diagnosed type by endoscopy, whereas undifferentiated carcinomas were the most frequently diagnosed in full-thickness samples. The most likely reason behind this difference is that, histologically, the diagnosis of SRC subtype is based upon identification of the characteristic isolated or small groups of malignant cells containing intracytoplasmic mucin with an eccentric nucleus (signet-ring cells) within the mucosa, and hence, diagnosis from an endoscopically retrieved (mucosa only) sample is possible. The diagnosis of other histopathological subtypes typically requires the evaluation of invasion beyond the mucosa, and thus, given the relatively high number of full-thickness biopsies in this study, this may have influenced the predominant subtype. Additionally, signet-ring cells were also present in other subtypes, i.e., mucinous and undifferentiated carcinoma; however, the predominant histological features and pattern did not favor a diagnosis of SRC carcinoma. This suggests that endoscopic samples alone can lead to diagnostic pitfalls between different subtypes [47,48,49,50]. In humans, novel techniques such as endoscopic surgical dissection are recommended in subtypes like SRC that spread subepithelially in the margins [51]. In combination with future novel imaging techniques, these findings should be taken into consideration to determine the appropriate sampling and therapeutic approach [52].
Cancer-related inflammation is one of the hallmarks of neoplasia, and once cancer develops, there is evidence of a substantial shift in the microenvironment affecting the immune response [53,54]. A simplified histopathologic scoring system was adapted to reduce variability in the diagnostic interpretation [37]. This scoring system captured and quantified chronic inflammatory changes in the gastric mucosa [55]. The TCIS for the carcinoma group, and individually for each CGC subtype (independent of helicobacter presence), was significantly greater when compared to the control group’s TCIS. Thus, there is a clear correlation between chronic inflammation and carcinoma for most CGC subtypes.
Given the link between the presence of helicobacter, inflammation and neoplasia in humans we aimed to quantitatively assess helicobacter presence in CGCs. In previous studies Helicobacter spp. have been reported in both neoplastic and non-neoplastic canine stomachs [56,57]. In this study, the TCIS of the carcinoma group with a concomitant presence of helicobacter was significantly greater compared to the helicobacter-negative carcinoma group. Furthermore, larger numbers of helicobacter were found in carcinoma cases than in control cases. Although the helicobacter presence does not prove a definite role in CGC pathogenesis, there is a clear association between increased chronic inflammation and higher numbers of helicobacter in the tumor cases versus controls. Whether the presence of Helicobacter spp. is associated with tumor development is not exactly clear, and thus further studies, perhaps to examine particular species of helicobacter in association with CGC, would be needed.
To understand the molecular pathways and investigate the cellular origin of CGC, the expression of cellular adhesion molecules (E-cadherin and CD44) and cell cycle regulators (p16 and 14-3-3σ) were investigated in a representative proportion of cases. CD44 and 14-3-3σ have not previously been studied in CGC.
E-cadherin controls cell motility and suppresses tumor growth and metastasis [58]. Immunohistochemical examination of E-cadherin in all tumor subtypes identified abnormalities of expression and localization. Dysplastic surface and glandular epithelia in immunopositive cases revealed a reduction in E-cadherin expression. Pleomorphic cells in the undifferentiated subtype showed E-cadherin expression, albeit in decreased intensity compared to the normal epithelium. Subcellular localization of E-cadherin was observed in the majority of neoplastic cells. Intracytoplasmic sequestration and the accumulation of E-cadherin have been previously associated with abnormalities in the intracytoplasmic transport and reuptake mechanisms of the molecule [59,60]. Aberrant nuclear expression of E-cadherin in humans has been previously described in several tumor types including gastric and colorectal carcinomas [19,61]. Similarly, increased intensity intracytoplasmic and nuclear expression was noted in the neoplastic emboli composed of pleomorphic neoplastic cells forming solid nests. Surprisingly, intravascular acinar structures did not express E-cadherin. Furthermore, tubular adenocarcinoma, which is composed of acini and tubules, had the lowest TIS compared to other subtypes. This could represent an inverse association between the reduction or loss of E-cadherin with an increasing degree of differentiation, where well-differentiated structures switch off and pleomorphic cells switch on or increase the expression of E-cadherin. Previous studies in human gastric adenocarcinoma showed that abnormalities of E-cadherin localization (internalization) in neoplastic cells lead to decreased adhesion, thus favoring invasion [59,60]. Neoplastic cells lacked E-cadherin expression in discohesive subtypes like mucinous adenocarcinoma, which is in accordance with a recent study [20].
