A Longitudinal Study of Hematology and Stress Biomarker Profiles in Young Asian Elephants (Elephas Maximus) in Relation to Elephant Endotheliotropic Herpesvirus (EEHV) in Thailand
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Elephants and Sample Collection
2.2. Blood Collection
2.3. Saliva and Fecal Analysis
2.4. Salivary Cortisol and FGM Analyses
2.5. SIgA and FIgA Analyses
2.6. EEHV Analysis
2.7. Statistical Analysis
3. Results
3.1. Animals
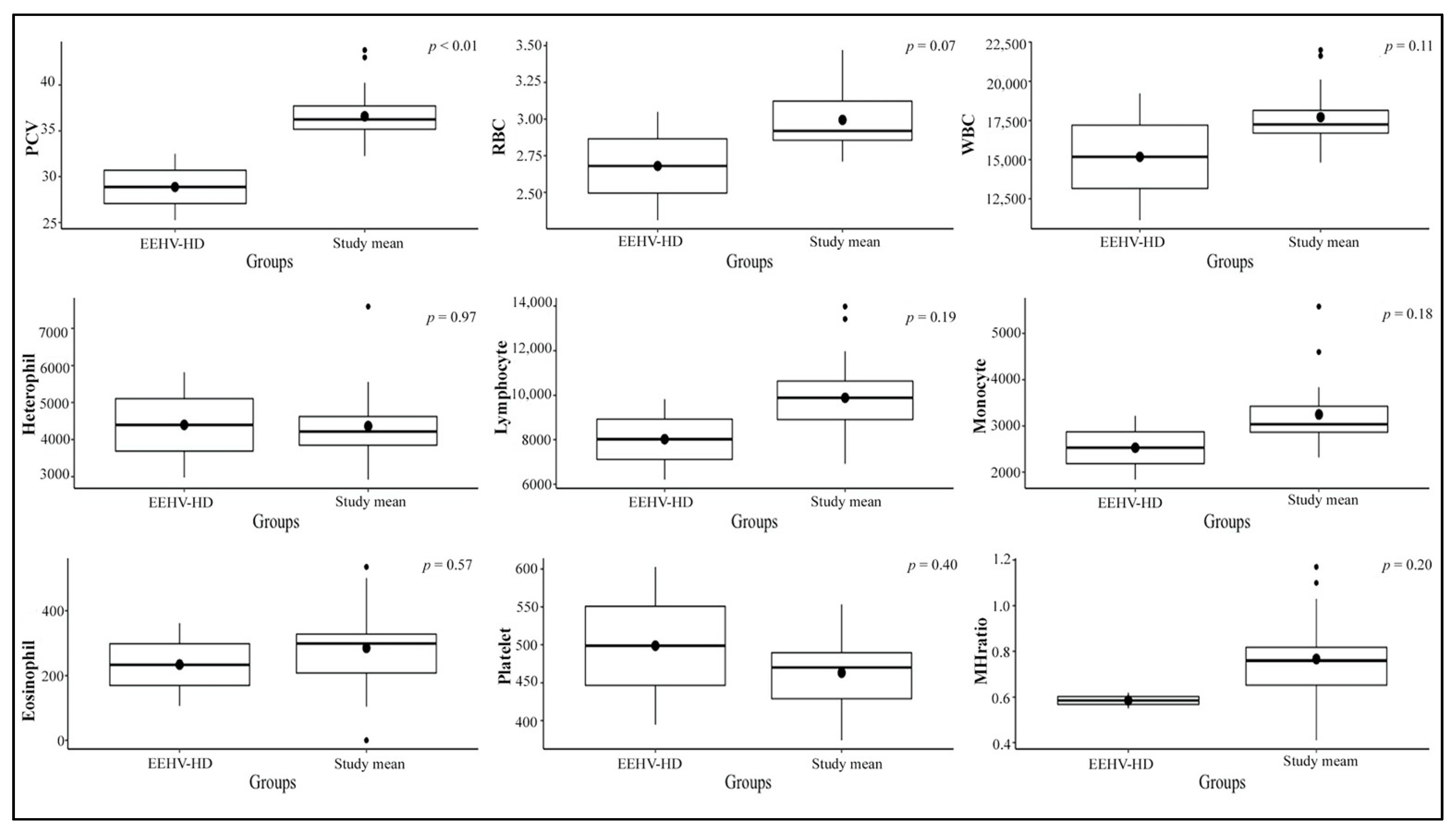
3.2. Hematological Values
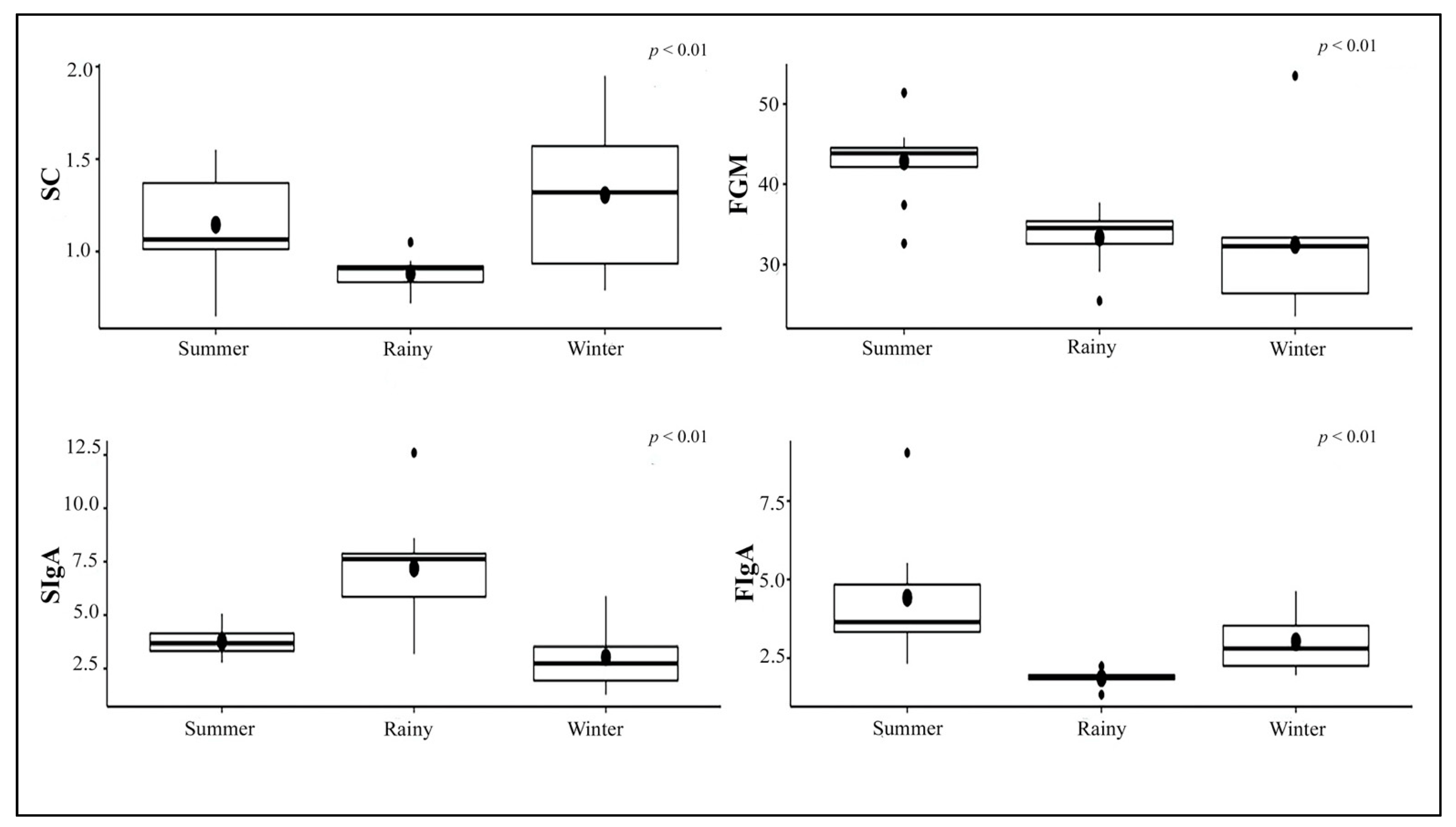
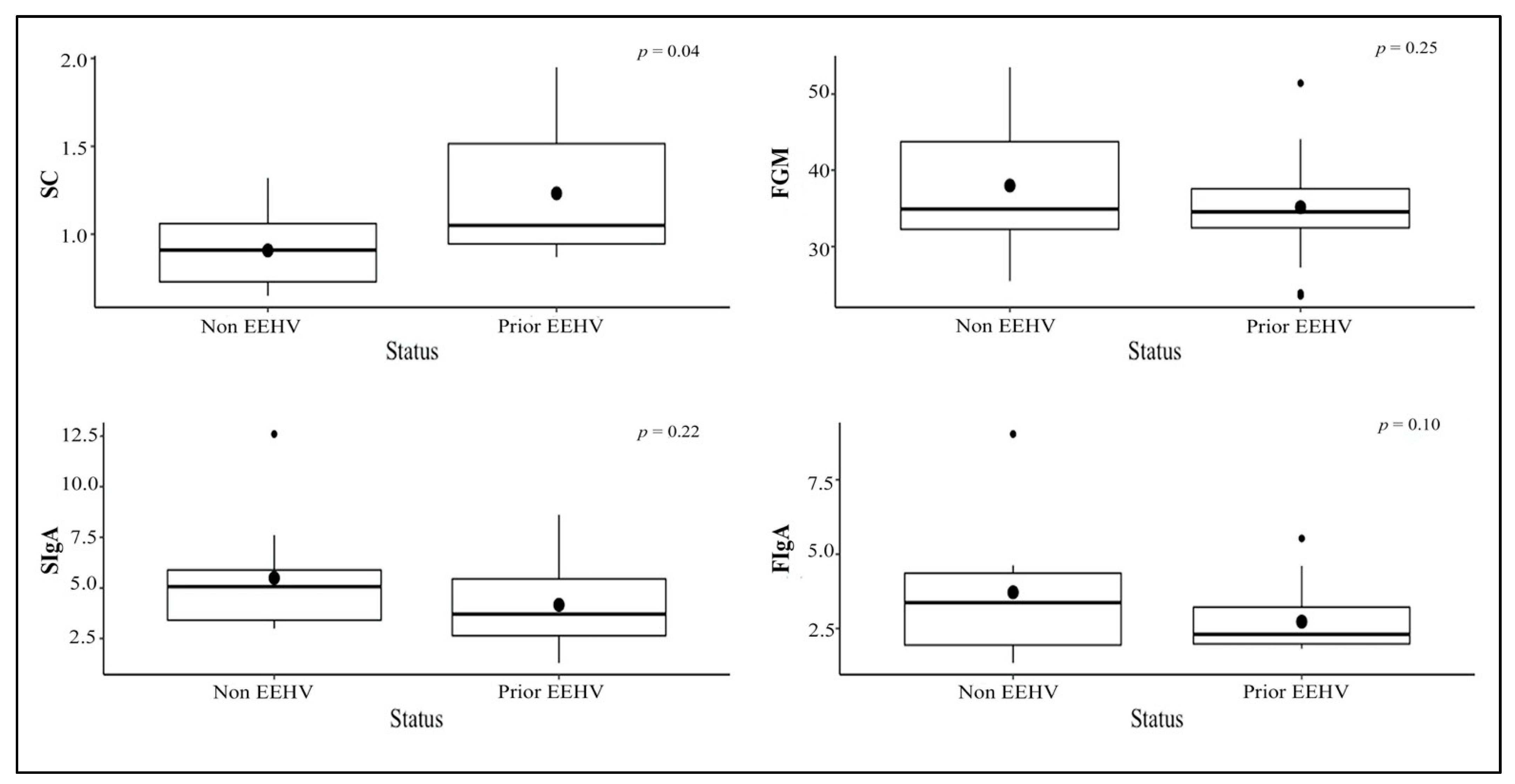
3.3. Stress Indicator Concentrations
3.4. Viremia Monitoring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, L.; Dunham, S.; Yon, L.; Chapman, S.; Kenaghan, M.; Purdie, L.; Tarlinton, R. Longitudinal study of Asian elephants, Elephas Maximus, indicates intermittent shedding of elephant endotheliotropic herpesvirus 1 during pregnancy. Vet. Rec. Open 2015, 2, e000088. [Google Scholar] [CrossRef] [Green Version]
- Wissink-Argilaga, N.; Dastjerdi, A.; Molenaar, F.M. Using in-house hematology to direct decision making in the successful treatment and monitoring of a clinical and subsequently subclinical case of elephant endotheliotropic herpesvirus 1B. J. Zoo Wildl. Med. 2019, 50, 498–502. [Google Scholar] [CrossRef]
- Garner, M.M.; Helmick, K.; Ochsenreiter, J.; Richman, L.K.; Latimer, E.; Wise, A.G.; Maes, R.K.; Kiupel, M.; Nordhausen, R.W.; Zong, J.C.; et al. Clinico-pathologic features of fatal disease attributed to new variants of endotheliotropic herpesviruses in two Asian elephants (Elephas maximus). Vet. Pathol. 2009, 46, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dastjerdi, A.; Seilern-Moy, K.; Darpel, K.; Steinbach, F.; Molenaar, F. Surviving and fatal elephant endotheliotropic herpesvirus-1A infections in juvenile Asian elephants—Lessons learned and recommendations on anti-herpesviral therapy. BMC Vet. Res. 2016, 12, 178. [Google Scholar] [CrossRef] [Green Version]
- Boonprasert, K.; Punyapornwithaya, V.; Tankaew, P.; Angkawanish, T.; Sriphiboon, S.; Titharam, C.; Brown, J.L.; Somgird, C. Survival analysis of confirmed elephant endotheliotropic herpes virus cases in Thailand from 2006–2018. PLoS ONE 2019, 14, e0219288. [Google Scholar] [CrossRef]
- Latimer, E.; Zong, J.C.; Heaggans, S.Y.; Richman, L.K.; Hayward, G.S. Detection and evaluation of novel herpesviruses in routine and pathological samples from Asian and African elephants: Identification of two new probosciviruses (EEHV5 and EEHV6) and two new gammaherpesviruses (EGHV3B and EGHV5). Vet. Microbiol. 2011, 147, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Abegglen, L.M.; Fuery, A.; Kiso, W.K.; Schmitt, D.L.; Ling, P.D.; Schiffman, J.D. Mammalia: Proboscidea: Elephant Immune System. In Advances in Comparative Immunology; Cooper, E.L., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 863–883. ISBN 978-3-319-76768-0. [Google Scholar]
- Atkins, L.; Zong, J.C.; Tan, J.; Mejia, A.; Heaggans, S.Y.; Nofs, S.A.; Stanton, J.J.; Flanagan, J.P.; Howard, L.; Latimer, E.; et al. Elephant endotheliotropic herpesvirus 5, a newly recognized elephant herpesvirus associated with clinical and subclinical infections in captive Asian elephants (Elephas maximus). J. Zoo Wildl. Med. 2013, 44, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanton, J.J.; Zong, J.C.; Eng, C.; Howard, L.; Flanagan, J.; Stevens, M.; Schmitt, D.; Wiedner, E.; Graham, D.; Junge, R.E.; et al. Kinetics of viral loads and genotypic analysis of elephant endotheliotropic herpesvirus-1 infection in captive Asian elephant (Elephas maximus). J. Zoo Wildl. Med. Off. Publ. Am. Assoc. Zoo Vet. 2013, 44, 42–54. [Google Scholar] [CrossRef] [Green Version]
