Simple Summary
Very little is known about the impact of equine-assisted interventions on equids’ perception of humans. Different factors can influence human–horse relationships: animal characteristics, daily interactions with the caretakers, and working and living conditions. In this study, 172 equids working in equine-assisted interventions, ‘classical’ riding school lessons, or both were submitted to a standardised human–horse relationship test in order to test if EAI had an impact on the equid reactions to humans. The possible influence of intrinsic (age, sex, type) or other extrinsic factors (housing and feeding conditions) was also considered. The results showed that the number (more than the type) of experimenter-directed behaviours varied significantly between individuals and that the activity was the most important factor of influence: Equids working in riding school lessons performed more interactive behaviours than those working in equine-assisted interventions or having mixed activity. Other factors such as daily hay quantity, the horses’ age, and sex also influenced secondarily the horse’s motivation to interact, although no interaction was found between factors. These results suggest that equine-assisted interventions do influence horses’ perception of humans outside work. Further studies are needed in order to understand the processes involved.
Abstract
Little is known about the impact of equine-assisted interventions (EAI) on equids’ perception of humans. In this study 172 equids, living in 12 riding centres, were submitted to a standardised human–horse relationship test: the motionless person test. Age, sex, type (horse/pony), housing, and feeding conditions of subjects were recorded. Overall, 17 equids worked in EAI, 95 in riding school lessons (RS), and 60 in both (EAI-RS). There were high inter-individual variations in the number of interactive behaviours directed towards the experimenter: negative binomial general linear models showed that activity was the most important factor: RS equids performed more interactive behaviours than EAI (p = 0.039) and EAI-RS (p < 0.001) equids. Daily quantity of hay appeared as the second most important factor (equids with more than 3 kg interacted more than equids with less than 3 kg, p = 0.013). Individual characteristics were also important as horses interacted more than ponies (p = 0.009), geldings more than mares (p = 0.032), and 3–15-year-old equids more than equids over 15 years (p = 0.032). However, there was no interaction between factors. The lower number of interactive behaviours of EAI equids leads to different hypotheses—namely, selection on temperament, specific training, or compromised welfare (apathy). In any case, our results raised new lines of questions on EAI.
1. Introduction
Animal-assisted interventions (AAIs), are defined by the International Association of Human–Animal Interaction Organisations [1] as ‘goal oriented and structured interventions that intentionally include or incorporate animals in health, education and human services (e.g., social work) for the purpose of therapeutic gains in humans’, and have become increasingly popular [2]. AAIs use various animals [2], amongst which horses are one of the most frequently used species (e.g., for children with autism spectrum disorders) [3]. The effects of these equine-assisted interventions (EAIs) on humans have been widely studied [4,5,6], but studies focusing on the animals involved remain scarce. Two main opinions prevail concerning how equids perceive these activities. One common belief is that horses ‘sense’ the ‘needs’ of persons, adapt to them, and appreciate these activities [7,8] —an anthropocentric view according to McGreevy et al. [9], whereas other authors argue that horses may find it difficult to deal with persons with unusual behaviours or postures [10,11] or even that an AAI animal’s welfare could be affected negatively [12].
Importantly, EAI horses have to also deal with the same constraints in their daily life as other working horses, with potential spatial and social restrictions and/or inappropriate feeding conditions that can compromise their welfare [13,14,15,16,17]. Researchers also suggested that repeated influences of poor working conditions can affect horses’ overall chronic welfare state [18] and even develop learned helplessness, a state where they have lost their ability to react to negatively perceived stimuli [19]. The reasons can be that riding may influence the comfort of horses during riding (e.g., rider’s posture [20,21]; equipment [22,23]), leading to potential dorsal problems [24,25]. Dorsal pain may induce aggressive reactions in human–horse relationship tests [26] and affect the cognitive assessment of their environment [27]. Horses experiencing compromised welfare can also become unresponsive to their environment, including humans [28,29,30]. Improvement of feeding/housing conditions is associated with more positive reactions towards humans [31,32,33]. Moreover, behavioural differences can be observed early in life and show some stability, leading some authors to propose that motivation to interact (i.e., interest in humans) can be considered as an equine temperament trait [34,35,36,37,38]. Thus, differences in reactions towards humans have been found according to the type of equid (ponies or horses), breed, or sire [39,40,41,42,43].
However, the reactions of horses in human–horse relationship tests reflect primarily their perception, positive or negative, of humans ([44] for a review) as horses are able to generalise the relationship they have with their owner, caretaker, trainer, or rider to an unknown human [30,39,45,46]. In addition, human–horse interactions are also influenced at the time of interaction by human characteristics, such as the human’s posture, attentional and emotional state, or experience with horses [47,48,49].
The human–horse relationship (HHR) is a crucial part of AAI, and the equids chosen for these activities are supposed to be calm and gentle, although experimental tests have shown that this is not necessarily the case [50]. During EAI, equids can be confronted with persons who have unpredictable behaviours [51], attention problems, aggression or irritability [10], negative moods [52], or balance problems [10]. As an example, in another species working in assisted intervention, it has been shown that while guinea pigs are first attracted when confronted for the first time with a child with autism spectrum disorders, they rapidly express discomfort (indicated by reduced frequency of feeding and exploration) [53]. However, Merkies et al. [48] observed no particular modification of the behaviour of horses moving freely towards patients with post-traumatic stress disorder or neurotypical humans. Most studies performed during EAI sessions suggest that the activity per se or type of person has no particular impact [48,51,52,54,55].
To our knowledge, however, no study has investigated the impact of EAI on the perception equids have of humans in general, i.e., outside working sessions, that is, as a reflection of the chronic impact these sessions might have (and not as immediate effects). One study showed that type of work can influence the reactions of horses in HHR tests outside work [56] and the quality of their working conditions has been shown to affect the reactions of horses towards unknown experimenters [28,57]. In low-income countries, horses used for draught work were less responsive to human approaches than riding horses [57,58], and breeding horses were more positive towards humans than working horses [59]. Training and working conditions have a major impact on the perception of humans that horses build, and this is the result of a generalisation process [46,60,61].
Therefore, we hypothesised that EAI horses, as a result of the particularities of these activities, would have a different perception of humans from that of riding school lessons horses beyond potential additional intrinsic (horse/pony, age or sex) or extrinsic (conditions of life amongst which housing and feeding conditions) factors. Standardised HHR tests have proved useful to evaluate this perception objectively [44]. Thus, to test this hypothesis, we chose a commonly used test, the motionless person (MP) test, to examine human-directed behaviours that reflect horses’ perception of humans and their motivation to interact (i.e., ‘interest’).
We expected differences in the reaction to an unknown human between equids according to their activity. We predicted differences in the valence of the behaviours displayed and differences in the ‘interest’ of equids for the unknown humans measured with the number of human-directed behaviours. We also expected an impact of conditions of life (i.e., feeding and housing conditions) with possibly more negative behaviours towards humans or fewer reactions in suboptimal conditions than in better conditions. Lastly, we also expected an impact of equids’ individual characteristics such as sex, age, or breed.
2. Materials and Methods
2.1. Ethical Statement
The experiments were carried out between 2009 and 2019 in accordance with the Directive 2010/63/UE of the European Parliament and the Council on the protection of animals used for scientific purposes. They complied with the current French laws related to animal experimentation (decree n°2013 ± 118 of 1 February 2013) and its five implementation orders (JO 7 February 2013, integrated into the Rural Code and the Code of maritime fishing under n° R. 214 ± 87 to n° R. 214–137). The experiments performed in this study were not within the scope of application of the European directive; thus, in accordance with this directive and the current French, Italian, and Irish laws, the following experiments did not require us to request authorisation. These experiments involved only behavioural observations and non-invasive interactions with the horses. The horses used in this research were not research animals. Animal husbandry and care were under the management of the riding school staff. The riding school managers gave the authors their informed consent for this study.
