Effects of Saccharomyces cerevisiae Culture on Ruminal Fermentation, Blood Metabolism, and Performance of High-Yield Dairy Cows
Abstract
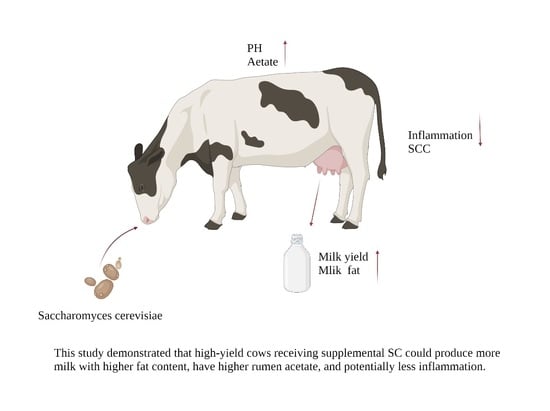
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Data and Sample Collection
2.2.1. Milk Yield and Milk Profile
2.2.2. Blood Sample Collection and Chemical Composition Determination
2.2.3. Rumen Fluid Collection and Analysis
2.3. Statistical Analysis
3. Results
3.1. Effects of SC on Milk Yield and Profile
3.2. Effects of SC on Ruminal pH and VFA
3.3. Effects of SC on Hepatic Function and Energy Metabolism
3.4. Effects of SC on Inflammatory Cytokine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oltenacu, P.A.; Broom, D.M. The impact of genetic selection for increased milk yield on the welfare of dairy cows. Anim. Welf. 2010, 19, 39–49. [Google Scholar]
- Walker, G.; Dunshea, F.; Doyle, P. Effects of nutrition and management on the production and composition of milk fat and protein: A review. Aust. J. Agric. Res. 2004, 55, 1009–1028. [Google Scholar] [CrossRef]
- Opsomer, G. Interaction between metabolic challenges and productivity in high yielding dairy cows. Jpn. J. Vet. Res. 2015, 63, S1–S14. [Google Scholar]
- Soltan, Y.; Morsy, A.; Hashem, N.; Sallam, S. Boswellia sacra resin as a phytogenic feed supplement to enhance ruminal fermentation, milk yield, and metabolic energy status of early lactating goats. Anim. Feed Sci. Technol. 2021, 277, 114963. [Google Scholar] [CrossRef]
- Hashem, N.M.; El-Zarkouny, S.Z. Metabolic attributes, milk production and ovarian activity of ewes supplemented with a soluble sugar or a protected-fat as different energy sources during postpartum period. Ann. Anim. Sci. 2017, 17, 229. [Google Scholar] [CrossRef] [Green Version]
- Olagaray, K.; Sivinski, S.; Saylor, B.; Mamedova, L.; Sauls-Hiesterman, J.; Yoon, I.; Bradford, B. Effect of Saccharomyces cerevisiae fermentation product on feed intake parameters, lactation performance, and metabolism of transition dairy cattle. J. Dairy Sci. 2019, 102, 8092–8107. [Google Scholar] [CrossRef]
- Bruno, R.G.; Rutigliano, H.M.; Cerri, R.; Robinson, P.H.; Santos, J.E. Effect of feeding Saccharomyces cerevisiae on performance of dairy cows during summer heat stress. Anim. Feed Sci. Technol. 2009, 150, 175–186. [Google Scholar] [CrossRef]
- Obeidat, B.S.; Mahmoud, K.Z.; Obeidat, M.D.; Ata, M.; Kridli, R.T.; Haddad, S.G.; Titi, H.H.; Jawasreh, K.I.; Altamimi, H.J.; Subih, H.S. The effects of Saccharomyces cerevisiae supplementation on intake, nutrient digestibility, and rumen fluid pH in Awassi female lambs. Vet. World 2018, 11, 1015. [Google Scholar] [CrossRef] [Green Version]
- Poppy, G.; Rabiee, A.; Lean, I.; Sanchez, W.; Dorton, K.; Morley, P. A meta-analysis of the effects of feeding yeast culture produced by anaerobic fermentation of Saccharomyces cerevisiae on milk production of lactating dairy cows. J. Dairy Sci. 2012, 95, 6027–6041. [Google Scholar] [CrossRef] [Green Version]
- Ogunade, I.M.; McCoun, M. Effects of adding live Saccharomyces cerevisiae and Aspergillus-based enzyme extracts on ruminal fermentation, plasma polyamine concentrations, and fiber digestibility in beef steers fed a high-forage diet. Appl. Anim. Sci. 2021, 37, 21–26. [Google Scholar] [CrossRef]
