Amino Acids Supplementation for the Milk and Milk Protein Production of Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
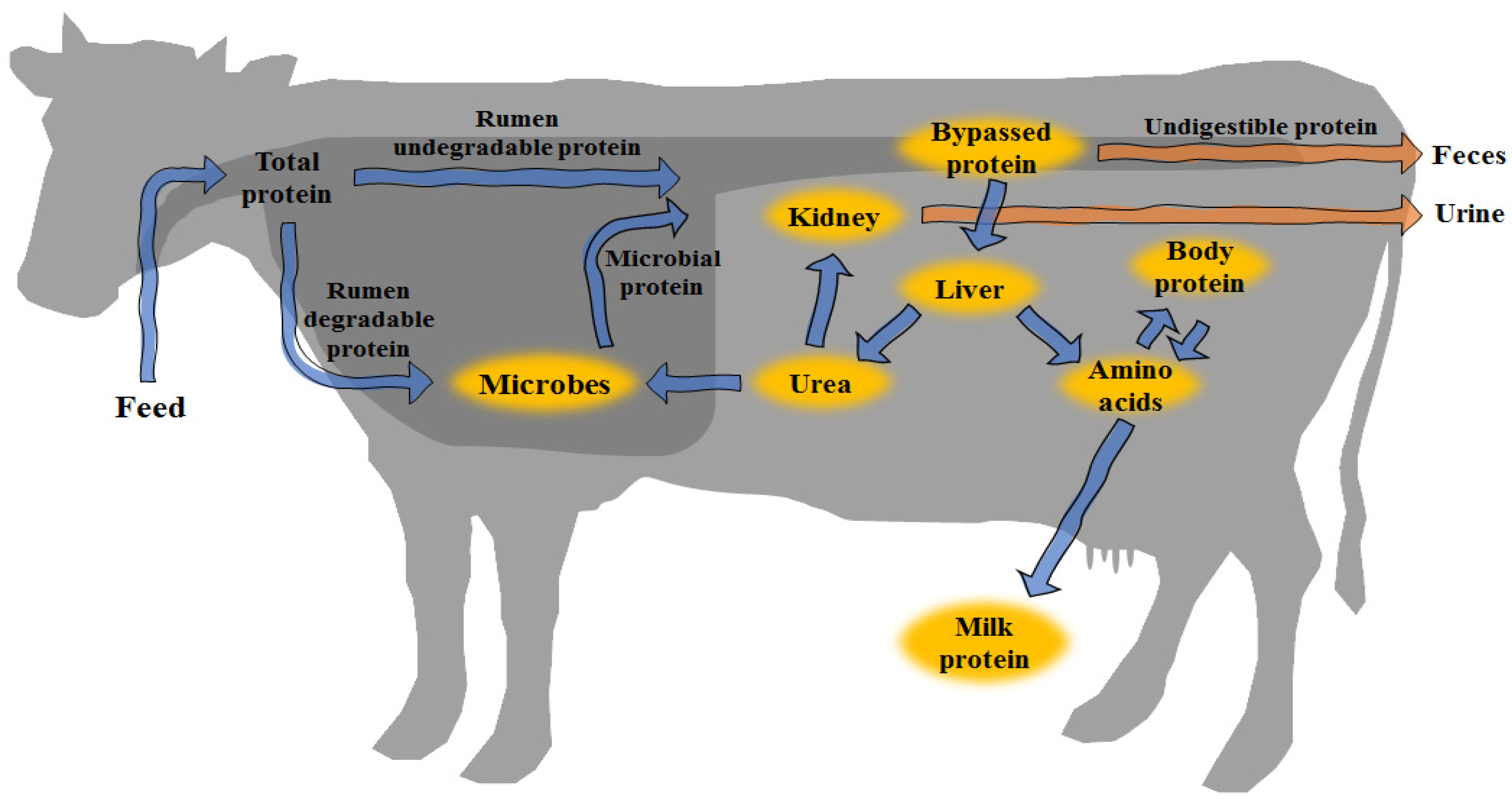
2. Protein Metabolism in Ruminants
3. Concept of AA Supplementation
3.1. Balancing AA
3.2. Limiting AA
3.3. Rumen-Protected AA
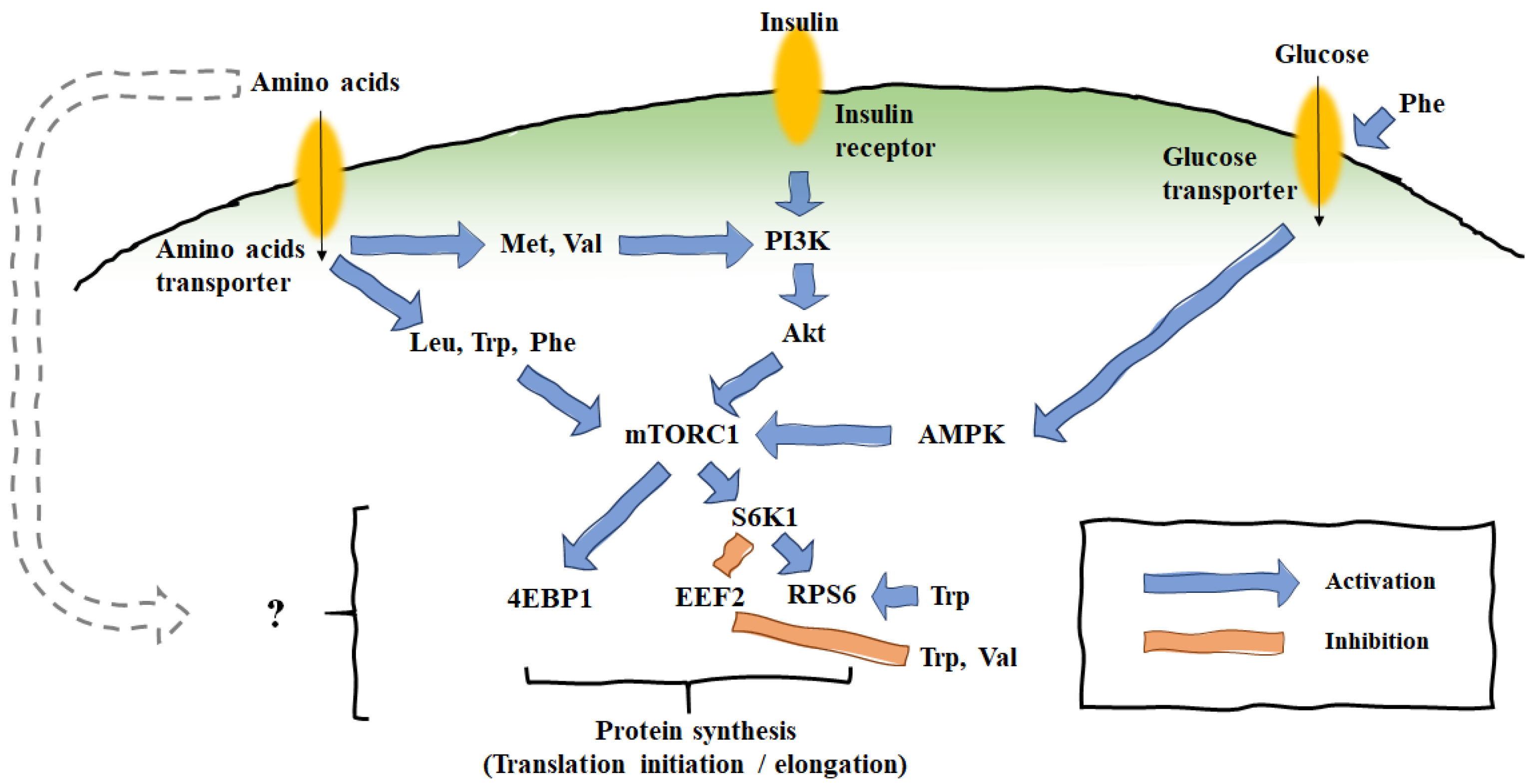
4. Effects of AA Supplementation on Mammary Translational Expression
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NRC. Nutrient Requirements of Dairy Cattle: 2001; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Schwab, C.G.; Broderick, G.A. A 100-Year Review: Protein and amino acid nutrition in dairy cows. J. Dairy Sci. 2017, 100, 10094–10112. [Google Scholar] [CrossRef]
- Gidlund, H.; Hetta, M.; Krizsan, S.J.; Lemosquet, S.; Huhtanen, P. Effects of soybean meal or canola meal on milk production and methane emissions in lactating dairy cows fed grass silage-based diets. J. Dairy Sci. 2015, 98, 8093–8106. [Google Scholar] [CrossRef]
- Metcalf, J.A.; Wray-Cahen, D.; Chettle, E.E.; Sutton, J.D.; Beever, D.E.; Crompton, L.A.; MacRae, J.C.; Bequette, B.J.; Backwell, F.R. The effect of dietary crude protein as protected soybean meal on mammary metabolism in the lactating dairy cow. J. Dairy Sci. 1996, 79, 603–611. [Google Scholar] [CrossRef]
- Korhonen, M.; Vanhatalo, A.; Huhtanen, P. Effect of protein source on amino acid supply, milk production, and metabolism of plasma nutrients in dairy cows fed grass silage. J. Dairy Sci. 2002, 85, 3336–3351. [Google Scholar] [CrossRef]
- Seymour, W.M.; Polan, C.E.; Herbein, J.H. Effects of dietary protein degradability and casein or amino acid infusions on production and plasma amino acids in dairy cows. J. Dairy Sci. 1990, 73, 735–748. [Google Scholar] [CrossRef]
- Raggio, G.; Lemosquet, S.; Lobley, G.E.; Rulquin, H.; Lapierre, H. Effect of casein and propionate supply on mammary protein metabolism in lactating dairy cows. J. Dairy Sci. 2006, 89, 4340–4351. [Google Scholar] [CrossRef]
- Danes, M.A.C.; Hanigan, M.D.; Arriola Apelo, S.I.; Dias, J.D.L.; Wattiaux, M.A.; Broderick, G.A. Post-ruminal supplies of glucose and casein, but not acetate, stimulate milk protein synthesis in dairy cows through differential effects on mammary metabolism. J. Dairy Sci. 2020, 103, 6218–6232. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, J.J.; Broderick, G.A. Effect of dietary crude protein concentration on milk production and nitrogen utilization in lactating dairy cows. J. Dairy Sci. 2006, 89, 1704–1712. [Google Scholar] [CrossRef]