2.2. Subjects
The subjects of this study were 172 equids (93 mares, 79 geldings, aged 4 to 29 years old, mean = 14.3 ± 0.4) that lived and were tested in 12 different riding centres (4 Italian, 1 Irish, and 7 French centres). They were 91 ponies ( ≤ 148cm at withers) and 81 horses ( > 148cm at withers) (International Federation for Equestrian Sport) from various breeds but mostly unregistered (Table 1). At the time of the study, they had been in their facility and had been involved in the same working practices for at least one year. Overall, 17 equids worked only in equine-assisted interventions (EAIs), 95 in ‘classical’ riding school lessons (RS), and 60 in both activities (EAI-RS; Table 1). The proportion of each activity could not be obtained in all mixed centres, but in the centres where the information could be collected, the proportion was between 7% and 86% of EAI activities, mean ± SE = 40.5 ± 5.1%. Each equid included in the study worked closely with at least three different persons. All centres used negative reinforcement for training. EAI activities were mostly grooming, groundwork, lunging, and riding. It was addressed to people with disabilities such as motor disabilities, visual, hearing, or cognitive impairments, crippling diseases, or other health diseases linked to psychosocial risks or social problems (e.g., jail, dropping out of school).

Table 1.
Subjects’ characteristics.
Equids were housed in either individual stalls (78%) (size between 6m ² and 25m ²) or group stalls (22%) (size between 5.3m ² and 11.7m ² per equid); 5% of equids had no bedding in their stall, 71.5% had straw, and 34.5% had wood shavings. Feeding practices varied between facilities. All horses were fed hay: less than 3 kg per day (19%), between 3 kg and 9 kg (37.8%), or more than 9 kg per day (43.2%); commercial pellets: none (25.9%), one (9.2%), two (45.4%), or three (19.5%) meals per day. Water was provided ad libitum in all facilities, mostly through automatic drinkers.
2.3. Experimental Test
This study included experiments conducted between November 2009 and May 2019. Four female experimenters aged 24 to 30 years (CL, CR, EG, and NL), trained by a senior author (MH) (until an inter-observer reliability kappa coefficient of 0.80 was reached for each behavior expressed by equids during the test), performed the tests. In accordance with the results of Merkies et al. [48] and suggestions of Nimer and Lundahl [62], the tests were performed by experimenters with neurotypical development who were experienced with horses but not familiar with our subjects. Each equid was tested once by only one person. Equids from the same riding school were all tested by the same person. The equid had never met the experimenter before the start of the test.
The motionless person (MP) test is a standardised test used in many studies on human–horse relationships, but the exact procedure can vary slightly between studies (review in [44]). The aim of this test is to assess the spontaneous reaction of equids to the mere presence of an unknown experimenter in its familiar home environment. Equids were tested in their own individual or group stall. When it was possible, subjects were tested individually in the stall; however, some equids (N = 31) had to be tested in groups. The experimenter chose a moment when the horse was feeding on the ground, facing towards the door to enter the stall. If the horses were tested in a group, the experimenter chose a time when they were at equal distance from the door. At the beginning of the test, the experimenter entered the stall and stood motionless with her back against the closed door with her arms by her side without interacting with the equid for five minutes.
The equid’s behaviours were recorded by the experimenter during the test using a voice recorder and a lapel microphone attached under the clothes. In addition, the test was filmed using a camera attached to the outside of the stalls (the animals had been accustomed to the camera on the previous days), in order to verify that all behaviours had been recorded.
Tests were conducted in calm conditions, at least 30 min before or after work sessions, between 8 a.m. and 6 p.m., and at least 30 minutes before or after feeding times, because behaviours change in this period [63].
2.4. Data Analyses
The occurrences of all behaviours directed towards the experimenter were recorded using focal continuous sampling [64] during the five minutes the MP test lasted. Recorded behaviours were divided into different categories: behaviours considered as positive towards humans were gazes, approaches, sniffs, licks, and nibbles with ears forwards, while behaviours considered negative towards humans were gazes, approaches, sniffs, licks, and nibbles with ears laid back, threats or actual bites, or kicks and avoidance (e.g., [45,46]). Invasive physical contacts (i.e., biting clothes, head rubbing, pushing with head, and jostling) were not included in the positive behaviours because they could not be separated from frustration behaviours [31]. Moreover, behaviours expressed with ears on the side or asymmetrical ears were considered as ‘other behaviours’ since their valence is unclear (see Table 2 for more details). Behaviours not directed towards the experimenter were not considered for this analysis. The total numbers of behaviours displayed by each equid towards the unknown human during the test were calculated.

Table 2.
List of behaviours recorded during the motionless person test (adapted from Fureix et al. [45]).
2.5. Statistical Analyses
Analyses were conducted using R software (version 3.5.0) [65]. The significance threshold was p = 0.05 and descriptive statistics were reported as means and standard error. The number of positive and negative behaviours towards humans during the test was compared between EAI horses and RS horses using Kruskal–Wallis tests, followed by Mann–Whitney tests as post hoc tests.
Negative binomial generalised linear mixed-effects models (GLMM.nb) (goft package) [66] were used to understand which factors explained best the individual differences in the number of human-directed interactive behaviours and to check for potential interactions between factors. GLMM.nb are classical models for count data (not normally distributed) and are adapted to situations where the explanatory variables are not balanced [67]. A first GLMM.nb was made including all following factors as fixed effects: test modality (in a group or alone), equid’s characteristics such as sex (mare or gelding), age (categorised according to Burn et al. (2010): < 15 years old or > 15 years old) and type (pony or horse), as well as the conditions of life factors such as type of housing (individual stall, group stall), hay quantity per day (0–3 kg, 3–9 kg or more than 9 kg), number of pellet meals per day (none, one, two or three) and type of activity (EAI, RS-EAI, or RS). Experimenters and centres were used as random effects. We followed a three-step procedure to test and select the best-fitting model. As the first step, in order to test if random effects are important for the model, a selection, based on the Akaike information criterion, (AICc) was made. Five models were tested: with centre and experimenter as random effects, with centre and experimenter as nested random effects, only with centre, only with the experimenter, and without any random effect. The model with the lower AICc was kept [68]. As the second step, we applied an automatic procedure based on the AICc (Package MuMIn) [69] on the previously chosen model to select which fixed effect to include in the model. The procedure tested all possible combinations of fixed effects and ranked the worst to the best fitting model. This procedure ensures that only significant factors are kept in the model. As a third step, all possible interactions were tested from the model chosen in the second step. The AICc was recalculated, and the interaction was only retained in the model if the AICc decreased by more than 2 points; this procedure ensures that only significant interactions are kept in the model. Once the final model was selected, an inspection of fitted values residuals was conducted using the plotresid function in RVAideMemoire package [70] in order to assess the independence and homogeneity of variances of the models, and the normality of the model residuals was tested using a Shapiro test. The estimate was used to assess the order of importance of the factors.
The first step of the model analyses revealed that the model was better without random effect (AICc: 1077.70, ΔAICc: 0.00) than with experimenter and centre (AICc: 1082.53, ΔAICc: 4.84), nested experimenter and centre (AICc: 1082.53, ΔAICc: 4.84), only experimenter (AICc: 1080.10, ΔAICc: 2.40), or only centre (AICc: 1080.92, ΔAICc: 3.22) as random effects. In the second step, the 256 possible models were tested, and significant factors appeared. The third step showed that there was no significant interaction; therefore, the best model, which was the model without interaction (AICc: 1066.5, ΔAICc: 0.00), was retained.
Finally, because the distribution of age was not comparable among the EAI, RS-EAI, and RS equids, the number of human-directed interactive behaviours was compared between EAI, RS-EAI, and RS equids over 15 years old and then between EAI, RS-EAI, and RS equids under 15 years old using Kruskal–Wallis tests, followed by Mann–Whitney tests as post hoc tests.