- Ding, G.; Chang, Y.; Zhao, L.; Zhou, Z.; Ren, L.; Meng, Q. Effect of Saccharomyces cerevisiae on alfalfa nutrient degradation characteristics and rumen microbial populations of steers fed diets with different concentrate-to-forage ratios. J. Anim. Sci. Biotechnol. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Yoon, I.; Scott, M.; Khafipour, E.; Plaizier, J. Impact of Saccharomyces cerevisiae fermentation product and subacute ruminal acidosis on production, inflammation, and fermentation in the rumen and hindgut of dairy cows. Anim. Feed Sci. Technol. 2016, 211, 50–60. [Google Scholar] [CrossRef]
- Lynch, H.; Martin, S. Effects of Saccharomyces cerevisiae culture and Saccharomyces cerevisiae live cells on in vitro mixed ruminal microorganism fermentation. J. Dairy Sci. 2002, 85, 2603–2608. [Google Scholar] [CrossRef]
- Lila, Z.; Mohammed, N.; Yasui, T.; Kurokawa, Y.; Kanda, S.; Itabashi, H. Effects of a twin strain of Saccharomyces cerevisiae live cells on mixed ruminal microorganism fermentation in vitro. J. Anim. Sci. 2004, 82, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Di Luccia, A.; Vincenti, D.; Cocconcelli, P.S. Effects of peptidic fractions from Saccharomyces cerevisiae culture on growth and metabolism of the ruminal bacteria Megasphaera elsdenii. Anim. Res. 2004, 53, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Cherdthong, A.; Prachumchai, R.; Supapong, C.; Khonkhaeng, B.; Wanapat, M.; Foiklang, S.; Milintawisamai, N.; Gunun, N.; Gunun, P.; Chanjula, P.; et al. Inclusion of yeast waste as a protein source to replace soybean meal in concentrate mixture on ruminal fermentation and gas kinetics using in vitro gas production technique. Anim. Prod. Sci. 2019, 59, 1682–1688. [Google Scholar] [CrossRef]
- Kim, H.S.; Ahn, B.S.; Chung, S.G.; Moon, Y.H.; Ha, J.K.; Seo, I.J.; Ahn, B.H.; Lee, S.S. Effect of yeast culture, fungal fermentation extract and non-ionic surfactant on performance of Holstein cows during transition period. Anim. Feed Sci. Technol. 2005, 126, 23–29. [Google Scholar] [CrossRef]
- Guedes, C.M.; Gonçalves, D.; Rodrigues, M.A.M.; Dias-da-Silva, A. Effects of a Saccharomyces cerevisiae yeast on ruminal fermentation and fibre degradation of maize silages in cows. Anim. Feed Sci. Technol. 2007, 145, 27–40. [Google Scholar] [CrossRef]
- Carpinelli, N.A.; Halfen, J.; Trevisi, E.; Chapman, J.D.; Sharman, E.D.; Anderson, J.L.; Osorio, J.S. Effects of peripartal yeast culture supplementation on lactation performance, blood biomarkers, rumen fermentation, and rumen bacteria species in dairy cows. J. Dairy Sci. 2021. [Google Scholar] [CrossRef]
- Paul, A.; Bhakat, C.; Mondal, S.; Mandal, A. An observational study investigating uniformity of manual body condition scoring in dairy cows. Ind. J. Dairy Sci. 2020, 73, 77–80. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle, 7th rev. ed.; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Shen, J.; Chai, Z.; Song, L.; Liu, J.; Wu, Y. Insertion depth of oral stomach tubes may affect the fermentation parameters of ruminal fluid collected in dairy cows. J. Dairy Sci. 2012, 95, 5978–5984. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Li, S.; Xing, J.; Ma, M.; Wang, L. Effects of maize grain and lucerne particle size on ruminal fermentation, digestibility and performance of cows in midlactation. J. Anim. Physiol. Anim. Nutr. 2008, 92, 157–167. [Google Scholar] [CrossRef]