- Richman, L.K.; Montali, R.J.; Cambre, R.C.; Schmitt, D.; Hardy, D.; Hildbrandt, T.; Bengis, R.G.; Hamzeh, F.M.; Shahkolahi, A.; Hayward, G.S. Clinical and pathological findings of a newly recognized disease of elephants caused by endotheliotropic herpesviruses. J. Wildl. Dis. 2000, 36, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuery, A.; Tan, J.; Peng, R.; Flanagan, J.P.; Tocidlowski, M.E.; Howard, L.L.; Ling, P.D. Clinical infection of two captive Asian elephants (Elephas maximus) with elephant endotheliotropic herpesvirus 1b. J. Zoo Wildl. Med. 2016, 47, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Fuery, A.; Browning, G.R.; Tan, J.; Long, S.; Hayward, G.S.; Cox, S.K.; Flanagan, J.P.; Tocidlowski, M.E.; Howard, L.L.; Ling, P.D. Clinical infection of captive Asian elephants (Elephas maximus) with elephant endotheliotropic herpesvirus 4. J. Zoo Wildl. Med. 2016, 47, 311–318. [Google Scholar] [CrossRef]
- Howard, L.L.; Schaftenaar, W. 95-Elephant Endotheliotropic Herpesvirus. In Fowler’s Zoo and Wild Animal Medicine Current Therapy; Miller, R.E., Lamberski, N., Calle, P.P., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2019; Volume 9, pp. 672–679. ISBN 978-0-323-55228-8. [Google Scholar]
- Hengtrakul, P.; Sudlapa, P.; Chaisurat, N.; Sodsaengthien, S.; Chamnankij, C.; Noimoon, S.; Punkong, C.; Phatthanakunanan, S.; Lertwatcharasarakul, P.; Sripiboon, S. Biological and environmental factors associated with the detection of elephant endotheliotropic herpesvirus in Asian elephants (Elephas maximus) in Thailand. J. Vet. Med. Sci. 2020, 82, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.E.; Mikota, S.K.; Hedges, S. Biology, Medicine, and Surgery of Elephants, 1st ed.; Blackwell Publishing Ltd.: Iowa, IA, USA, 2006; pp. 325–345. [Google Scholar]
- Janyamethakul, T.; Sripiboon, S.; Somgird, C.; Pongsopawijit, P.; Panyapornwithaya, V.; Klinhom, S.; Loythong, J.; Thitaram, C. Hematologic and biochemical reference intervals for captive Asian elephants (Elephas maximus) in Thailand. Kafkas Univ. Vet. Fak. Derg. 2017, 23, 665–669. [Google Scholar] [CrossRef]
- Perrin, K.L.; Kristensen, A.T.; Gray, C.; Nielsen, S.S.; Bertelsen, M.F.; Kjelgaard-Hansen, M. Biological variation of hematology and biochemistry parameters for the Asian elephant (Elephas maximus), and applicability of population derived reference intervals. J. Zoo Wildl. Med. 2020, 51, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Schaftenaar, W.; Reid, C.; Martina, B.; Fickel, J.; Osterhaus, A.D. Nonfatal clinical presentation of elephant endotheliotropic herpes virus discovered in a group of captive Asian elephants (Elephas maximus). J. Zoo Wildl. Med. 2010, 41, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Hardman, K.; Dastjerdi, A.; Gurrala, R.; Routh, A.; Banks, M.; Steinbach, F.; Bouts, T. Detection of elephant endotheliotropic herpesvirus type 1 in asymptomatic elephants using TaqMan real-time PCR. Vet. Rec. 2012, 170, 205. [Google Scholar] [CrossRef]
- Sripiboon, S.; Tankaew, P.; Lungka, G.; Thitaram, C. The occurrence of elephant endotheliotropic herpesvirus in captive Asian elephants (Elephas maximus): First case of eehv 4 in Asia. J. Zoo Wildl. Med. 2013, 44, 100–104. [Google Scholar] [CrossRef]
- Sripiboon, S.; Angkawanish, T.; Boonprasert, K.; Sombutputorn, P.; Langkaphin, W.; Ditcham, W.; Warren, K. Successful treatment of a clinical elephant endotheliotropic herpesvirus Infection: The dynamics of viral load, genotype analysis, and treatment with acyclovir. J. Zoo Wildl. Med. 2017, 48, 1254–1259. [Google Scholar] [CrossRef]