3. Results
The number of behaviours directed towards the experimenter during the 5 minutes of a test varied significantly between subjects (n = 172 equines; range of 0–51 behaviours; mean ± SE = 8.3 ± 0.8). During a test, 81% of the subjects displayed at least one positive behaviour and 27% at least one negative behaviour. Interestingly, 12% of the subjects tested did not express any behaviour towards the experimenter. Overall, the equids displayed a higher proportion of positive (ears forwards, sniffs: 26.1%; gazes: 26.0%; nibbles: 12.5%), than negative (ears backwards, gazes: 5.8%; sniffs: 4.9%) behaviours (Figure 1). Equids involved in riding school lessons (RS) displayed more positive behaviours (mean ± SE = 7.9 ± 0.9) than the equids involved in equine-assisted interventions whether it was only part of (EAI-RS) (mean ± SE positive = 3.9 ± 0.6) or their whole (EAI) (mean ± SE positive = 4.4 ± 2.0) activity (Kruskal–Wallis chi-squared = 12.4, df = 2, p-value = 0.002; Mann–Whitney tests as post hoc tests: RS vs. EAI-RS: W = 3716, p-value = 0.001; RS vs. EAI: W = 1074.5, p-value = 0.030; EAI-RS vs. EAI: W = 531.5, p-value = 0.793) (Figure 2). No significant difference was observed for the negative behaviours (mean ± SE: RS = 1.7 ± 0.4; EAI-RS = 0.5 ± 0.1; EAI = 0.5 ± 0.2) (Kruskal–Wallis chi-squared = 2.9, df = 2, p-value = 0.235) (Figure 2).
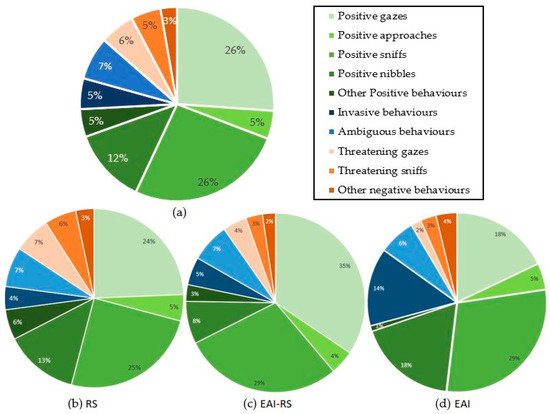
Figure 1.
Proportion of behaviours expressed during the motionless person test by (a) all equids, (b) riding school lesson equids, (c) equids with mixed activities, and (d) assisted-intervention equids.
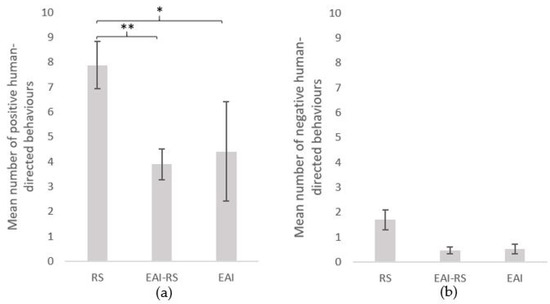
Figure 2.
(a) Positive and (b) negative human-directed behaviours (mean ± SE) during the motionless person test. Tests followed by Mann–Whitney tests as post hoc tests. RS = riding school horses (N = 95), EAI = assisted-intervention horses (N = 17), EAI-RS = horse with mixed activity (N = 60). **: p < 0.01, *: p < 0.05.
Since the most variable aspect was the total number of interactive behaviours, we tested further the possible factors of influence on this parameter. The comparison between the different GLM models revealed that five out of the eight factors tested had a significant influence: age, sex, type of equid (pony or horse), the quantity of hay, and type of activity (EAI, EAI-RS, or RS) (AICc: 1066.5, ΔAICc: 0.00), whereas single versus group housing (1068.0, ΔAICc: 1.5), individual versus group testing (AICc: 1070.4, ΔAICc: 3.9), and the number of pellet meals per day (AICc: 1071.0, ΔAICc: 4.5) did not seem to influence the number of behaviours towards the experimenter. There was no interaction between factors, the best model was the model without interaction (AICc: 1066.5, ΔAICc: 0.00).
Type of activity was the most important factor (Figure 3). RS equids exhibited more interactive behaviours towards the experimenter (mean = 10.8 ± 1.1) than EAI (mean ± SE = 6.2 ± 1.6) (GLMnb: estimate = 0.58, z-value = 2.06, p-value = 0.039) or EAI-RS (mean ± SE = 5.0 ± 0.7) (GLMnb: estimate = 0.722, z-value = 4. 28, p-value < 0.001) equids, but the number of interactive behaviours did not differ significantly between EAI and EAI-RS equids (GLMnb: estimate = 0.15, z-value = 0. 49, p-value = 0.632). The second most important factor was the quantity of hay (Figure 4): equids receiving more than 3kgs hay per day produced more interactive behaviours (mean = 9.4 ± 0.8) than equids having less hay (mean = 4.1 ± 1.7) (GLMnb: estimate = 0.52, z-value = 2.47, p-value = 0.014).
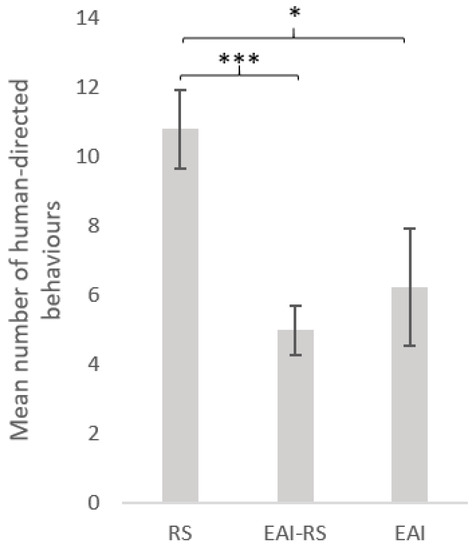
Figure 3.
Behaviour differences of horses during the motionless person test according to the horses’ usual activity. Numbers (mean ± SE) of human-directed behaviours according to the horses’ usual activity. GLM negative binomial. RS = riding school horses (N = 95), EAI = assisted-intervention horses (N = 17), EAI-RS = horse with mixed activity (N = 60). ***: p < 0.001, *: p < 0.05.
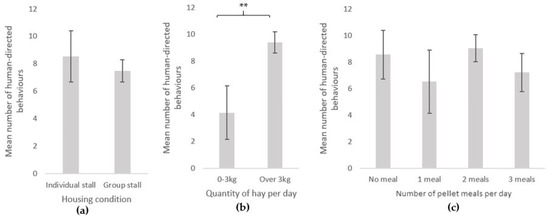
Figure 4.
Human-directed behaviours (mean ± SE) during the motionless person test according to equids’ conditions of life parameters: (a) housing condition (individual stall: N = 134, group stall: N = 38; (b) hay quantity per day (0–3 kg: N = 35, Over 3 kg: N = 137); (c) Number of pellet meals per day (No meal: N = 39, 1 meal: N = 17, 2 meals: N = 81 , 3 meals: N = 35). GLM negative binomial. **: p < 0.01.
The equids’ individual characteristics (type, age and sex) also influenced their interactions with humans (Figure 5). Horses performed more behaviours (mean = 10.3 ± 1.1) than ponies (mean = 6.6 ± 1.0) towards the experimenter (GLMnb: estimate = 0.42, z-value = 2.60, p-value = 0.009). Younger equids were more interactive: equids under 15 years performed more interactive behaviours than equids over 15 years of age (mean = 6.3 ± 0.9) towards the experimenter (mean = 9.7 ± 1.1) (GLMnb: estimate = 0.35, z-value = 2.15, p-value = 0.032). Geldings (mean = 9.3 ± 1.2) performed more interactive behaviours than mares (mean = 7.5 ± 1.0) towards the experimenter (mean = 10.6 ± 1.2 and 7.7 ± 1.0, respectively; GLMnb: estimate = 0.33, z-value = 2.14, p-value = 0.032).