In the context of these findings, we postulate that the reduction or loss of E-cadherin expression on the cell membrane could potentially facilitate invasion, and that the re-expression of E-cadherin, including on the cell membrane, in neoplastic emboli could lead to the development of solid cohesive intravascular structures, enhancing their survival. These findings reinforce the notion that E-cadherin in CGC is a context-dependent adhesion molecule that can either be up- or down-regulated or re-expressed, depending on the stage of tumor progression [62]. Further studies analyzing ligands of E-cadherin and possible mutations are required to clarify its precise role in CGC.
CD44 serves as a signaling platform, with transmembrane and cytoplasmic domains as a co-receptor for various types of cell surface receptors that modulate cell adhesion, migration, proliferation, differentiation and survival [63,64,65,66]. In humans, its role in the adaptive plasticity and survival of cancer cells in processes like epithelial to mesenchymal transition, invasion and metastasis has been widely studied [67,68]. In CGC, CD44 was variably expressed in all tumor subtypes. Similar to E-cadherin, the expression of CD44 was enhanced in pleomorphic neoplastic cells and was absent in well-differentiated acinar structures within neoplastic emboli. In the context of these findings, we postulate that CD44 expression could potentially facilitate invasion and expression in neoplastic emboli and, alongside E-cadherin, could lead to the development of cohesive intravascular structures, enhancing their survival. In previous studies in humans, CD44 positive cells in gastric cell lines were associated with increased chemoresistance and invasiveness [66]. Additionally, CD44 positive gastric tumors were associated with larger tumor size, a lower grade of differentiation, tumor relapse, lymph node invasion, distant metastasis and reduced survival [63,64,65,66]. It seems that CD44 may represent an important biomarker and a promising therapeutic target in canine gastric carcinomas.
14-3-3σ is a cell cycle regulator that may serve as either a tumor suppressor or an oncogene involved in tissue invasion and metastasis. Histological features of malignancy have been previously associated with the overexpression and/or neo-expression of the protein [69,70]. The role of this protein as a tissue differentiation marker and as an oncoprotein in veterinary medicine has been previously studied in canine mammary, urinary bladder, renal cell and equine penile squamous cell carcinomas [33,34,71,72]. In the current study, immunohistochemical analysis revealed the absence of 14-3-3σ immunolabeling in a normal stomach and in SRC and mucinous carcinomas. The latter finding was consistent for all cases of the two subtypes and likely demonstrates a 14-3-3σ independent molecular pathway in carcinogenesis. In contrast, tubular and undifferentiated subtypes showed strong intracytoplasmic neoexpression, and occasionally nuclear expression of 14-3-3σ. The single papillary carcinoma had weak intracytoplasmic neoexpression. Undifferentiated carcinomas demonstrated strong intracytoplasmic, and occasionally nuclear, expression in the most pleomorphic neoplastic cells. Similarly, in those tumors with neoplastic emboli formed by pleomorphic cells forming solid nests, there was strong intracytoplasmic and occasionally nuclear expression of 14-3-3σ. However, acinar structures within the intravascular emboli lacked expression of 14-3-3σ. Aberrant nuclear expression of 14-3-3σ, in a subset of cases of renal cell carcinomas, was associated with a malignant phenotype and shorter survival rate [43]. Thus, we hypothesize, in view of these findings, that expression of 14-3-3σ is associated with features of malignancy, and that neoexpression of the molecule is essential in the stage of intravascular invasion. Expression of the protein in the extracellular milieu, as previously demonstrated for other canine carcinomas, was not observed in this study [72].