- Azab, W.; Damiani, A.M.; Ochs, A.; Osterrieder, N. Subclinical infection of a young captive Asian elephant with elephant endotheliotropic herpesvirus 1. Arch. Virol. 2018, 163, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Kochagul, V.; Srivorakul, S.; Boonsri, K.; Somgird, C.; Sthitmatee, N.; Thitaram, C.; Pringproa, K. Production of antibody against elephant endotheliotropic herpesvirus (EEHV) unveils tissue tropisms and routes of viral transmission in EEHV-infected Asian elephants. Sci. Rep. 2018, 8, 4675. [Google Scholar] [CrossRef]
- Yun, Y.; (Center of Elephant and Wildlife Research, Faculty of Veterinary Medicine, Chiang Mai University, Chiang Mai, Thailand). Personal communication, 2021.
- Kendall, R.; Howard, L.; Masters, N.; Grant, R. The impact of elephant endotheliotropic herpesvirus on the captive Asian elephant (Elephas maximus) population of the United Kingdom and Ireland (1995–2013). J. Zoo Wildl. Med. 2016, 47, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Walker, S.L.; Moeller, T. Comparative endocrinology of cycling and non-cycling Asian (Elephas maximus) and African (Loxodonta africana) Elephants. Gen. Comp. Endocrinol. 2004, 136, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.A.; Papageorge, S.; Wasser, S.K. Noninvasive stress and reproductive measures of social and ecological pressures in free-ranging African elephants. Conserv. Biol. 2001, 15, 1134–1142. [Google Scholar] [CrossRef]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Millspaugh, J.J.; Burke, T.; Van Dyk, G.; Slotow, R.; Washburn, B.E.; Woods, R.J. Stress response of working African elephants to transportation and safari adventures. J. Wildl. Manag. 2007, 71, 1257–1260. [Google Scholar] [CrossRef] [Green Version]
- Dathe, H.H.; Kuckelkorn, B.; Minnemann, D. Salivary cortisol assessment for stress detection in the Asian elephant (Elephas maximus): A pilot study. Zoo Biol. 1992, 11, 285–289. [Google Scholar] [CrossRef]
- Laws, N.; Ganswindt, A.; Heistermann, M.; Harris, M.; Harris, S.; Sherwin, C. A case study: Fecal corticosteroid and behavior as indicators of welfare during relocation of an Asian elephant. J. Appl. Anim. Welf. Sci. 2007, 10, 349–358. [Google Scholar] [CrossRef]
- Marcilla, A.; Urios, V.; Mauri, M. Welfare assessment of captive Asian elephants (Elephas Maximus) and Indian rhinoceros (Rhinoceros unicornis) using salivary cortisol measurement. Anim. Welf. 2008, 17, 305–312. [Google Scholar]
- Menargues, A.; Urios, V.; Limiñana, R.; Mauri, M. Circadian rhythm of salivary cortisol in Asian elephants (Elephas maximus): A factor to consider during welfare assessment. J. Appl. Anim. Welf. Sci. 2012, 15, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansiddhi, P.; Brown, J.L.; Khonmee, J.; Norkaew, T.; Nganvongpanit, K.; Punyapornwithaya, V.; Angkawanish, T.; Somgird, C.; Thitaram, C. Management factors affecting adrenal glucocorticoid activity of tourist camp elephants in Thailand and implications for elephant welfare. PLoS ONE 2019, 14, e0221537. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Pradheeps, M.; Kokkiligadda, A.; Niyogi, R.; Umapathy, G. Non-invasive assessment of physiological stress in captive Asian elephants. Animals 2019, 9, 553. [Google Scholar] [CrossRef] [Green Version]
- Staley, M.; Conners, M.G.; Hall, K.; Miller, L.J. Linking stress and immunity: Immunoglobulin A as a non-invasive physiological biomarker in animal welfare studies. Horm. Behav. 2018, 102, 55–68. [Google Scholar] [CrossRef]