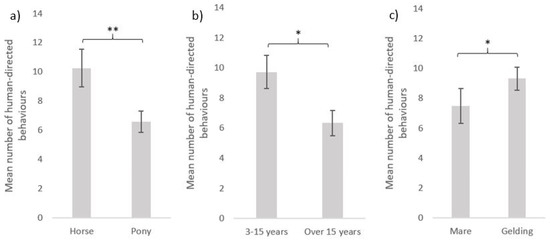
Figure 5.
Human-directed behaviours (mean ± SE) during the motionless person test according to equids’ characteristics: (a) equine type (horses: N = 81, ponies: N = 91); (b) age (3–15 years old: N = 101, over 15 years old: N = 71); (c) sex (mare: N = 93, gelding: N = 79). GLM negative binomial. **: p < 0.01, *: p < 0.05.
Distribution of sex and type of equid (pony or horses) were comparable among the three groups (Sex: khi ²: X-squared = 0.25, df = 2, p-value = 0.880, type: khi ²: X-squared = 4.18, df = 2, p-value = 0.124), but equids used for EAI were overall older (over 15 years) than RS equids (khi ²: X-squared = 5.85, df = 1, p-value = 0.016) and tended to be older than EAI-RS (khi ²: X-squared = 3.36, df = 1, p-value = 0.067).
Since EAI equids were on average older than RS equids, we performed further statistical tests to test whether age may have influenced the major impact of activities on the behaviours of equids during the MP test. However, within age–class comparisons of the number of behaviours performed by equids during the test confirmed this significant impact of activity (young: Kruskal–Wallis chi-squared = 8.47, df = 2, p-value = 0.015; old: Kruskal–Wallis chi-squared = 8.10, df = 2, p-value = 0.017). RS tended to perform more interactive behaviours than EAI for older equids (W = 276, p-value = 0.073) and differed significantly from EAI-RS for both older (W = 598, p-value = 0.007) and younger (W = 1445.5, p-value = 0.004) equids. No difference was found between EAI and EAI-RS for either older (W = 142, p-value = 0.805) or younger (W = 83, p-value = 0.869) equids.
4. Discussion
The results of this study, in which a large sample of riding school and EAI equids underwent the same standardised human–horse relationship test, showed that even though subjects performed overall more positive than negative behaviours towards the experimenter, clear differences appeared according to activity (EAI vs. RS), feeding (hay quantity per day), and equids’ intrinsic characteristics (sex, age, and type). The riding school equids performed more positive behaviours but, most of all, performed, in total, more human-directed behaviours during a test than equids involved in EAI, even if this was only part of their working time. In other words, EAI subjects appeared less interested in humans, i.e., less interactive. Moreover, equids receiving small quantities of hay per day, ponies, older equids, and females were also less interactive than equids with more hay per day, horses, geldings, and younger animals. However, no significant interaction was found between these factors.
4.1. The Valence of Horses’ Behaviours towards Humans
Overall, 30% of our study population displayed at least one negative behaviour, 80% at least one positive behaviour, and 10% did not show any human-directed behaviour; this result was close to the results of some other studies on riding horses using tests with no forced contact, e.g., motionless person test: 60% positive and 15% negative [45]. However, there are large discrepancies in the valence of horses’ reactions during human–horse relationship (HHR) tests, ranging from almost no negative reaction to a majority of negative behaviours [71]. Factors influencing reaction discrepancies include welfare state [26,72,73], feeding conditions [31], health status [74], and working conditions [56,57,58,59], as well as the type of test. Fureix et al. [45] showed that the valence of the reactions in an MP test may be less predictive of the valence of a horse’s reactions, especially when positive, than in other more ‘intrusive’ tests such as approaching or touching the animal, a finding also reported by Burn et al. [28]. Moreover, the relatively high prevalence of positive behaviours in our study may also be due to the fact that we included here measures of positive gazes, which have not been considered in previous studies [45,56] or at least not in the same way [39]. This could be a further interesting measure for future studies. Therefore, the results of our study in terms of valence have to be interpreted with caution even though the finding that RS equines showed more positive behaviours than EAI animals is intriguing and would deserve further consideration.
4.2. On the Meaning of Unresponsiveness
In the present study, animals were free to show their interest in humans and their motivation to interact. The lower proportion of interactive responses to the test of EAI equids is remarkable. Unresponsiveness to a human approach has been interpreted as ‘neutral’ by [75,76], whereas it is considered a welfare problem in other studies where unreactive horses were more at risk of presenting skin lesions, low body-condition scores, abnormal mucous membrane colours, or abnormal gaits [28,30,57,77]. Other studies showed that the more horses are depressed, the more they are unresponsive to a human approach [28,29].
Popescu and Diugan [59] reported that unresponsiveness changed into negative behaviours when the human approach became more invasive, suggesting that indifference may be a ‘mild’ version or another version of negative valence, expressed when free to initiate the interaction or not. Indeed, in different studies using a motionless person test, the equids produced mostly either positive behaviours and visual attention towards the experimenter or avoidance/indifference but rarely clear negative behaviours [46,61,78,79], contrary to observations performed with more invasive tests [45].
4.3. Housing and Feeding Conditions
Contrary to our expectations, we found no significant impact of single versus group housing on the results in the MP test. This is surprising as different studies have shown that social restrictions are deleterious for equids’ welfare [80,81,82] and the human–horse interactions [83,84]. Visser [85], for example, found that horses housed in individual stalls developed more stereotypic behaviours than horses kept in pairs. Group-housing in stalls, contrary to group-housing in larger spaces, may, however, induce tensions due to space limitations and group composition, which may counterbalance the positive effect of social contact. Further studies are needed on this aspect, but overall, studies converge to show that permanent stall housing is not appropriate and may alter equids’ behaviour, in particular during training and work sessions [32,86] and during tests [77].
Feeding modalities, in terms of hay availability, appeared, on the contrary, to have an important impact in the present study. Permanent access to roughage allows equids to express a more normal time budget, and many studies have shown its importance for horse welfare [13,17,81,82] and to prevent the emergence of pathologies such as gastric ulcers or colitis [16,87,88]. Horses allowed to graze or feed on roughage all day long appear to show less aggressiveness towards conspecifics [17,89] and to express more positive behaviours towards humans [31]. In the present study, welfare indicators were not collected, and therefore, we cannot definitely conclude on a possible link between welfare state and equids’ reactions to the MP test, but we can hypothesise that a better welfare state could explain the differences we found here, suggesting further that being interactive with humans may reflect a more positive state. Indeed, Lansade et al. [33] showed that equids in more appropriate living conditions were more interactive during a motionless person test than equids in an impoverished environment.
4.4. Individual Behavioural Characteristics
Some researchers suggest that horses’ reactions to humans are temperament traits [34], and individual differences have been demonstrated [44]. These individual differences are partly related to genetic factors such as breed or sire. At 6 months, foals’ facilitation of human contact by their dam is modulated by their sire origin [41]. A study of adult horses all living in the same facility showed that French saddlebreds were more friendly in a sudden approach test than Angloarabs, whereas Thoroughbreds were more indifferent [39]. Cold-blooded horses were less interactive than warm-blooded ones in a motionless test [90]. Our study equids came from too many breeds and too many were unregistered to test for a breed or sire effect, but ponies appeared less reactive towards humans than horses. This is in line with the study by Henriksson et al. [40], although Schrimpf et al. [43] and Maros et al. [91] found no differences or even opposite results. However, their results were obtained in tests not involving human relationships. These mitigated results may be due also to the side-effects of selection for other traits or to different equid managements.