The protein p16 acts as a tumor suppressor and a cell cycle regulator, by slowing progression from the G1 to S phase through the inhibition of cyclinD-CDK4/6. Its role and possible relationship to tumor progression and prognosis have been studied in a variety of human tumors [73,74,75]. In human gastric carcinomas, loss of p16 expression has been associated with malignant characteristics and poor prognosis [28,76,77]. In the CGCs studied here, the expression of p16 seemed to be an event common to all subtypes, and thus appears fundamental for neoplasia development, with expression most strong in mucinous and SRC carcinomas. Half of the tumors showed cytoplasmic, and 36.7% both nuclear and cytoplasmic, immunolabeling. Within neoplastic emboli, expression was cytoplasmic and only in one tubular carcinoma case was both cytoplasmic and nuclear. Similar to the other markers, when acinar structures were present within the emboli, p16 expression was absent. These results contradict a previous canine study, where lack of p16 expression in tubular, SRC and undifferenced subtypes was significantly associated with histological criteria of malignancy in 14 cases [29]. In humans, the overexpression of p16 is associated with mutations in genes encoding retinoblastoma protein (Rb) and p53, and is considered to be a mechanism to arrest the uncontrolled proliferation caused by failure of the Rb pathway [78]. Furthermore, the significance of the p16 expression within the cell is not clear, and few studies have associated cytoplasmic expression with malignant features [79]. Currently, similar to humans, the significance of p16 expression patterns in CGC remains unclear [78].
5. Conclusions
In conclusion, CGC affects female and male dogs in relatively equal proportions, with a mean age of nine years, and the highest frequency in Staffordshire bull terriers. The most common CGC histological subtype was the undifferentiated carcinoma. CGCs were associated with increased chronic inflammation parameters and with a greater chronic inflammatory score when Helicobacter spp. were present. Understanding the altered molecular pathways associated with gastric carcinoma development including its histological subtype remains a challenge. The significance of the findings from the cell cycle regulators and cell adhesion molecules in the subtypes examined, should be combined with clinicopathological data and additional molecular analysis to further understand the molecular mechanisms and similarities between dogs and humans.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ani11051409/s1, Table S1: 149 cases of canine gastric carcinoma by sex and breed frequency.
Author Contributions
Conceptualization, S.L.P., A.S.-B. and A.H.; methodology, S.L.P., A.S.-B. and A.H.; investigation, A.H. and W.E.B.; resources, S.B. and G.A.R.; data curation, A.H. and W.E.B.; writing—original draft preparation, A.H.; writing—review and editing, A.H., S.L.P. and A.S.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study is contained within the manuscript and Supplementary Table S1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Patnaik, A.K.; Hurvitz, A.I.; Johnson, G.F. Canine gastrointestinal neoplasms. Vet. Pathol. 1977, 14, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Löhr, C.V.; Kiupel, M. Tumors of the alimentary tract. Tumors Domest. Anim. 2016, 499–601. [Google Scholar] [CrossRef]
- Fonda, D.; Gualtieri, M.; Scanziani, E. Gastric-carcinoma in the dog—A clinicopathological study of 11 cases. J. Small Anim. Pract. 1989, 30, 353–360. [Google Scholar] [CrossRef]