- Tsujita, S.; Morimoto, K. Secretory IgA in saliva can be a useful stress marker. Environ. Health Prev. Med. 1999, 4, 1–8. [Google Scholar] [CrossRef]
- Chintalacharuvu, K.R.; Morrison, S.L. Production of secretory immunoglobulin A by a single mammalian cell. Proc. Natl. Acad. Sci. USA 1997, 94, 6364–6368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muneta, Y.; Yoshikawa, T.; Minagawa, Y.; Shibahara, T.; Maeda, R.; Omata, Y. Salivary IgA as a useful non-invasive marker for restraint stress in pigs. J. Vet. Med. Sci. 2010, 72, 1295–1300. [Google Scholar] [CrossRef] [Green Version]
- Escribano, D.; Gutiérrez, A.M.; Tecles, F.; Cerón, J.J. Changes in saliva biomarkers of stress and immunity in domestic pigs exposed to a psychosocial stressor. Res. Vet. Sci. 2015, 102, 38–44. [Google Scholar] [CrossRef]
- Campos-Rodríguez, R.; Godínez-Victoria, M.; Abarca-Rojano, E.; Pacheco-Yépez, J.; Reyna-Garfias, H.; Barbosa-Cabrera, R.E.; Drago-Serrano, M.E. Stress modulates intestinal secretory immunoglobulin A. Front. Integr. Neurosci. 2013, 7, 86. [Google Scholar] [CrossRef] [Green Version]
- Kosaruk, W.; Brown, J.L.; Plangsangmas, T.; Towiboon, P.; Punyapornwithaya, V.; Silva-Fletcher, A.; Thitaram, C.; Khonmee, J.; Edwards, K.L.; Somgird, C. Effect of tourist activities on fecal and salivary glucocorticoids and immunoglobulin A in female captive Asian elephants in Thailand. Animals 2020, 10, 1928. [Google Scholar] [CrossRef]
- Casares, M.; Silván, G.; Carbonell, M.D.; Gerique, C.; Martinez-Fernandez, L.; Cáceres, S.; Illera, J.C. Circadian rhythm of salivary cortisol secretion in female zoo-kept African elephants (Loxodonta africana). Zoo Biol. 2016, 35, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Plangsangmas, T.; Brown, J.L.; Thitaram, C.; Silva-Fletcher, A.; Edwards, K.L.; Punyapornwithaya, V.; Towiboon, P.; Somgird, C. Circadian rhythm of salivary immunoglobulin A and associations with cortisol as a stress biomarker in captive Asian elephants (Elephas maximus). Animals 2020, 10, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, V.; Koh, D.; Chan, G.; Ong, H.Y.; Chia, S.E.; Ong, C.N. Are salivary immunoglobulin A and lysozyme biomarkers of stress among nurses? J. Occup. Environ. Med. 1999, 41, 920–927. [Google Scholar] [CrossRef]
- Edwards, K.L.; Bansiddhi, P.; Paris, S.; Galloway, M.; Brown, J.L. The development of an immunoassay to measure immunoglobulin A in Asian elephant feces, saliva, urine, and serum as a potential biomarker of well-being. Conserv. Physiol. 2019, 7, coy077. [Google Scholar] [CrossRef] [PubMed]
- Chuammitri, P.; Srikok, S.; Saipinta, D.; Boonyayatra, S. The effects of quercetin on microRNA and inflammatory gene expression in lipopolysaccharide-stimulated bovine neutrophils. Vet. World 2017, 10, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Seilern-Moy, K.; Darpel, K.; Steinbach, F.; Dastjerdi, A. Distribution and load of elephant endotheliotropic herpesviruses in tissues from associated fatalities of Asian elephants. Virus Res. 2016, 220, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Loy, A.; Hofmann, H. Qqplotr: Quantile-Quantile Plot Extensions for “ggplot2”. 2020. Available online: https://cran.r-project.org/package=qqplotr (accessed on 11 August 2020).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Nlme: Linear and Nonlinear Mixed Effects Models. 2020. Available online: https://cran.r-project.org/package=nlme (accessed on 11 August 2020).