We detected other individual characteristics that modulated horses’ reactions to humans. The sex differences found here, with geldings being more interactive, have not been recorded in other studies using the same test [36,37,38,78]. Popescu et al. [92] reported that stallions appeared to be more indifferent and mares more aggressive, but since these animals had different housing conditions, it is difficult to draw definitive conclusions. Similar results were reported by Wulf et al. [93] for yearling horses, but males and females had different handlers in this study and, as Fureix et al. [45] showed, daily interactions with their caretaker can impact a human–horse relationship. Overall, studies of the social behaviour of domestic horses report that geldings are more interactive socially [94,95]. Although to our knowledge it is still not clear, one hypothesis would be that this extends to the interspecific relationship context.
Younger horses are generally more socially interactive [96] and explore more [97], which may explain the age differences found here that are similar to those found by Burn et al. [28] and Popescu and Diugan [30]. There was no indication that older horses had more health problems (caretakers’ reports, direct observations), and earlier studies have shown, for example, that the prevalence and extent of back disorders were not correlated with age (e.g., review in [25]).
4.5. EAI as a Working Activity
As mentioned above, working conditions, when inappropriate, can have a negative impact on horses, such as an increased prevalence of abnormal behaviours, higher emotionality, or physical health problems [18,20,21,25,98,99,100,101]. Positive training leads on the contrary to improved HHR [46,61].
While research studies on EAI mention no particular problem with this activity [48,51,52,54,55], the questions of the impact of the characteristics of the clients involved and of the modalities of interventions still remain [10,11]. Indeed, during EAI, horses are often requested to interact with persons who may have unpredictable behaviours [51], attention problems, be aggressive or irritable [10], and are in a negative mood [52]. In other cases, persons with a balance problem [10] can have poor stability and an asymmetrical position on the saddle that can cause lameness or back pain in horses [102,103]. As there are discrepancies in the methods and indicators used for assessing how horses react during the EAI sessions, further studies are needed in order to elucidate whether there may be some aspects of the EAI that are more difficult to deal with for the horses than others.
5. Conclusions
Overall, it is not possible at this stage to know why the EAI equids in our study were less interactive with the experimenter in the test, i.e., whether they are more depressed due to their activity, whether they may have been trained for not reacting in any human-related situation, or if they had been chosen for EAI because of their overall low reactivity, including their reactions to humans (e.g., in order to decrease the risks for the clients of EAI). However, studies aiming at testing EAI equids’ temperament have not led to any conclusive difference with other working horses [50,104].
Further studies are needed that will include different types of tests, welfare assessments, and observations during EAI sessions (Lerch et al. in preparation). In any case, since it is well known that positive training can lead to improved human–horse relationships [46,61], it could be interesting to associate EAI with positive reinforcement, as recommended by the International Association of Human–Animal Interaction Organisations [1], in order to have more positively interactive and attentive horses and, at the same time, improve their welfare.
Author Contributions
Conceptualisation, M.G., M.H., and N.L.; methodology, M.G., M.H., and N.L.; validation, M.G. and M.H.; formal analysis, N.L.; investigation, E.G., N.L., C.L., and C.R.; writing—original draft preparation, N.L.; writing—review and editing, M.B., F.C., L.C., M.G., M.H., N.L., and C.R.; supervision, M.G. and M.H.; funding acquisition, M.G. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ifce – Institut Français du Cheval et de l’Equitation (IFCE contrat: CS_2018_18 ; CS_2017_32 ; CS_2015_01), Fondation Adrienne et Pierre Sommer, Région Bretagne (ARED 20007248) and Caisse Centrale de la Mutualité Sociale Agricole.
Institutional Review Board Statement
Ethical review and approval were waived for this study, and the experiments were carried out between 2009 and 2019 in accordance with the Directive 2010/63/UE of the European Parliament and the Council on the protection of animals used for scientific purposes. They complied with the current French laws related to animal experimentation (decree n°2013 ± 118 of 1 February 2013) and its five implementation orders (JO 7 February 2013, integrated into the Rural Code and the Code of maritime fishing under n° R. 214 ± 87 to n° R. 214-137). The experiments performed in this study were not within the scope of application of the European directive, and thus, in accordance with this directive and the current French, Italian, and Irish laws, the following experiments did not require us to request authorisation. These experiments involved only behavioural observations and non-invasive interactions with the horses. The horses used in this research were not research animals. Animal husbandry and care were under the management of the riding school staff. The riding school managers gave the authors their informed consent for this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated and analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank the owner and staff of the riding schools for allowing them to work with their horses and for their understanding, and are grateful to Ann Cloarec for correcting the English writing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jegatheesan, B.; Beetz, A.; Choi, G.; Dudzik, C.; Fine, A.; Garcia, R.M.; Johnson, R.; Ormerod, E.; Winkle, E.; Yamazaki, K. The IAHAIO Definitions for Animal Assisted Intervention and Guidelines for Wellness of Animals Involved in AAI. In Handbook on Animal-Assisted Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 499–504. ISBN 978-0-12-815395-6. [Google Scholar]
- Fine, A.H. Handbook on Animal-Assisted Therapy: Foundations and Guidelines for Animal-Assisted Interventions, 4th ed.; Elsevier Science: Amsterdam, The Netherlands, 2015; ISBN 978-0-12-801292-5. [Google Scholar]