- Crow, S.E. Tumors of the alimentary tract. Vet Clin. N. Am. Small Anim. Pract. 1985, 15, 577–596. [Google Scholar] [CrossRef]
- De Stefani, E.; Correa, P.; Boffetta, P.; Deneo-Pellegrini, H.; Ronco, A.L.; Mendilaharsu, M. Dietary patterns and risk of gastric cancer: A case-control study in uruguay. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2004, 7, 211–220. [Google Scholar] [CrossRef]
- Seim-Wikse, T.; Jorundsson, E.; Nodtvedt, A.; Grotmol, T.; Bjornvad, C.R.; Kristensen, A.T.; Skancke, E. Breed predisposition to canine gastric carcinoma—A study based on the norwegian canine cancer register. Acta. Vet. Scand. 2013, 55, 25. [Google Scholar] [CrossRef]
- Sullivan, M.; Lee, R.; Fisher, E.W.; Nash, A.S.; McCandlish, I.A. A study of 31 cases of gastric carcinoma in dogs. Vet. Rec. 1987, 120, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Head, K. Tumors of the alimentary system of domestic animals. WHO Collab. Cent. Worldw. Ref. Comp. Oncol. 2003, 54–55. [Google Scholar]
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma: An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Morrison, W.B. Inflammation and cancer: A comparative view. J. Vet. Intern. Med. 2012, 26, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer. Oncology (Williston Park) 2002, 16, 217–226. [Google Scholar] [PubMed]
- Piazuelo, M.B.; Epplein, M.; Correa, P. Gastric cancer: An infectious disease. Infect. Dis. Clin. N. Am. 2010, 24, 853–869. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M. Structure and function of helicobacter pylori caga, the first-identified bacterial protein involved in human cancer. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 196–219. [Google Scholar] [CrossRef] [PubMed]
- Amorim, I.; Smet, A.; Alves, O.; Teixeira, S.; Saraiva, A.L.; Taulescu, M.; Reis, C.; Haesebrouck, F.; Gartner, F. Presence and significance of helicobacter spp. In the gastric mucosa of portuguese dogs. Gut Pathog. 2015, 7, 12. [Google Scholar] [CrossRef]
- Priestnall, S.L.; Wiinberg, B.; Spohr, A.; Neuhaus, B.; Kuffer, M.; Wiedmann, M.; Simpson, K.W. Evaluation of “helicobacter heilmannii” subtypes in the gastric mucosas of cats and dogs. J. Clin. Microbiol. 2004, 42, 2144–2151. [Google Scholar] [CrossRef]
- Rossi, G.; Rossi, M.; Vitali, C.G.; Fortuna, D.; Burroni, D.; Pancotto, L.; Capecchi, S.; Sozzi, S.; Renzoni, G.; Braca, G.; et al. A conventional beagle dog model for acute and chronic infection with helicobacter pylori. Infect. Immun. 1999, 67, 3112–3120. [Google Scholar] [CrossRef]
- Pugacheva, E.N.; Roegiers, F.; Golemis, E.A. Interdependence of cell attachment and cell cycle signaling. Curr. Opin. Cell Biol. 2006, 18, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Berx, G.; van Roy, F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb. Perspect. Biol. 2009, 1, a003129. [Google Scholar] [CrossRef]
- Rodriguez, F.J.; Lewis-Tuffin, L.J.; Anastasiadis, P.Z. E-cadherin’s dark side: Possible role in tumor progression. Biochim. Biophys. Acta 2012, 1826, 23–31. [Google Scholar] [CrossRef]
- Saito, T.; Chambers, J.K.; Nakashima, K.; Nibe, K.; Ohno, K.; Tsujimoto, H.; Uchida, K.; Nakayama, H. Immunohistochemical analysis of beta-catenin, e-cadherin and p53 in canine gastrointestinal epithelial tumors. J. Vet. Med. Sci. 2020, 82, 1277–1286. [Google Scholar] [CrossRef]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. Cd44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Orian-Rousseau, V.; Sleeman, J. Cd44 is a multidomain signaling platform that integrates extracellular matrix cues with growth factor and cytokine signals. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 123, pp. 231–254. [Google Scholar]