- Reid, C.E.; Hildebrandt, T.B.; Marx, N.; Hunt, M.; Thy, N.; Reynes, J.M.; Schaftenaar, W.; Fickel, J. Endotheliotropic elephant herpes Virus (EEHV) infection. The first PCR-confirmed fatal case in Asia. Vet. Q. 2006, 28, 61–64. [Google Scholar] [CrossRef]
- Boyd, J.W. The relationships between blood haemoglobin concentration, packed cell volume and plasma protein concentration in dehydration. Br. Vet. J. 1981, 137, 166–172. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, S.H.; Ryu, J.K. Changes in the blood components caused by water intake. Korean J. Clin. Lab. Sci. 2017, 49, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Fielding, C.L.; Magdesian, K.G. Review of packed cell volume and total protein for use in equine practice. In Proceedings of the AAEP Annual Convention-San Antonio, San Antonio, TX, USA, 20 November; 2011; Volume 57, pp. 318–321. [Google Scholar]
- Liu, B.; Taioli, E. Seasonal variations of complete blood count and inflammatory biomarkers in the US population—Analysis of NHANES data. PLoS ONE 2015, 10, e0142382. [Google Scholar] [CrossRef]
- Lombardi, G.; Ricci, C.; Banfi, G. Effects of winter swimming on haematological parameters. Biochem. Medica. 2011, 21, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Ahmad, N.; Ahmad, I.; Mahmood, S.A.; Andrabi, S.M.; Idris, M. Effect of Seasonal variations on the haematochemical profile of cholistani service bulls. J. Appl. Anim. Res. 2017, 45, 85–89. [Google Scholar] [CrossRef]
- Giri, A.; Bharti, V.K.; Kalia, S.; Ravindran, V.; Ranjan, P.; Kundan, T.; Kumar, B. Seasonal changes in haematological and biochemical profile of dairy cows in high altitude cold desert. Indian J. Anim. Sci. 2017, 87, 723–727. [Google Scholar]
- Norkaew, T.; Brown, J.L.; Thitaram, C.; Bansiddhi, P.; Somgird, C.; Punyapornwithaya, V.; Punturee, K.; Vongchan, P.; Somboon, N.; Khonmee, J. Associations among tourist camp management, high and low tourist seasons, and welfare factors in female Asian elephants in Thailand. PLoS ONE 2019, 14, e0218579. [Google Scholar] [CrossRef]
- Dorsey, C.; Dennis, P.; Guagnano, G.; Wood, T.; Brown, J.L. Decreased baseline fecal glucocorticoid concentrations associated with skin and oral lesions in black rhinoceros (Diceros bicornis). J. Zoo Wildl. Med. Off. Publ. Am. Assoc. Zoo Vet. 2010, 41, 616–625. [Google Scholar] [CrossRef]
- Maeda, S.; Ohno, K.; Uchida, K.; Nakashima, K.; Fukushima, K.; Tsukamoto, A.; Nakajima, M.; Fujino, Y.; Tsujimoto, H. Decreased immunoglobulin A concentrations in feces, duodenum, and peripheral blood mononuclear cells of dogs with inflammatory bowel disease. J. Vet. Intern. Med. 2013, 27, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; Mormède, P. Stress in farm animals: A need for reevaluation. J. Anim. Sci. 1983, 57, 6–18. [Google Scholar] [CrossRef]
- Huber, S.; Palme, R.; Arnold, W. Effects of season, sex, and sample collection on concentrations of fecal cortisol metabolites in Red Deer (Cervus elaphus). Gen. Comp. Endocrinol. 2003, 130, 48–54. [Google Scholar] [CrossRef]
- Norkaew, T.; Brown, J.L.; Bansiddhi, P.; Somgird, C.; Thitaram, C.; Punyapornwithaya, V.; Punturee, K.; Vongchan, P.; Somboon, N.; Khonmee, J. Body condition and adrenal glucocorticoid activity affects metabolic marker and lipid profiles in captive female elephants in Thailand. PLoS ONE 2018, 13, e0204965. [Google Scholar] [CrossRef] [Green Version]