- Philippe-Peyroutet, C.; Grandgeorge, M. Animal-Assisted Interventions for Children with Autism Spectrum Disorders: A Survey of French Facilities. People Anim. Int. J. Res. Pract. 2017, 1, 8. [Google Scholar]
- Borgi, M.; Loliva, D.; Cerino, S.; Chiarotti, F.; Venerosi, A.; Bramini, M.; Nonnis, E.; Marcelli, M.; Vinti, C.; Santis, C.; et al. Effectiveness of a Standardized Equine-Assisted Therapy Program for Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kendall, E.; Maujean, A.; Pepping, C.A.; Downes, M.; Lakhani, A.; Byrne, J.; Macfarlane, K. A Systematic Review of the Efficacy of Equine-Assisted Interventions on Psychological Outcomes. Eur. J. Psychother. Couns. 2015, 17, 57–79. [Google Scholar] [CrossRef]
- Srinivasan, S.; Dt, C.; An, B. Effects of Equine Therapy on Individuals with Autism Spectrum Disorder: A Systematic Review. Rev. J. Autism Dev. Disord. 2018, 5, 156–175. [Google Scholar] [CrossRef]
- Kendall, E.; Maujean, A.; Pepping, C.A.; Wright, J.J. Hypotheses about the Psychological Benefits of Horses. Explore 2014, 10, 81–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kieson, E.; Abramson, C.I. Equines as Tools vs. Partners: A Critical Look at the Uses and Beliefs Surrounding Horses in Equine Therapies and Argument for Mechanical Horses. J. Vet. Behav. 2016, 15, 94–95. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Oddie, C.; Burton, F.L.; McLean, A.N. The Horse–Human Dyad: Can We Align Horse Training and Handling Activities with the Equid Social Ethogram? Vet. J. 2009, 181, 12–18. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.; Contalbrigo, L.; Borgi, M.; Cirulli, F.; Luzi, F.; Redaelli, V.; Stefani, A.; Toson, M.; Odore, R.; Vercelli, C.; et al. Equine Assisted Interventions (EAIs): Methodological Considerations for Stress Assessment in Horses. Vet. Sci. 2017, 4, 44. [Google Scholar] [CrossRef]
- Grandgeorge, M.; Hausberger, M. La médiation équine. Qu’en pensent les scientifiques? In Choix, Éducation et Bien-Être des Chevaux de Médiation; IFCE: Geneva, Switzerland, 2018; pp. 161–181. ISBN 978-2-915250-63-3. [Google Scholar]
- Wensley, S.P. Animal Welfare and the Human–Animal Bond: Considerations for Veterinary Faculty, Students, and Practitioners. J. Vet. Med. Educ. 2008, 35, 532–539. [Google Scholar] [CrossRef]
- Lesimple, C.; Poissonnet, A.; Hausberger, M. How to Keep Your Horse Safe? An Epidemiological Study about Management Practices. Appl. Anim. Behav. Sci. 2016, 181, 105–114. [Google Scholar] [CrossRef]
- Raabymagle, P.; Ladewig, J. Lying Behavior in Horses in Relation to Box Size. J. Equine Vet. Sci. 2006, 26, 11–17. [Google Scholar] [CrossRef]
- Mills, D.S.; Riezebos, M. The Role of the Image of a Conspecific in the Regulation of Stereotypic Head Movements in the Horse. Appl. Anim. Behav. Sci. 2005, 91, 155–165. [Google Scholar] [CrossRef]
- Luthersson, N.; Nielsen, K.H.; Harris, P.; Parkin, T.D.H. Risk Factors Associated with Equine Gastric Ulceration Syndrome (EGUS) in 201 Horses in Denmark. Equine Vet. J. 2009, 41, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Benhajali, H.; Richard-Yris, M.-A.; Ezzaouïa, M.; Charfi, F.; Hausberger, M. Foraging Opportunity: A Crucial Criterion for Horse Welfare? Animal 2009, 3, 1308–1312. [Google Scholar] [CrossRef]
- Hausberger, M.; Gautier, E.; Biquand, V.; Lunel, C.; Jégo, P. Could Work Be a Source of Behavioural Disorders? A Study in Horses. PLoS ONE 2009, 4, e7625. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Goodwin, D.; Heleski, C.; Randle, H.; Waran, N. Is There Evidence of Learned Helplessness in Horses? J. Appl. Anim. Welf. Sci. 2008, 11, 249–266. [Google Scholar] [CrossRef]
- Greve, L.; Dyson, S. The Horse–Saddle–Rider Interaction. Vet. J. 2013, 195, 275–281. [Google Scholar] [CrossRef]
- Lesimple, C.; Fureix, C.; Menguy, H.; Hausberger, M. Human Direct Actions May Alter Animal Welfare, a Study on Horses (Equus caballus). PLoS ONE 2010, 5, e10257. [Google Scholar] [CrossRef]
- Clayton, H.M.; Nauwelaerts, S. Effect of Blindfolding on Centre of Pressure Variables in Healthy Horses during Quiet Standing. Vet. J. 2014, 199, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Cook, W.R. Pathophysiology of Bit Control in the Horse. J. Equine Vet. Sci. 1999, 19, 196–204. [Google Scholar] [CrossRef]
- Jeffcott, L.B.; Holmes, M.A.; Townsend, H.G.G. Validity of Saddle Pressure Measurements Using Force-Sensing Array Technology—Preliminary Studies. Vet. J. 1999, 158, 113–119. [Google Scholar] [CrossRef]
- Lesimple, C.; Fureix, C.; Aube, L.; Hausberger, M. Detecting and Measuring Back Disorders in Nonverbal Individuals: The Example of Domestic Horses. Anim. Behav. Cogn. 2016, 3, 159–179. [Google Scholar] [CrossRef]
- Fureix, C.; Menguy, H.; Hausberger, M. Partners with Bad Temper: Reject or Cure? A Study of Chronic Pain and Aggression in Horses. PLoS ONE 2010, 5, e12434. [Google Scholar] [CrossRef]
- Stomp, M.; d’Ingeo, S.; Henry, S.; Lesimple, C.; Cousillas, H.; Hausberger, M. EEG Individual Power Profiles Correlate with Tension along Spine in Horses. PLoS ONE 2020, 15, e0243970. [Google Scholar] [CrossRef] [PubMed]
- Burn, C.C.; Dennison, T.L.; Whay, H.R. Relationships between Behaviour and Health in Working Horses, Donkeys, and Mules in Developing Countries. Appl. Anim. Behav. Sci. 2010, 126, 109–118. [Google Scholar] [CrossRef]
- Fureix, C.; Jego, P.; Henry, S.; Lansade, L.; Hausberger, M. Towards an Ethological Animal Model of Depression? A Study on Horses. PLoS ONE 2012, 7, e39280. [Google Scholar] [CrossRef] [PubMed]
- Popescu, S.; Diugan, E.-A. The Relationship Between Behavioral and Other Welfare Indicators of Working Horses. J. Equine Vet. Sci. 2013, 33, 1–12. [Google Scholar] [CrossRef]
- Rochais, C.; Sébilleau, M.; Menoret, M.; Oger, M.; Henry, S.; Hausberger, M.; Cousillas, H. Attentional State and Brain Processes: State-Dependent Lateralization of EEG Profiles in Horses. Sci. Rep. 2018, 8, 10153. [Google Scholar] [CrossRef] [PubMed]
- Losonci, Z.; Berry, J.; Paddison, J. Do Stabled Horses Show More Undesirable Behaviors during Handling than Field-Kept Ones? J. Vet. Behav. 2016, 15, 93. [Google Scholar] [CrossRef]
- Lansade, L.; Valenchon, M.; Foury, A.; Neveux, C.; Cole, S.W.; Layé, S.; Cardinaud, B.; Lévy, F.; Moisan, M.-P. Behavioral and Transcriptomic Fingerprints of an Enriched Environment in Horses (Equus caballus). PLoS ONE 2014, 9, e114384. [Google Scholar] [CrossRef]