- Tesfaye, A.; Hailu, Y. The effects of probiotics supplementation on milk yield and composition of lactating dairy cows. J. Phytopharm. 2019, 8, 12–17. [Google Scholar] [CrossRef]
- Davidson, S.; Hopkins, B.; Odle, J.; Brownie, C.; Fellner, V.; Whitlow, L. Supplementing limited methionine diets with rumen-protected methionine, betaine, and choline in early lactation Holstein cows. J. Dairy Sci. 2008, 91, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Z.; Cheng, Y.Y.; Wang, S.Q.; Ge, J.Z.; Shi, H.P.; Kou, J.C. Positive effects of dietary supplementation of three probiotics on milk yield, milk composition and intestinal flora in Sannan dairy goats varied in kind of probiotics. J. Anim. Physiol. Anim. Nutr. 2020, 104, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.M.; Oetzel, G.R. Understanding and preventing subacute ruminal acidosis in dairy herds: A review. Anim. Feed Sci. Technol. 2006, 126, 215–236. [Google Scholar] [CrossRef]
- Callaway, E.; Martin, S. Effects of a Saccharomyces cerevisiae culture on ruminal bacteria that utilize lactate and digest cellulose. J. Dairy Sci. 1997, 80, 2035–2044. [Google Scholar] [CrossRef]
- Williams, P.; Tait, C.; Innes, G.; Newbold, C. Effects of the inclusion of yeast culture (Saccharomyces cerevisiae plus growth medium) in the diet of dairy cows on milk yield and forage degradation and fermentation patterns in the rumen of steers. J. Anim. Sci. 1991, 69, 3016–3026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, G.; Hemken, R.; Dawson, K.; Harmon, R.; Barker, K. Influence of addition of yeast culture supplement to diets of lactating cows on ruminal fermentation and microbial populations. J. Dairy Sci. 1988, 71, 2967–2975. [Google Scholar] [CrossRef]
- Yoon, I.; Garrett, J.E. Yeast Culture and Processing Effects on 24-Hour in Situ Ruminal Degradation of Corn Silage. In Proceedings of the Proceeding of World Animal Production Conference, Seoul, Korea, 28 June–4 July 1998; pp. 322–323. [Google Scholar]
- Russell, J. The importance of pH in the regulation of ruminal acetate to propionate ratio and methane production in vitro. J. Dairy Sci. 1998, 81, 3222–3230. [Google Scholar] [CrossRef]
- Urrutia, N.L.; Harvatine, K.J. Acetate dose-dependently stimulates milk fat synthesis in lactating dairy cows. J. Nutr. 2017, 147, 763–769. [Google Scholar] [CrossRef] [Green Version]
- Urrutia, N.; Bomberger, R.; Matamoros, C.; Harvatine, K. Effect of dietary supplementation of sodium acetate and calcium butyrate on milk fat synthesis in lactating dairy cows. J. Dairy Sci. 2019, 102, 5172–5181. [Google Scholar] [CrossRef] [PubMed]
- Desnoyers, M.; Giger-Reverdin, S.; Bertin, G.; Duvaux-Ponter, C.; Sauvant, D. Meta-analysis of the influence of Saccharomyces cerevisiae supplementation on ruminal parameters and milk production of ruminants. J. Dairy Sci. 2009, 92, 1620–1632. [Google Scholar] [CrossRef]
- Thrune, M.; Bach, A.; Ruiz-Moreno, M.; Stern, M.; Linn, J. Effects of Saccharomyces cerevisiae on ruminal pH and microbial fermentation in dairy cows: Yeast supplementation on rumen fermentation. Livest. Sci. 2009, 124, 261–265. [Google Scholar] [CrossRef]