- Mumby, H.S.; Mar, K.U.; Thitaram, C.; Courtiol, A.; Towiboon, P.; Min-Oo, Z.; Htut-Aung, Y.; Brown, J.L.; Lummaa, V. Stress and body condition are associated with climate and demography in Asian elephants. Conserv. Physiol. 2015, 3, cov030. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.L.; Paris, S.; Prado-Oviedo, N.A.; Meehan, C.L.; Hogan, J.N.; Morfeld, K.A.; Carlstead, K. Reproductive health assessment of female elephants in north American zoos and association of husbandry practices with reproductive dysfunction in African elephants (Loxodonta africana). PLoS ONE 2016, 11, e0145673. [Google Scholar] [CrossRef]
- Rees, A.; Fischer-Tenhagen, C.; Heuwieser, W. Effect of heat stress on concentrations of faecal cortisol metabolites in dairy cows. Reprod. Domest. Anim. 2016, 51, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Veissier, I.; Van laer, E.; Palme, R.; Moons, C.P.; Ampe, B.; Sonck, B.; Andanson, S.; Tuyttens, F.A. Heat stress in cows at pasture and benefit of shade in a temperate climate region. Int. J. Biometeorol. 2018, 62, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Narayan, E.; Sawyer, G.; Parisella, S. Faecal glucocorticoid metabolites and body temperature in Australian merino ewes (Ovis aries) during summer artificial insemination (AI) program. PLoS ONE 2018, 13, e0191961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keay, J.; Singh, J.; Gaunt, M.; Kaur, T. Fecal glucocorticoids, and their metabolites as indicators of stress in various mammalian species: A literature review. J. Zoo Wildl. Med. 2006, 37, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.C. Essentials of Veterinary Hematology, 1st ed.; Wiley-Blackwell: Philadelphia, PA, USA, 1993; ISBN 978-0-8121-1437-9. [Google Scholar]
- Kumar, B.; Pachauri, S.P. Haematological profile of crossbred dairy cattle to monitor herd health status at medium elevation in central himalayas. Res. Vet. Sci. 2000, 69, 141–145. [Google Scholar] [CrossRef] [PubMed]



| ID | Camp | Gender | Weaning Age (Months) | Age at Start of Study (Months) | EEHV Category | Detection of EEHV | EEHV Subtype | Clinical Signs and Treatment | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Date | Age (Months) | Test | Highest Viral Load (vgc/mL) | ||||||||
| E1 | A | Female | 36 | 77 | Prior-EEHV 1 | July 2017 | 60 | Conventional PCR | negative | 4 | Signs: Depression and diarrhea |
| Tx: Acyclovir, fluids, and supportive therapies 4 | |||||||||||
| E2 | B | Male | 30 | 60 | Non-EEHV 2 | N/A | N/A | N/A | |||
| E3 | C | Female | 30 | 39 | Prior-EEHV | October 2018 | 36 | Real-time PCR | 1,152,837 | 1A | Signs: Lethargy, depression, and facial edema |
| Tx: Acyclovir, fluids, and supportive therapies 4 | |||||||||||
| E4 | D | Female | 15 | 35 | Prior-EEHV | July 2018 | 28 | Real-time PCR | 25,607 | 4 | Signs: Depressing and diarrhea |
| Tx: Acyclovir, fluids, and supportive therapies 4 | |||||||||||
| E5 | E | Male | 12 | 20 | Prior-EEHV 3 | June 2018 and June 2019 | 14 and 26 | Real-time PCR | 13 and 6,902,328 | 1A | 1st infection |
| Signs: Lethargy, depression, and facial edema | |||||||||||
| Tx: Famciclovir, fluids, and supportive therapies | |||||||||||
| 2nd infection | |||||||||||
| Signs: Lethargy, depression, fever, and facial edema, diarrhea | |||||||||||
| Tx: Acyclovir, fluids, and supportive therapies 4 | |||||||||||
| E6 | A | Female | 35 | 78 | Prior-EEHV | July 2018 | 72 | Conventional PCR | negative | 4 | Signs: Depression and diarrhea |
| Tx: Acyclovir, fluids, and supportive therapies 4 | |||||||||||
| E7 | F | Male | 12 | 24 | Prior-EEHV | July 2018 | 18 | Real-time PCR | 1,306,864 | 4 | Signs: Depression and diarrhea |
| Tx: Acyclovir, fluids, and supportive therapies 4 | |||||||||||
| E8 | B | Male | 34 | 60 | Non-EEHV | N/A | N/A | N/A | |||
| E9 | B | Male | 34 | 48 | Non-EEHV | N/A | N/A | N/A | |||