- Lansade, L.; Bouissou, M.-F. Reactivity to Humans: A Temperament Trait of Horses Which Is Stable across Time and Situations. Appl. Anim. Behav. Sci. 2008, 114, 492–508. [Google Scholar] [CrossRef]
- McCann, J.S.; Heird, J.C.; Bell, R.W.; Lutherer, L.O. Normal and More Highly Reactive Horses. I. Heart Rate, Respiration Rate and Behavioral Observations. Appl. Anim. Behav. Sci. 1988, 19, 201–214. [Google Scholar] [CrossRef]
- Visser, E.K.; van Reenen, C.G.; Hopster, H.; Schilder, M.B.H.; Knaap, J.H.; Barneveld, A.; Blokhuis, H.J. Quantifying Aspects of Young Horses’ Temperament: Consistency of Behavioural Variables. Appl. Anim. Behav. Sci. 2001, 74, 241–258. [Google Scholar] [CrossRef]
- Visser, E.K.; van Reenen, C.G.; van der Werf, J.T.N.; Schilder, M.B.H.; Knaap, J.H.; Barneveld, A.; Blokhuis, H.J. Heart Rate and Heart Rate Variability during a Novel Object Test and a Handling Test in Young Horses. Physiol. Behav. 2002, 76, 289–296. [Google Scholar] [CrossRef]
- Visser, E.K.; Reenen, C.G.V.; Rundgren, M.; Zetterqvist, M.; Morgan, K.; Blokhuis, H.J. Responses of Horses in Behavioural Tests Correlate with Temperament Assessed by Riders. Equine Vet. J. 2003, 35, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Hausberger, M.; Muller, C. A Brief Note on Some Possible Factors Involved in the Reactions of Horses to Humans. Appl. Anim. Behav. Sci. 2002, 76, 339–344. [Google Scholar] [CrossRef]
- Henriksson, J.; Sauveroche, M.; Roth, L.S.V. Effects of Size and Personality on Social Learning and Human-Directed Behaviour in Horses (Equus caballus). Anim. Cogn. 2019, 22, 1001–1011. [Google Scholar] [CrossRef]
- Henry, S.; Briefer, S.; Richard-Yris, M.-A.; Hausberger, M. Are 6-Month-Old Foals Sensitive to Dam’s Influence? Dev. Psychobiol. 2007, 49, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Houpt, K.; Kusunose, R. Genetics of Behaviour. In The Genetics of the Horse; CABI: Wallingford, UK, 2000; ISBN 978-0-85199-925-8. [Google Scholar]
- Schrimpf, A.; Single, M.-S.; Nawroth, C. Social Referencing in the Domestic Horse. Animals 2020, 10, 164. [Google Scholar] [CrossRef]
- Hausberger, M.; Roche, H.; Henry, S.; Visser, E.K. A Review of the Human–Horse Relationship. Appl. Anim. Behav. Sci. 2008, 109, 1–24. [Google Scholar] [CrossRef]
- Fureix, C.; Jego, P.; Sankey, C.; Hausberger, M. How Horses (Equus caballus) See the World: Humans as Significant “Objects. ” Anim. Cogn. 2009, 12, 643–654. [Google Scholar] [CrossRef]
- Sankey, C.; Richard-Yris, M.-A.; Henry, S.; Fureix, C.; Nassur, F.; Hausberger, M. Reinforcement as a Mediator of the Perception of Humans by Horses (Equus caballus). Anim. Cogn. 2010, 13, 753–764. [Google Scholar] [CrossRef]
- Smith, A.V.; Wilson, C.; McComb, K.; Proops, L. Domestic Horses (Equus caballus) Prefer to Approach Humans Displaying a Submissive Body Posture Rather than a Dominant Body Posture. Anim. Cogn. 2018, 21, 307–312. [Google Scholar] [CrossRef]
- Merkies, K.; McKechnie, M.J.; Zakrajsek, E. Behavioural and Physiological Responses of Therapy Horses to Mentally Traumatized Humans. Appl. Anim. Behav. Sci. 2018, 205, 61–67. [Google Scholar] [CrossRef]
- Merkies, K.; Sievers, A.; Zakrajsek, E.; MacGregor, H.; Bergeron, R.; von Borstel, U.K. Preliminary Results Suggest an Influence of Psychological and Physiological Stress in Humans on Horse Heart Rate and Behavior. J. Vet. Behav. 2014, 9, 242–247. [Google Scholar] [CrossRef]
- Anderson, M.K.; Friend, T.H.; Evans, J.W.; Bushong, D.M. Behavioral Assessment of Horses in Therapeutic Riding Programs. Appl. Anim. Behav. Sci. 1999, 63, 11–24. [Google Scholar] [CrossRef]
- Pluta, M.; Kędzierski, W. Emotional Responses of Horses to Patients Requiring Therapy. Soc. Anim. 2018, 26, 426–436. [Google Scholar] [CrossRef]
- Fazio, E.; Medica, P.; Cravana, C.; Ferlazzo, A. Hypothalamic-Pituitary-Adrenal Axis Responses of Horses to Therapeutic Riding Program: Effects of Different Riders. Physiol. Behav. 2013, 118, 138–143. [Google Scholar] [CrossRef]
- Grandgeorge, M.; Dubois, E.; Alavi, Z.; Bourreau, Y.; Hausberger, M. Do Animals Perceive Human Developmental Disabilities? Guinea Pigs’ Behaviour with Children with Autism Spectrum Disorders and Children with Typical Development. A Pilot Study. Animals 2019, 9, 522. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.A.; Johnson, P.J.; Megarani, D.V.; Patel, S.D.; Yaglom, H.D.; Osterlind, S.; Grindler, K.; Vogelweid, C.M.; Parker, T.M.; Pascua, C.K.; et al. Horses Working in Therapeutic Riding Programs: Cortisol, Adrenocorticotropic Hormone, Glucose, and Behavior Stress Indicators. J. Equine Vet. Sci. 2017, 57, 77–85. [Google Scholar] [CrossRef]
- Kaiser, L.; Heleski, C.R.; Siegford, J.; Smith, K.A. Stress-Related Behaviors among Horses Used in a Therapeutic Riding Program. J. Am. Vet. Med. Assoc. 2006, 228, 39–45. [Google Scholar] [CrossRef]
- Diugan, E.A.; Spinu, M.; Popescu, S. Human-Animal Relationship Assessment in Horses (Equus caballus) with Different Uses. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Vet. Med. 2014, 71, 441–444. [Google Scholar] [CrossRef][Green Version]
- Pritchard, J.C.; Lindberg, A.C.; Main, D.C.J.; Whay, H.R. Assessment of the Welfare of Working Horses, Mules and Donkeys, Using Health and Behaviour Parameters. Prev. Vet. Med. 2005, 69, 265–283. [Google Scholar] [CrossRef]
- Ali, A.B.A.; El Sayed, M.A.; Matoock, M.Y.; Fouad, M.A.; Heleski, C.R. A Welfare Assessment Scoring System for Working Equids—A Method for Identifying at Risk Populations and for Monitoring Progress of Welfare Enhancement Strategies (Trialed in Egypt). Appl. Anim. Behav. Sci. 2016, 176, 52–62. [Google Scholar] [CrossRef]
- Popescu, S.; Diugan, E.A. The Relationship between the Welfare Quality and Stress Index in Working and Breeding Horses. Res. Vet. Sci. 2017, 115, 442–450. [Google Scholar] [CrossRef]
- Hausberger, M.; Stomp, M.; Sankey, C.; Brajon, S.; Lunel, C.; Henry, S. Mutual Interactions between Cognition and Welfare: The Horse as an Animal Model. Neurosci. Biobehav. Rev. 2019. [CrossRef] [PubMed]
- Sankey, C.; Richard-Yris, M.-A.; Leroy, H.; Henry, S.; Hausberger, M. Positive Interactions Lead to Lasting Positive Memories in Horses, Equus caballus. Anim. Behav. 2010, 79, 869–875. [Google Scholar] [CrossRef]
- Nimer, J.; Lundahl, B. Animal-Assisted Therapy: A Meta-Analysis. Anthrozoös 2015, 20, 225–238. [Google Scholar] [CrossRef]
- Fureix, C.; Gorecka-Bruzda, A.; Gautier, E.; Hausberger, M. Cooccurrence of Yawning and Stereotypic Behaviour in Horses (Equus caballus). ISRN Zool. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 22 August 2021).