- Castillo, A.; Kebreab, E.; Beever, D.; France, J. A review of efficiency of nitrogen utilisation in lactating dairy cows and its relationship with environmental pollution. J. Anim. Feed. Sci. 2000, 9, 1–32. [Google Scholar] [CrossRef]
- Kebreab, E.; France, J.; Beever, D.; Castillo, A. Nitrogen pollution by dairy cows and its mitigation by dietary manipulation. Nutr. Cycl. Agroecosyst. 2001, 60, 275–285. [Google Scholar] [CrossRef]
- Broderick, G.A.; Faciola, A.P.; Armentano, L.E. Replacing dietary soybean meal with canola meal improves production and efficiency of lactating dairy cows. J. Dairy Sci. 2015, 98, 5672–5687. [Google Scholar] [CrossRef]
- Lage, C.F.A.; Raisanen, S.E.; Stefenoni, H.; Melgar, A.; Chen, X.; Oh, J.; Fetter, M.E.; Kniffen, D.M.; Fabin, R.A.; Hristov, A.N. Lactational performance, enteric gas emissions, and plasma amino acid profile of dairy cows fed diets with soybean or canola meals included on an equal protein basis. J. Dairy Sci. 2021, 104, 3052–3066. [Google Scholar] [CrossRef]
- Ying, F.; Lin, X.; Ma, W.; Chi, H.; Yan, Z.; Song, Y.; Wang, Z. Metabolic responses to the deficiency of Lys, Arg, Met, or His in the mammary gland of lactating goats. Small Rumin. Res. 2013, 113, 219–230. [Google Scholar] [CrossRef]
- Weekes, T.L.; Luimes, P.H.; Cant, J.P. Responses to amino acid imbalances and deficiencies in lactating dairy cows. J. Dairy Sci. 2006, 89, 2177–2187. [Google Scholar] [CrossRef]
- Stahel, P.; Purdie, N.; Cant, J. Use of dietary feather meal to induce histidine deficiency or imbalance in dairy cows and effects on milk composition. J. Dairy Sci. 2014, 97, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.G.; Satter, L.; Clay, A. Response of lactating dairy cows to abomasal infusion of amino acids. J. Dairy Sci. 1976, 59, 1254–1270. [Google Scholar] [CrossRef]
- Schwab, C.G.; Bozak, C.K.; Whitehouse, N.L.; Mesbah, M.M. Amino acid limitation and flow to duodenum at four stages of lactation. 1. Sequence of lysine and methionine limitation. J. Dairy Sci. 1992, 75, 3486–3502. [Google Scholar] [CrossRef]