- Ogata, T.; Makino, H.; Ishizuka, N.; Iwamoto, E.; Masaki, T.; Ikuta, K.; Kim, Y.-H.; Sato, S. Long-term high-grain diet altered the ruminal pH, fermentation, and composition and functions of the rumen bacterial community, leading to enhanced lactic acid production in Japanese Black beef cattle during fattening. PLoS ONE 2019, 14, e0225448. [Google Scholar] [CrossRef]
- Kumprechtová, D.; Illek, J.; Julien, C.; Homolka, P.; Jančík, F.; Auclair, E. Effect of live yeast (Saccharomyces cerevisiae) supplementation on rumen fermentation and metabolic profile of dairy cows in early lactation. J. Anim. Physiol. Anim. Nutr. 2019, 103, 447–455. [Google Scholar] [CrossRef]
- Burdick Sanchez, N.C.; Broadway, P.R.; Carroll, J.A. Influence of yeast products on modulating metabolism and immunity in cattle and swine. Animals 2021, 11, 371. [Google Scholar] [CrossRef]
- Reist, M.; Erdin, D.; Von Euw, D.; Tschuemperlin, K.; Leuenberger, H.; Chilliard, Y.; Hammon, H.; Morel, C.; Philipona, C.; Zbinden, Y. Estimation of energy balance at the individual and herd level using blood and milk traits in high-yielding dairy cows. J. Dairy Sci. 2002, 85, 3314–3327. [Google Scholar] [CrossRef]
- Larsen, T.; Moyes, K. Are free glucose and glucose-6-phosphate in milk indicators of specific physiological states in the cow? Animal 2015, 9, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Lemosquet, S.; Delamaire, E.; Lapierre, H.; Blum, J.; Peyraud, J.-L. Effects of glucose, propionic acid, and nonessential amino acids on glucose metabolism and milk yield in Holstein dairy cows. J. Dairy Sci. 2009, 92, 3244–3257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khafipour, E.; Krause, D.; Plaizier, J. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J. Dairy Sci. 2009, 92, 1060–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmanuel, D.; Madsen, K.; Churchill, T.; Dunn, S.; Ametaj, B. Acidosis and lipopolysaccharide from Escherichia coli B: 055 cause hyperpermeability of rumen and colon tissues. J. Dairy Sci. 2007, 90, 5552–5557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozho, G.; Plaizier, J.; Krause, D.; Kennedy, A.; Wittenberg, K. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J. Dairy Sci. 2005, 88, 1399–1403. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zhu, W.; Jiang, L.; Mao, S. Comparative metabolome analysis of ruminal changes in Holstein dairy cows fed low-or high-concentrate diets. Metabolomics 2017, 13, 1–15. [Google Scholar] [CrossRef]
- Anzhi, L.; Changming, Q.; Hualin, C.; Lin, B.; Xiuqin, W.; Wencheng, W.; Qing, Y. Influence of Yikang XP on Plasma Endotoxin Concentration and Additional Indexes in Lactating Cows. China Dairy Cattle 2005, 2, 12–15. [Google Scholar]
- Li, S.; Khafipour, E.; Krause, D.; Kroeker, A.; Rodriguez-Lecompte, J.; Gozho, G.; Plaizier, J. Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows. J. Dairy Sci. 2012, 95, 294–303. [Google Scholar] [CrossRef]
- Ametaj, B.N.; Zebeli, Q.; Iqbal, S. Nutrition, microbiota, and endotoxin-related diseases in dairy cows. Rev. Bras. Zootec. 2010, 39, 433–444. [Google Scholar] [CrossRef] [Green Version]
- Russell, J.B.; Rychlik, J.L. Factors that alter rumen microbial ecology. Science 2001, 292, 1119–1122. [Google Scholar] [CrossRef]

| Items | Basal Diet (%) |
|---|---|
| Ingredients | |
| Whole corn silage | 26.8 |
| Alfalfa hay | 12.5 |
| Oat grass | 1.6 |
| Steamflaked corn | 11.6 |
| Extruded soybean meal | 1.6 |
| High yield concentrate 1 | 39.1 |
| Soybean hull | 1.2 |
| Fat power | 2.1 |