- Gonzalez-Estrada, E.; Villasenor-Alva, J.A. Tests of Fit for Some Probability Distributions. R Package Version 2017, 1, 4. [Google Scholar]
- Gardner, W.; Mulvey, E.P.; Shaw, E.C. Regression Analyses of Counts and Rates: Poisson, Overdispersed Poisson, and Negative Binomial Models. Psychol. Bull. 1995, 118, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004. [CrossRef]
- Barton, K. Package ‘Mumin’. Version 2020, 1, 439. [Google Scholar]
- Hervé, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R Package Version 2019, 0.9, 72. [Google Scholar]
- Hausberger, M.; Lerch, N.; Guilbaud, E.; Stomp, M.; Grandgeorge, M.; Henry, S.; Lesimple, C. On-Farm Welfare Assessment of Horses: The Risks of Putting the Cart before the Horse. Animals 2020, 10, 371. [Google Scholar] [CrossRef]
- Henry, S.; Fureix, C.; Rowberry, R.; Bateson, M.; Hausberger, M. Do Horses with Poor Welfare Show ‘Pessimistic’ Cognitive Biases? Sci. Nat. 2017, 104, 8. [Google Scholar] [CrossRef] [PubMed]
- Stomp, M.; Leroux, M.; Cellier, M.; Henry, S.; Lemasson, A.; Hausberger, M. An Unexpected Acoustic Indicator of Positive Emotions in Horses. PLoS ONE 2018, 13, e0197898. [Google Scholar] [CrossRef]
- Hausberger, M.; Fureix, C.; Lesimple, C. Detecting Horses’ Sickness: In Search of Visible Signs. Appl. Anim. Behav. Sci. 2016, 175, 41–49. [Google Scholar] [CrossRef]
- Dalla Costa, E.; Minero, M.; Lebelt, D.; Stucke, D.; Canali, E.; Leach, M.C. Development of the Horse Grimace Scale (HGS) as a Pain Assessment Tool in Horses Undergoing Routine Castration. PLoS ONE 2014, 9, e92281. [Google Scholar] [CrossRef]
- Dalla Costa, E.; Dai, F.; Lebelt, D.; Scholz, P.; Barbieri, S.; Canali, E.; Minero, M. Initial Outcomes of a Harmonized Approach to Collect Welfare Data in Sport and Leisure Horses. Animal 2017, 11, 254–260. [Google Scholar] [CrossRef]
- Popescu, S.; Diugan, E.A.; Spinu, M. The Interrelations of Good Welfare Indicators Assessed in Working Horses and Their Relationships with the Type of Work. Res. Vet. Sci. 2014, 96, 406–414. [Google Scholar] [CrossRef]
- Henry, S.; Hemery, D.; Richard, M.-A.; Hausberger, M. Human–Mare Relationships and Behaviour of Foals toward Humans. Appl. Anim. Behav. Sci. 2005, 93, 341–362. [Google Scholar] [CrossRef]
- Rochais, C.; Sébilleau, M.; Houdebine, M.; Bec, P.; Hausberger, M.; Henry, S. A Novel Test for Evaluating Horses’ Spontaneous Visual Attention Is Predictive of Attention in Operant Learning Tasks. Sci. Nat. 2017, 104, 1–6. [Google Scholar] [CrossRef]
- Heleski, C.R.; Shelle, A.C.; Nielsen, B.D.; Zanella, A.J. Influence of Housing on Weanling Horse Behavior and Subsequent Welfare. Appl. Anim. Behav. Sci. 2002, 78, 291–302. [Google Scholar] [CrossRef]
- Bachmann, I.; Audigé, L.; Stauffacher, M. Risk Factors Associated with Behavioural Disorders of Crib-Biting, Weaving and Box-Walking in Swiss Horses. Equine Vet. J. 2003, 35, 158–163. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, P.D.; Cripps, P.J.; French, N.P.; Green, L.E.; Nicol, C.J. Management Factors Associated with Stereotypic and Redirected Behaviour in the Thoroughbred Horse. Equine Vet. J. 1995, 27, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, E.; Halekoh, U. Young Horses’ Reactions to Humans in Relation to Handling and Social Environment. Appl. Anim. Behav. Sci. 2003, 84, 265–280. [Google Scholar] [CrossRef]
- Søndergaard, E.; Ladewig, J. Group Housing Exerts a Positive Effect on the Behaviour of Young Horses during Training. Appl. Anim. Behav. Sci. 2004, 87, 105–118. [Google Scholar] [CrossRef]
- Visser, E.K.; Ellis, A.D.; Van Reenen, C.G. The Effect of Two Different Housing Conditions on the Welfare of Young Horses Stabled for the First Time. Appl. Anim. Behav. Sci. 2008, 114, 521–533. [Google Scholar] [CrossRef]
- Rivera, E.; Benjamin, S.; Nielsen, B.; Shelle, J.; Zanella, A.J. Behavioral and Physiological Responses of Horses to Initial Training: The Comparison between Pastured versus Stalled Horses. Appl. Anim. Behav. Sci. 2002, 78, 235–252. [Google Scholar] [CrossRef]
- Tinker, M.K.; White, N.A.; Lessard, P.; Thatcher, C.D.; Pelzer, K.D.; Davis, B.; Carmel, D.K. Prospective Study of Equine Colic Incidence and Mortality. Equine Vet. J. 1997, 29, 448–453. [Google Scholar] [CrossRef]
- Murray, M.J.; Schusser, G.F.; Pipers, F.S.; Gross, S.J. Factors Associated with Gastric Lesions in Thoroughbred Racehorses. Equine Vet. J. 1996, 28, 368–374. [Google Scholar] [CrossRef]
- Fureix, C.; Bourjade, M.; Henry, S.; Sankey, C.; Hausberger, M. Exploring Aggression Regulation in Managed Groups of Horses Equus caballus. Appl. Anim. Behav. Sci. 2012, 138, 216–228. [Google Scholar] [CrossRef]
- Górecka-Bruzda, A.; Jastrzębska, E.; Sosnowska, Z.; Jaworski, Z.; Jezierski, T.; Chruszczewski, M.H. Reactivity to Humans and Fearfulness Tests: Field Validation in Polish Cold Blood Horses. Appl. Anim. Behav. Sci. 2011, 133, 207–215. [Google Scholar] [CrossRef]
- Maros, K.; Gácsi, M.; Miklósi, Á. Comprehension of Human Pointing Gestures in Horses (Equus caballus). Anim. Cogn. 2008, 11, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Popescu, S.; Borda, C.; Oros, D.; Sandru, D.C.; Spinu, M.; Giupina, R.; Diugan, E. Human-Animal Relationship: A Comparative Study in Working and Breeding Horses. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Vet. Med. 2016, 73, 301. [Google Scholar] [CrossRef][Green Version]
- Wulf, M.; Aurich, J.; May, A.-C.; Aurich, C. Sex Differences in the Response of Yearling Horses to Handling by Unfamiliar Humans. J. Vet. Behav. 2013, 8, 238–244. [Google Scholar] [CrossRef]
- Snorrason, S.; Sigurjónsdóttir, H.; Thórhallsdóttir, A.; van Dierendonck, M. Social Relationships in a Group of Horses without a Mature Stallion. Behaviour 2003, 140, 783–804. [Google Scholar] [CrossRef]
- VanDierendonck, M.C.; de Vries, H.; Schilder, M.B.H.; Colenbrander, B.; Þorhallsdóttir, A.G.; Sigurjónsdóttir, H. Interventions in Social Behaviour in a Herd of Mares and Geldings. Appl. Anim. Behav. Sci. 2009, 116, 67–73. [Google Scholar] [CrossRef]
- Waring, G.H. Horse Behavior, 2nd ed.; Noyes Publishing: Norwich, NY, USA, 2003; ISBN 978-0-8155-1484-8. [Google Scholar]
- Boy, V.; Duncan, P. Time-Budgets of Camargue Horses I. Developmental Changes in the Time-Budgets of Foals. Behaviour 1979, 71, 187–201. [Google Scholar] [CrossRef]
- Christie, J.L.; Hewson, C.J.; Riley, C.B.; McNiven, M.A.; Dohoo, I.R.; Bate, L.A. Management Factors Affecting Stereotypies and Body Condition Score in Nonracing Horses in Prince Edward Island. Can. Vet. J. 2006, 47, 136–143. [Google Scholar]
- Hausberger, M.; Muller, C.; Lunel, C. Does Work Affect Personality? A Study in Horses. PLoS ONE 2011, 6, e14659. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.S.; Alston, R.D.; Rogers, V.; Longford, N.T. Factors Associated with the Prevalence of Stereotypic Behaviour amongst Thoroughbred Horses Passing through Auctioneer Sales. Appl. Anim. Behav. Sci. 2002, 78, 115–124. [Google Scholar] [CrossRef]
- Peham, C.; Hofmann, A.; Molsner, J.; Borkenhagen, B.; Kuhnke, S.; Baltacis, A. Forces Acting on the Horses Back and the Stability of the Rider in Sitting and Rising Trot—A Comparison. Pferdeheilkunde Equine Med. 2008, 24, 337–342. [Google Scholar] [CrossRef]
- Greve, L.; Dyson, S.J. The Interrelationship of Lameness, Saddle Slip and Back Shape in the General Sports Horse Population: Saddle Slip and Lameness. Equine Vet. J. 2014, 46, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Mackechnie-Guire, R.; Mackechnie-Guire, E.; Fisher, M.; Mathie, H.; Bush, R.; Pfau, T.; Weller, R. Relationship Between Saddle and Rider Kinematics, Horse Locomotion, and Thoracolumbar Pressures in Sound Horses. J. Equine Vet. Sci. 2018, 69, 43–52. [Google Scholar] [CrossRef]
- Minero, M.; Zucca, D.; Canali, E. A Note on Reaction to Novel Stimulus and Restraint by Therapeutic Riding Horses. Appl. Anim. Behav. Sci. 2006, 97, 335–342. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).