| Parameters | Study Means | 18 December 2018 | 9 January 2019 | 8 February 2019 | 8 March 2019 | 6 April 2019 | 6 May 2019 | 4 June 2019 | 16 June 2019 | 17 June 2019 | 18 June 2019 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Viral load (vgc/mL) | - | Undetected | Undetected | Undetected | Undetected | Undetected | Undetected | Undetected | 6,902,328 | 4,845,925 | 5,744,321 |
| PCV (%) | 37.3 ± 0.38 | 30 | 31 | 36 | 33 | 36 | 33 | 32 | 29 | 30 | 31 |
| RBC (×10 6 cells/μL) | 3.10 ± 0.03 | 2.81 | 2.93 | 3.37 | 3.08 | 3.33 | 3.02 | 2.88 | 2.73 | 3.29 | 2.95 |
| WBC (cells/μL) | 18825 ± 351 | 16,230 | 17,760 | 20,640 | 22,300 | 15,370 | 15,010 | 14,140 | 10,110 | 14,300 | 8330 |
| Heterophils (cells/μL) | 4247 ± 136 | 3246 | 2309 | 7018 | 10,704 | 4765 | 3753 | 3394 | 5561 | 8065 | 5081 |
| Lymphocytes (cells/μL) | 10,675 ± 358 | 10,063 | 12,077 | 8050 | 9143 | 9222 | 8256 | 7353 | 2325 | 2054 | 1833 |
| Monocytes (cells/μL) | 3459 ± 219 | 2434 | 2842 | 5366 | 2230 | 1383 | 3002 | 2969 | 2224 | 700 | 1333 |
| Eosinophile (cells/μL) | 296 ± 28 | 487 | 532 | 206 | 618 | 0 | 0 | 428 | 143 | 0 | 83 |
| Platelets (×10 3 cells/μL) | 458 ± 8 | 515 | 553 | 802 | 541 | 459 | 580 | 540 | 194 | 98 | 74 |
| Monocyte/Heterophil ratio | 0.87 ± 0.05 | 0.75 | 1.23 | 0.76 | 0.21 | 0.29 | 0.80 | 0.87 | 0.40 | 0.09 | 0.26 |
| Salivary cortisol (ng/mL) | 1.17 ± 0.08 | 1.03 | 0.91 | 1.57 | 0.78 | 1.27 | 0.40 | 0.39 | No sample | No sample | No sample |
| FGM (ng/g) | 36.1 ± 3.19 | 72.30 | 62.59 | 34.37 | 39.26 | 103.11 | 48.73 | 15.56 | No sample | No sample | No sample |
| SIgA (µg/mL) | 3.53 ± 0.31 | 3.70 | 4.98 | 5.50 | 5.04 | 5.49 | 2.78 | 1.70 | No sample | No sample | No sample |
| FIgA (µg/mL) | 1.85 ± 0.10 | 2.42 | 2.57 | 1.41 | 1.55 | 12.22 | 2.57 | 0.94 | No sample | No sample | No sample |
| Parameter | Elephant ID (Blood Sample Number) | Range | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E1 (n = 13) | E2 (n = 13) | E3 (n = 13) | E4 (n = 13) | E5 * (n = 7) | E6 (n = 13) | E7 (n = 13) | E8 (n = 13) | E9 (n = 13) | ||
| PCV (%) | 42.40 ± 1.08 | 33.50 ± 0.80 | 37.60 ± 1.18 | 36.00 ± 0.30 | 28.90 ± 3.62 | 36.90 ± 0.57 | 35.40 ± 1.11 | 35.70 ± 1.03 | 35.40 ± 1.38 | 32.25–43.80 |
| RBC (×10 6 cells/μL) | 3.37 ± 0.08 | 2.84 ± 0.08 | 3.10 ± 0.12 | 2.88 ± 0.02 | 2.68 ± 0.37 | 3.00 ± 0.06 | 2.93 ± 0.99 | 2.95 ± 0.09 | 2.89 ± 0.08 | 2.17–3.47 |
| WBC (cells/μL) | 17,731 ± 1245 | 19,700 ± 1236 | 17,979 ± 942 | 16,124 ± 709 | 15,181 ± 4051 | 17,262 ± 611 | 17,523 ± 489 | 17,165 ± 910 | 18,257 ± 3088 | 14,810–21,990 |
| Heterophils (cells/μL) | 5147 ± 1246 | 4467 ± 303 | 3687 ± 384 | 4629 ± 500 | 4300 ± 1421 | 4176 ± 539 | 4421 ± 274 | 4421 ± 282 | 3989 ± 274 | 2920–7587 |
| Lymphocyte (cells/μL) | 10,051 ± 1033 | 11,928 ± 877 | 10,633 ± 751 | 7982 ± 956 | 8020 ± 181 | 8725 ± 813 | 9698 ± 301 | 9367 ± 569 | 10,706 ± 1865 | 6918–13,980 |
| Monocytes (cells/μL) | 3059 ± 192 | 3817 ± 892 | 3176 ± 153 | 3298 ± 678 | 2528 ± 690 | 3175 ± 143 | 3176 ± 333 | 3167 ± 342 | 3111 ± 352 | 2316–5578 |
| Eosinophils (cells/μL) | 374 ± 80 | 321 ± 53 | 265 ± 33 | 143 ± 96 | 2234 ± 128 | 413 ± 50 | 282 ± 37 | 276 ± 35 | 208 ± 35 | 0–535 |
| Platelets (×10 3 cells/μL) | 552 ± 23 | 465 ± 14 | 464 ± 18 | 463 ± 20 | 499 ± 104 | 424 ± 39 | 466 ± 15 | 465 ± 18 | 416 ± 8 | 374–554 |
| M:H ratio | 0.66 ± 0.16 | 0.84 ± 0.13 | 0.90 ± 0.14 | 0.74 ± 0.17 | 0.58 ± 0.03 | 0.77 ± 0.07 | 0.72 ± 0.05 | 0.74 ± 0.05 | 0.78 ± 0.13 | 0.41–1.17 |
| Variables | Mean Value in the Study (n = 104) | Range | Fowler et al. (2006) | Janyamethakul et al. (2017) | |
|---|---|---|---|---|---|
| Male | Female | ||||