- Yan, Y.; Zuo, X.; Wei, D. Concise review: Emerging role of cd44 in cancer stem cells: A promising biomarker and therapeutic target. Stem Cells Transl. Med. 2015, 4, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, C.B.; Oliveira, C.; Wen, X.; Gomes, B.; Sousa, S.; Suriano, G.; Grellier, M.; Huntsman, D.G.; Carneiro, F.; Granja, P.L.; et al. De novo expression of cd44 variants in sporadic and hereditary gastric cancer. Lab. Investig. 2010, 90, 1604–1614. [Google Scholar] [CrossRef]
- Li, H.; Guo, L.; Li, J.W.; Liu, N.; Qi, R.; Liu, J. Expression of hyaluronan receptors cd44 and rhamm in stomach cancers: Relevance with tumor progression. Int. J. Oncol. 2000, 17, 927–932. [Google Scholar] [CrossRef]
- Li, M.; Zhang, B.; Zhang, Z.; Liu, X.; Qi, X.; Zhao, J.; Jiang, Y.; Zhai, H.; Ji, Y.; Luo, D. Stem cell-like circulating tumor cells indicate poor prognosis in gastric cancer. Biomed. Res. Int. 2014, 2014, 981261. [Google Scholar] [CrossRef]
- Fang, M.; Wu, J.; Lai, X.; Ai, H.; Tao, Y.; Zhu, B.; Huang, L. Cd44 and cd44v6 are correlated with gastric cancer progression and poor patient prognosis: Evidence from 42 studies. Cell Physiol. Biochem. 2016, 40, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Myung, N.; Kim, M.R.; Chung, I.P.; Kim, H.; Jang, J.J. Loss of p16 and p27 is associated with progression of human gastric cancer. Cancer. Lett. 2000, 153, 129–136. [Google Scholar] [CrossRef]
- Carrasco, V.; Canfran, S.; Rodriguez-Franco, F.; Benito, A.; Sainz, A.; Rodriguez-Bertos, A. Canine gastric carcinoma: Immunohistochemical expression of cell cycle proteins (p53, p21, and p16) and heat shock proteins (hsp27 and hsp70). Vet. Pathol. 2011, 48, 322–329. [Google Scholar] [CrossRef]
- Muhlmann, G.; Ofner, D.; Zitt, M.; Muller, H.M.; Maier, H.; Moser, P.; Schmid, K.W.; Zitt, M.; Amberger, A. 14-3-3 sigma and p53 expression in gastric cancer and its clinical applications. Dis. Markers 2010, 29, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Liu, L.; Xiao, Y.; Zeng, T.; Zeng, C. 14-3-3σ is an independent prognostic biomarker for gastric cancer and is associated with apoptosis and proliferation in gastric cancer. Oncol. Lett. 2015, 9, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Lodygin, D.; Hermeking, H. Epigenetic silencing of 14-3-3sigma in cancer. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2006; pp. 214–224. [Google Scholar]
- Suarez-Bonnet, A.; Herraez, P.; de las Mulas, J.M.; Rodriguez, F.; Deniz, J.M.; de los Monteros, A.E. Expression of 14-3-3 sigma protein in normal and neoplastic canine mammary gland. Vet. J. 2011, 190, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Bonnet, A.; Herráez, P.; Aguirre, M.; Suárez-Bonnet, E.; Andrada, M.; Rodríguez, F.; de los Monteros, A.E. Expression of cell cycle regulators, 14-3-3σ and p53 proteins, and vimentin in canine transitional cell carcinoma of the urinary bladder. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 332.e1. [Google Scholar]
- Fenoglio-Preiser, C.; Carneiro, F.; Correa, P.; Guilford, P.; Lambert, R.; Megraud, F.; Muñoz, N.; Powell, S.M.; Rugge, M.; Sasako, M.; et al. Tumours of the stomach. In World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System; Hamilton, S., Aaltonen, L., Eds.; IARC Press: Lyon, France, 2000; pp. 37–67. [Google Scholar]