- Kim, C.H.; Choung, J.J.; Chamberlain, D.G. Determination of the first-limiting amino acid for milk production in dairy cows consuming a diet of grass silage and a cereal-based supplement containing feather meal. J. Sci. Food Agric. 1999, 79, 1703–1708. [Google Scholar] [CrossRef]
- Kim, C.H.; Choung, J.J.; Chamberlain, D.G. Variability in the ranking of the three most-limiting amino acids for milk protein production in dairy cows consuming grass silage and a cereal-based supplement containing feather meal. J. Sci. Food Agric. 2000, 80, 1386–1392. [Google Scholar] [CrossRef]
- Korhonen, M.; Vanhatalo, A.; Varvikko, T.; Huhtanen, P. Responses to graded postruminal doses of histidine in dairy cows fed grass silage diets. J. Dairy Sci. 2000, 83, 2596–2608. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Huhtanen, P.; Toivonen, V.; Varvikko, T. Response of dairy cows fed grass silage diets to abomasal infusions of histidine alone or in combinations with methionine and lysine. J. Dairy Sci. 1999, 82, 2674–2685. [Google Scholar] [CrossRef]
- Fisher, L. Response of lactating cows to the intravenous infusion of amino acids. Can. J. Anim. Sci. 1972, 52, 377–384. [Google Scholar] [CrossRef][Green Version]
- Sok, M.; Ouellet, D.R.; Firkins, J.L.; Pellerin, D.; Lapierre, H. Amino acid composition of rumen bacteria and protozoa in cattle. J. Dairy Sci. 2017, 100, 5241–5249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Mackle, T.R.; Dwyer, D.A.; Bauman, D.E. Effects of branched-chain amino acids and sodium caseinate on milk protein concentration and yield from dairy cows. J. Dairy Sci. 1999, 82, 161–171. [Google Scholar] [CrossRef]
- Korhonen, M.; Vanhatalo, A.; Huhtanen, P. Evaluation of isoleucine, leucine, and valine as a second-limiting amino acid for milk production in dairy cows fed grass silage diet. J. Dairy Sci. 2002, 85, 1533–1545. [Google Scholar] [CrossRef]
- Huhtanen, P.; Vanhatalo, A.; Varvikko, T. Effects of abomasal infusions of histidine, glucose, and leucine on milk production and plasma metabolites of dairy cows fed grass silage diets. J. Dairy Sci. 2002, 85, 204–216. [Google Scholar] [CrossRef]