| Beet pulp | 1.1 |
| Sunflower meal | 0.8 |
| Molasses cane | 1.6 |
| Total | 100 |
| Nutrient levels | |
| NEL/(Mcal/kg) 2 | 1.8 |
| NDF | 30.7 |
| ADF | 20.4 |
| CP | 17.8 |
| EE | 5.9 |
| Starch | 27.3 |
| Calcium | 1.0 |
| Phosphorus | 0.5 |
| Items | CON 3 | SC 4 | p-Value |
|---|---|---|---|
| Milk yield (kg/d) | 46.58 ± 0.36 | 47.94 ± 0.29 | 0.003 |
| 3.5% FCM (kg/d) 1 | 50.03 ± 0.57 | 52.66 ± 0.52 | <0.001 |
| Milk composition | |||
| Milk fat (%) | 3.96 ± 0.16 | 4.11 ± 0.15 | 0.005 |
| Milk protein (%) | 3.29 ± 0.0.06 | 3.23 ± 0.05 | 0.54 |
| Milk lactose (%) | 5.19 ± 0.03 | 5.29 ± 0.03 | 0.046 |
| DM content (%) | 13.14 ± 0.16 | 13.84 ± 0.19 | 0.006 |
| SCC/(×104/mL) 2 | 32.4 ± 1.37 | 23.4 ± 0.98 | 0.005 |
| Item 1 | CON 2 | SC 3 | SEM (±) 4 | p-Value | ||
|---|---|---|---|---|---|---|
| Treatment | Time 5 | Interaction 6 | ||||
| TBIL (umol/L) | 7.75 | 7.18 | 1.68 | 0.185 | <0.001 | 0.204 |
| TP(g/L) | 77.47 | 73.97 | 3.48 | <0.001 | 0.088 | 0.575 |
| ALB (g/L) | 40.07 | 40.7 | 1.16 | 0.100 | 0.475 | 0.749 |
| ALP (U/L) | 50.75 | 55.95 | 13.88 | 0.225 | 0.227 | 0.167 |
| ALT (U/L) | 30.60 | 30.03 | 4.66 | 0.513 | <0.001 | 0.014 |
| Item 1 | CON 2 | SC 3 | SEM (±) 4 | p-Value | ||
|---|---|---|---|---|---|---|
| Treatment | Time 5 | Interaction 6 | ||||
| NEFA (umol/L) | 42.74 | 41.96 | 7.45 | 0.790 | 0.445 | 0.117 |
| BHBA(mmol/L) | 0.37 | 0.32 | 0.08 | 0.125 | 0.918 | 0.396 |
| GLU (mmol/L) | 4.25 | 4.34 | 0.50 | 0.493 | 0.001 | 0.568 |
| TG (mmol/L) | 0.25 | 0.27 | 0.04 | 0.253 | 0.772 | 0.296 |
| Item 1 | CON 2 | SC 3 | SEM (±) 4 | p-Value | ||
|---|---|---|---|---|---|---|
| Treatment | Time 5 | Interaction 6 | ||||
| IL-1β (ng/L) | 44.51 | 39.60 | 7.62 | 0.061 | 0.820 | 0.487 |
| IL-2 (pg/mL) | 205.73 | 180.30 | 33.17 | 0.025 | 0.928 | 0.978 |
| IL-6 (ng/L) | 519.06 | 454.81 | 72.67 | 0.009 | 0.994 | 0.854 |
| IL-10 (ng/L) | 214.84 | 201.77 | 26.69 | 0.161 | 0.896 | 0.901 |
| γ-IFN (pg/mL) | 87.93 | 77.69 | 14.03 | 0.028 | 0.703 | 0.684 |
| TNF-α (ng/L) | 370.49 | 324.79 | 57.49 | 0.018 | 0.953 | 0.772 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Wang, Y.; Wang, E.; Zhang, S.; Wang, Q.; Zhang, Y.; Wang, Y.; Cao, Z.; Yang, H.; Wang, W.; et al. Effects of Saccharomyces cerevisiae Culture on Ruminal Fermentation, Blood Metabolism, and Performance of High-Yield Dairy Cows. Animals 2021, 11, 2401. https://doi.org/10.3390/ani11082401
Sun X, Wang Y, Wang E, Zhang S, Wang Q, Zhang Y, Wang Y, Cao Z, Yang H, Wang W, et al. Effects of Saccharomyces cerevisiae Culture on Ruminal Fermentation, Blood Metabolism, and Performance of High-Yield Dairy Cows. Animals. 2021; 11(8):2401. https://doi.org/10.3390/ani11082401
Chicago/Turabian StyleSun, Xiaoge, Yue Wang, Erdan Wang, Shu Zhang, Qianqian Wang, Yan Zhang, Yajing Wang, Zhijun Cao, Hongjian Yang, Wei Wang, and et al. 2021. "Effects of Saccharomyces cerevisiae Culture on Ruminal Fermentation, Blood Metabolism, and Performance of High-Yield Dairy Cows" Animals 11, no. 8: 2401. https://doi.org/10.3390/ani11082401
APA StyleSun, X., Wang, Y., Wang, E., Zhang, S., Wang, Q., Zhang, Y., Wang, Y., Cao, Z., Yang, H., Wang, W., & Li, S. (2021). Effects of Saccharomyces cerevisiae Culture on Ruminal Fermentation, Blood Metabolism, and Performance of High-Yield Dairy Cows. Animals, 11(8), 2401. https://doi.org/10.3390/ani11082401