- Head, K.W.; Armed Forces Institute of Pathology (U.S.); American Registry of Pathology; WHO Collaborating Center for Worldwide Reference on Comparative Oncology. Histological Classification of Tumors of the Alimentary System of Domestic Animals; International Histological Classification of Tumors of Domestic Animals Series; Armed Forces Institute of Pathology: Washington, DC, USA, 2003. [Google Scholar]
- Allenspach, K.A.; Mochel, J.P.; Du, Y.; Priestnall, S.L.; Moore, F.; Slayter, M.; Rodrigues, A.; Ackermann, M.; Krockenberger, M.; Mansell, J.; et al. Correlating gastrointestinal histopathologic changes to clinical disease activity in dogs with idiopathic inflammatory bowel disease. Vet. Pathol. 2019, 56, 435–443. [Google Scholar] [CrossRef]
- Day, M.J.; Bilzer, T.; Mansell, J.; Wilcock, B.; Hall, E.J.; Jergens, A.; Minami, T.; Willard, M.; Washabau, R. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the world small animal veterinary association gastrointestinal standardization group. J. Comp. Pathol. 2008, 138, S1–S43. [Google Scholar] [CrossRef]
- Querzoli, P.; Coradini, D.; Pedriali, M.; Boracchi, P.; Ambrogi, F.; Raimondi, E.; La Sorda, R.; Lattanzio, R.; Rinaldi, R.; Lunardi, M. An immunohistochemically positive e-cadherin status is not always predictive for a good prognosis in human breast cancer. Br. J. Cancer 2010, 103, 1835–1839. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, I.W.; Souza, M.J.L.; da Costa, W.H.; Amâncio, A.M.; Fonseca, F.P.; de Cassio Zequi, S.; Lopes, A.; Guimarães, G.C.; Soares, F. Epithelial-mesenchymal transition (emt) phenotype at invasion front of squamous cell carcinoma of the penis influences oncological outcomes. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 433.e19. [Google Scholar]
- Rogez, B.; Pascal, Q.; Bobillier, A.; Machuron, F.; Lagadec, C.; Tierny, D.; Le Bourhis, X.; Chopin, V. Cd44 and cd24 expression and prognostic significance in canine mammary tumors. Vet. Pathol. 2019, 56, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Roma, A.A.; Goldblum, J.R.; Fazio, V.; Yang, B. Expression of 14-3-3σ, p16 and p53 proteins in anal squamous intraepithelial neoplasm and squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2008, 1, 419. [Google Scholar] [PubMed]
- Suárez-Bonnet, A.; Lara-García, A.; Stoll, A.L.; Carvalho, S.; Priestnall, S.L. 14-3-3σ protein expression in canine renal cell carcinomas. Vet. Pathol. 2018, 55, 233–240. [Google Scholar] [CrossRef]
- Patnaik, A.K.; Lieberman, P.H. Gastric squamous cell carcinoma in a dog. Vet. Pathol. 1980, 17, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Washabau, R.; Day, M. Canine and Feline Gastroenterology-e-Book; Elsevier Health Sciences: Philadelphia, PA, USA, 2012. [Google Scholar]
- Fléjou, J.-F. Classification oms 2010 des tumeurs digestives: La quatrième édition. Ann. Pathol. 2011, 31, S27–S31. (In French) [Google Scholar] [CrossRef]
- Jergens, A.E.; Willard, M.D.; Allenspach, K. Maximizing the diagnostic utility of endoscopic biopsy in dogs and cats with gastrointestinal disease. Vet. J. 2016, 214, 50–60. [Google Scholar] [CrossRef]
- Standards of Practice Comittee; Faulx, A.L.; Kothari, S.; Acosta, R.D.; Agrawal, D.; Bruining, D.H.; Chandrasekhara, V.; Eloubeidi, M.A.; Fanelli, R.D.; Gurudu, S.R.; et al. The role of endoscopy in subepithelial lesions of the gi tract. Gastrointest. Endosc. 2017, 85, 1117–1132. [Google Scholar] [CrossRef]