- Appuhamy, J.; Knapp, J.; Becvar, O.; Escobar, J.; Hanigan, M. Effects of jugular-infused lysine, methionine, and branched-chain amino acids on milk protein synthesis in high-producing dairy cows. J. Dairy Sci. 2011, 94, 1952–1960. [Google Scholar] [CrossRef]
- Yoder, P.S.; Huang, X.; Teixeira, I.A.; Cant, J.P.; Hanigan, M.D. Effects of jugular infused methionine, lysine, and histidine as a group or leucine and isoleucine as a group on production and metabolism in lactating dairy cows. J. Dairy Sci. 2020, 103, 2387–2404. [Google Scholar] [CrossRef]
- Broderick, G.A.; Stevenson, M.J.; Patton, R.A.; Lobos, N.E.; Olmos Colmenero, J.J. Effect of supplementing rumen-protected methionine on production and nitrogen excretion in lactating dairy cows. J. Dairy Sci. 2008, 91, 1092–1102. [Google Scholar] [CrossRef]
- Tamura, T.; Inoue, K.; Nishiki, H.; Sakata, M.; Seki, M.; Koga, T.; Ookubo, Y.; Akutsu, K.; Sato, S.; Saitou, K.; et al. Effects of rumen-protected methionine on milk production in early lactation dairy cattle fed with a diet containing 14.5% crude protein. Anim. Sci. J. 2019, 90, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.; Swanepoel, N.; Shinzato, I.; Juchem, S. Productive responses of lactating dairy cattle to supplementing high levels of ruminally protected lysine using a rumen protection technology. Anim. Feed. Sci. Technol. 2011, 168, 30–41. [Google Scholar] [CrossRef]
- Paz, H.A.; de Veth, M.J.; Ordway, R.S.; Kononoff, P.J. Evaluation of rumen-protected lysine supplementation to lactating dairy cows consuming increasing amounts of distillers dried grains with solubles. J. Dairy Sci. 2013, 96, 7210–7222. [Google Scholar] [CrossRef] [PubMed]
- Armentano, L.E.; Bertics, S.J.; Ducharme, G.A. Response of lactating cows to methionine or methionine plus lysine added to high protein diets based on alfalfa and heated soybeans. J. Dairy Sci. 1997, 80, 1194–1199. [Google Scholar] [CrossRef]
- Socha, M.T.; Putnam, D.E.; Garthwaite, B.D.; Whitehouse, N.L.; Kierstead, N.A.; Schwab, C.G.; Ducharme, G.A.; Robert, J.C. Improving intestinal amino acid supply of pre- and postpartum dairy cows with rumen-protected methionine and lysine. J. Dairy Sci. 2005, 88, 1113–1126. [Google Scholar] [CrossRef]
- Třináctý, J.; Křížová, L.; Richter, M.; Černý, V.; Říha, J. Effect of rumen-protected methionine, lysine or both on milk production and plasma amino acids of high-yielding dairy cows. Czech J. Anim. Sci. 2009, 54, 239–248. [Google Scholar] [CrossRef]