| PCV (%) | 37.3 ± 0.38 | 32.25–43.80 | 30–40 | 29.40–40.70 | 27.80–43.00 |
| RBC (×10 6 cells/μL) | 3.10 ± 0.03 | 2.17–3.47 | 2.00–5.00 | 1.90–3.20 | 1.90–3.10 |
| WBC (cells/μL) | 18,825 ± 351 | 14,810–21,990 | 10,000–18,000 | 7924–21,890 | 7202.50–23,220.50 |
| Heterophils (cells/μL) | 4247 ± 136 | 2920–7587 | 2000–4000 | 967–13426 | 828.70–13,514.30 |
| Lymphocytes (cells/μL) | 10,675 ± 358 | 6918–13,980 | 5000–8000 | 1672–11,179 | 1064.10–12,032.80 |
| Monocytes (cells/μL) | 3459 ± 219 | 2316–5578 | 2000–4000 | 0–2391 | 0–3298 |
| Eosinophils (cells/μL) | 296 ± 28 | 0–535 | 100–1000 | 0–867 | 0–1170 |
| Platelets (×10 3 cells/μL) | 458 ± 8.38 | 374–554 | 200–600 | 102–578 | 105.30–598.70 |
| Monocyte/Heterophil ratio | 0.87 ± 0.05 | 0.41–1.17 | 1.18–3.57 | ND | ND |
| Salivary cortisol (ng/mL) | 1.17 ± 0.08 | 0.65–1.95 | ND | ND | ND |
| FGM (ng/mL) | 36.1 ± 3.19 | 23.55–53.51 | ND | ND | ND |
| SIgA (µg/mL) | 3.53 ± 0.31 | 1.28–12.60 | ND | ND | ND |
| FIgA (µg/mL) | 1.85 ± 0.10 | 1.33 9.04 | ND | ND | ND |
| Variable | Elephant ID | Range | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E1 (n = 12) | E2 (n = 12) | E3 (n = 12) | E4 (n = 12) | E5 * (n = 7) | E6 (n = 12) | E7 (n = 12) | E8 (n = 12) | E9 (n = 12) | ||
| Salivary cortisol (ng/mL) | 1.40 ± 0.18 | 0.72 ± 0.04 | 0.97 ± 0.02 | 1.40 ± 0.30 | 0.75 ± 0.32 | 1.33 ± 0.21 | 1.06 ± 0.13 | 1.10 ± 0.12 | 0.90 ± 0.01 | 0.65–1.95 |
| FGM (ng/mL) | 31.3 ± 4.14 | 36.10 ± 5.02 | 36.00 ± 4.85 | 39.8 ± 5.83 | 47.00 ± 5.14 | 32.00 ± 4.10 | 36.70 ± 3.72 | 37.00 ± 3.47 | 40.90 ± 8.21 | 23.55–53.51 |
| SIgA (µg/mL) | 2.63 ± 0.66 | 7.85 ± 293 | 4.66 ± 1.18 | 4.328 ± 1.70 | 3.66 ± 1.15 | 4.33 ± 184 | 5.14 ± 1.75 | 4.72 ± 1.45 | 3.91 ± 0.61 | 1.28–12.60 |
| FIgA (µg/mL) | 2.43 ± 0.38 | 5.00 ± 2.23 | 3.38 ± 1.08 | 2.46 ± 0.55 | 4.38 ± 1.75 | 2.16 ± 0.15 | 3.23 ± 0.80 | 2.97 ± 0.53 | 3.18 ± 0.74 | 1.33–9.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonprasert, K.; Yun, Y.; Kosaruk, W.; Towiboon, P.; Tankaew, P.; Punyapornwithaya, V.; Janyamathakul, T.; Muanghong, P.; Brown, J.L.; Thitaram, C.; et al. A Longitudinal Study of Hematology and Stress Biomarker Profiles in Young Asian Elephants (Elephas Maximus) in Relation to Elephant Endotheliotropic Herpesvirus (EEHV) in Thailand. Animals 2021, 11, 2530. https://doi.org/10.3390/ani11092530
Boonprasert K, Yun Y, Kosaruk W, Towiboon P, Tankaew P, Punyapornwithaya V, Janyamathakul T, Muanghong P, Brown JL, Thitaram C, et al. A Longitudinal Study of Hematology and Stress Biomarker Profiles in Young Asian Elephants (Elephas Maximus) in Relation to Elephant Endotheliotropic Herpesvirus (EEHV) in Thailand. Animals. 2021; 11(9):2530. https://doi.org/10.3390/ani11092530
Chicago/Turabian StyleBoonprasert, Khajohnpat, Yaoprapa Yun, Worapong Kosaruk, Patcharapa Towiboon, Pallop Tankaew, Veerasak Punyapornwithaya, Thittaya Janyamathakul, Panida Muanghong, Janine L. Brown, Chatchote Thitaram, and et al. 2021. "A Longitudinal Study of Hematology and Stress Biomarker Profiles in Young Asian Elephants (Elephas Maximus) in Relation to Elephant Endotheliotropic Herpesvirus (EEHV) in Thailand" Animals 11, no. 9: 2530. https://doi.org/10.3390/ani11092530
APA StyleBoonprasert, K., Yun, Y., Kosaruk, W., Towiboon, P., Tankaew, P., Punyapornwithaya, V., Janyamathakul, T., Muanghong, P., Brown, J. L., Thitaram, C., & Somgird, C. (2021). A Longitudinal Study of Hematology and Stress Biomarker Profiles in Young Asian Elephants (Elephas Maximus) in Relation to Elephant Endotheliotropic Herpesvirus (EEHV) in Thailand. Animals, 11(9), 2530. https://doi.org/10.3390/ani11092530