- Papanikolaou, I.S.; Triantafyllou, K.; Kourikou, A.; Rosch, T. Endoscopic ultrasonography for gastric submucosal lesions. World J. Gastrointest. Endosc. 2011, 3, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Oh, S.J. Signet ring cell carcinoma mimicking gastric gastrointestinal stromal tumor: A case report. Case Rep. Oncol. 2020, 13, 538–543. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.-H.; Lee, Y.C.; Kim, H.; Youn, Y.H.; Park, H.; Choi, S.H.; Noh, S.H.; Gotoda, T. Growth patterns of signet ring cell carcinoma of the stomach for endoscopic resection. Gut Liver 2015, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Akiyoshi, H.; Mie, K.; Okamoto, M.; Yoshida, Y.; Kurokawa, S. Contrast-enhanced computed tomography may be helpful for characterizing and staging canine gastric tumors. J. Vet. Radiol. Ultrasound 2019, 60, 7–18. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Macarthur, M.; Hold, G.L.; El-Omar, E.M. Inflammation and cancer ii. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G515–G520. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.E.; Evans, R.B.; Ackermann, M.; Hostetter, J.; Willard, M.; Mansell, J.; Bilzer, T.; Wilcock, B.; Washabau, R.; Hall, E.J.; et al. Design of a simplified histopathologic model for gastrointestinal inflammation in dogs. Vet. Pathol. 2014, 51, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Poutahidis, T.; Doulberis, M.; Karamanavi, E.; Angelopoulou, K.; Koutinas, C.K.; Papazoglou, L.G. Primary gastric choriocarcinoma in a dog. J. Comp. Pathol. 2008, 139, 146–150. [Google Scholar] [CrossRef]
- Taulescu, M.A.; Valentine, B.A.; Amorim, I.; Gartner, F.; Dumitrascu, D.L.; Gal, A.F.; Sevastre, B.; Catoi, C. Histopathological features of canine spontaneous non-neoplastic gastric polyps—A retrospective study of 15 cases. Histol. Histopathol. 2014, 29, 65–75. [Google Scholar]
- Harris, T.J.; Tepass, U. Adherens junctions: From molecules to morphogenesis. Nat. Rev. Mol. Cell Biol 2010, 11, 502–514. [Google Scholar] [CrossRef]
- Handschuh, G.; Candidus, S.; Luber, B.; Reich, U.; Schott, C.; Oswald, S.; Becke, H.; Hutzler, P.; Birchmeier, W.; Hofler, H.; et al. Tumour-associated e-cadherin mutations alter cellular morphology, decrease cellular adhesion and increase cellular motility. Oncogene 1999, 18, 4301–4312. [Google Scholar] [CrossRef]
- Gabbert, H.E.; Mueller, W.; Schneiders, A.; Meier, S.; Moll, R.; Birchmeier, W.; Hommel, G. Prognostic value of e-cadherin expression in 413 gastric carcinomas. Int. J. Cancer. 1996, 69, 184–189. [Google Scholar] [CrossRef]
- Moon, K.C.; Cho, S.Y.; Lee, H.S.; Jeon, Y.K.; Chung, J.H.; Jung, K.C.; Chung, D.H. Distinct expression patterns of e-cadherin and beta-catenin in signet ring cell carcinoma components of primary pulmonary adenocarcinoma. Arch. Pathol. Lab. Med. 2006, 130, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R. Cancer: Context is key for e-cadherin in invasion and metastasis. Curr. Biol. 2019, 29, R1140–R1142. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lu, S.; Wen, Y.G.; Yu, F.D.; Zhu, X.W.; Qiu, G.Q.; Tang, H.M.; Peng, Z.H.; Zhou, C.Z. Overexpression of hoxa10 promotes gastric cancer cells proliferation and hoxa10(+)/cd44(+) is potential prognostic biomarker for gastric cancer. Eur. J. Cell Biol. 2015, 94, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Isozaki, H.; Ohyama, T.; Mabuchi, H. Expression of cell adhesion molecule cd44 and sialyl lewis a in gastric carcinoma and colorectal carcinoma in association with hepatic metastasis. Int. J. Oncol. 1998, 13, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Okumura, T.; Hirano, K.; Yamaguchi, T.; Sekine, S.; Nagata, T.; Tsukada, K. Circulating tumor cells expressing cancer stem cell marker cd44 as a diagnostic biomarker in patients with gastric cancer. Oncol. Lett. 2017, 13, 281–288. [Google Scholar] [CrossRef]