- Patton, R.A. Effect of rumen-protected methionine on feed intake, milk production, true milk protein concentration, and true milk protein yield, and the factors that influence these effects: A meta-analysis. J. Dairy Sci. 2010, 93, 2105–2118. [Google Scholar] [CrossRef]
- Zang, Y.; Silva, L.H.P.; Ghelichkhan, M.; Miura, M.; Whitehouse, N.L.; Chizzotti, M.L.; Brito, A.F. Incremental amounts of rumen-protected histidine increase plasma and muscle histidine concentrations and milk protein yield in dairy cows fed a metabolizable protein-deficient diet. J. Dairy Sci. 2019, 102, 4138–4154. [Google Scholar] [CrossRef]
- Morris, D.L.; Kononoff, P.J. Effects of rumen-protected lysine and histidine on milk production and energy and nitrogen utilization in diets containing hydrolyzed feather meal fed to lactating Jersey cows. J. Dairy Sci. 2020, 103, 7110–7123. [Google Scholar] [CrossRef]
- Giallongo, F.; Harper, M.T.; Oh, J.; Lopes, J.C.; Lapierre, H.; Patton, R.A.; Parys, C.; Shinzato, I.; Hristov, A.N. Effects of rumen-protected methionine, lysine, and histidine on lactation performance of dairy cows. J. Dairy Sci. 2016, 99, 4437–4452. [Google Scholar] [CrossRef]
- Giallongo, F.; Hristov, A.N.; Oh, J.; Frederick, T.; Weeks, H.; Werner, J.; Lapierre, H.; Patton, R.A.; Gehman, A.; Parys, C. Effects of slow-release urea and rumen-protected methionine and histidine on performance of dairy cows. J. Dairy Sci. 2015, 98, 3292–3308. [Google Scholar] [CrossRef]
- Lee, C.; Hristov, A.N.; Cassidy, T.W.; Heyler, K.S.; Lapierre, H.; Varga, G.A.; de Veth, M.J.; Patton, R.A.; Parys, C. Rumen-protected lysine, methionine, and histidine increase milk protein yield in dairy cows fed a metabolizable protein-deficient diet. J. Dairy Sci. 2012, 95, 6042–6056. [Google Scholar] [CrossRef] [PubMed]
- Arriola Apelo, S.I.; Bell, A.L.; Estes, K.; Ropelewski, J.; de Veth, M.J.; Hanigan, M.D. Effects of reduced dietary protein and supplemental rumen-protected essential amino acids on the nitrogen efficiency of dairy cows. J. Dairy Sci. 2014, 97, 5688–5699. [Google Scholar] [CrossRef] [PubMed]