- Yoon, C.; Park, D.J.; Schmidt, B.; Thomas, N.J.; Lee, H.J.; Kim, T.S.; Janjigian, Y.Y.; Cohen, D.J.; Yoon, S.S. Cd44 expression denotes a subpopulation of gastric cancer cells in which hedgehog signaling promotes chemotherapy resistance. Clin. Cancer Res. 2014, 20, 3974–3988. [Google Scholar] [CrossRef]
- Thorne, R.F.; Legg, J.W.; Isacke, C.M. The role of the cd44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J. Cell Sci. 2004, 117, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Orian-Rousseau, V. Cd44 acts as a signaling platform controlling tumor progression and metastasis. Front. Immunol. 2015, 6, 154. [Google Scholar] [CrossRef]
- Ko, S.; Kim, J.Y.; Jeong, J.; Lee, J.E.; Yang, W.I.; Jung, W.H. The role and regulatory mechanism of 14-3-3 sigma in human breast cancer. J. Breast Cancer 2014, 17, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Maruyama, S.; Abé, T.; Kobayashi, T.; Yamazaki, M.; Funayama, A.; Shingaki, S.; Kobayashi, T.; Jun, C.; Saku, T. Keratin 17 is co-expressed with 14-3-3 sigma in oral carcinoma in situ and squamous cell carcinoma and modulates cell proliferation and size but not cell migration. Virchows Arch. 2015, 466, 559–569. [Google Scholar] [CrossRef]
- Suárez-Bonnet, A.; de las Mulas, J.M.; Herráez, P.; Rodríguez, F.; de los Monteros, A.E. Immunohistochemical localisation of 14-3-3 σ protein in normal canine tissues. Vet. J. 2010, 185, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Bonnet, A.; Willis, C.; Pittaway, R.; Smith, K.; Mair, T.; Priestnall, S.L. Molecular carcinogenesis in equine penile cancer: A potential animal model for human penile cancer. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 532.e9. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Merlo, A.; Mao, L.; Lapidus, R.G.; Issa, J.P.J.; Davidson, N.E.; Sidransky, D.; Baylin, S.B. Inactivation of the cdkn2/p16/mts1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995, 55, 4525–4530. [Google Scholar]
- Otterson, G.A.; Kratzke, R.A.; Coxon, A.; Kim, Y.W.; Kaye, F.J. Absence of p16(ink4) protein is restricted to the subset of lung-cancer lines that retains wildtype rb. Oncogene 1994, 9, 3375–3378. [Google Scholar]
- Kelley, M.J.; Nakagawa, K.; Steinberg, S.M.; Mulshine, J.L.; Kamb, A.; Johnson, B.E. Differential inactivation of cdkn2 and rb protein in non—Small-cell and small-cell lung cancer cell lines. J. Natl. Cancer Inst. 1995, 87, 756–761. [Google Scholar] [CrossRef][Green Version]
- Rocco, A.; Schandl, L.; Nardone, G.; Tulassay, Z.; Staibano, S.; Malfertheiner, P.; Ebert, M. Loss of expression of tumor suppressor p16ink4 protein in human primary gastric cancer is related to the grade of differentiation. J. Dig. Dis. 2002, 20, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.F.; Shao, J.C.; Wang, D.B.; Qin, R.; Zhang, H. Expression and significance of cell cycle regulators in gastric carcinoma. Ai Zheng Chin. J. Cancer 2005, 24, 175–179. [Google Scholar]
- Romagosa, C.; Simonetti, S.; Lopez-Vicente, L.; Mazo, A.; Lleonart, M.E.; Castellvi, J.; Ramon y Cajal, S. P16(ink4a) overexpression in cancer: A tumor suppressor gene associated with senescence and high-grade tumors. Oncogene 2011, 30, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Song, L.J.; Xu, W.Q.; Zhao, L.; Zheng, L.; Yan, Z.W.; Fu, G.H. Expression of cytoplasmic p16 and anion exchanger 1 is associated with the invasion and absence of lymph metastasis in gastric carcinoma. Mol. Med. Rep. 2009, 2, 169–174. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).