- Swanepoel, N.; Robinson, P.; Erasmus, L.J. Effects of ruminally protected methionine and/or phenylalanine on performance of high producing Holstein cows fed rations with very high levels of canola meal. Anim. Feed. Sci. Technol. 2015, 205, 10–22. [Google Scholar] [CrossRef][Green Version]
- Swanepoel, N.; Robinson, P.; Erasmus, L.J. Impacts of adding ruminally protected phenylalanine to rations containing high levels of canola meal on performance of high producing Holstein cows. Anim. Feed. Sci. Technol. 2016, 216, 108–120. [Google Scholar] [CrossRef][Green Version]
- Swanepoel, N.; Robinson, P.; Erasmus, L.J. Production responses of high producing Holstein cows to ruminally protected phenylalanine and tyrosine supplemented to diets containing high levels of canola meal. Anim. Feed. Sci. Technol. 2018, 243, 90–101. [Google Scholar] [CrossRef]
- Kollmann, M.T.; Locher, M.; Hirche, F.; Eder, K.; Meyer, H.H.; Bruckmaier, R.M. Effects of tryptophan supplementation on plasma tryptophan and related hormone levels in heifers and dairy cows. Domest. Anim. Endocrinol. 2008, 34, 14–24. [Google Scholar] [CrossRef]
- Robinson, P.; Swanepoel, N.; Evans, E. Effects of feeding a ruminally protected lysine product, with or without isoleucine, valine and histidine, to lactating dairy cows on their productive performance and plasma amino acid profiles. Anim. Feed. Sci. Technol. 2010, 161, 75–84. [Google Scholar] [CrossRef]
- Proud, C.G. mTOR-mediated regulation of translation factors by amino acids. Biochem. Biophys. Res. Commun. 2004, 313, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Proud, C.G. The mTOR pathway in the control of protein synthesis. Physiology 2006, 21, 362–369. [Google Scholar] [CrossRef]
- Kim, S.G.; Buel, G.R.; Blenis, J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol. Cells 2013, 35, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, C.; Yoshino, K.; Yonezawa, K. mTOR integrates amino acid- and energy-sensing pathways. Biochem. Biophys. Res. Commun. 2004, 313, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.R.; Jefferson, L.S. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J. Nutr. 2006, 136, 227S–231S. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H. Leucine and protein synthesis: mTOR and beyond. Nutr. Rev. 2007, 65, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Conejos, J.R.V.; Ghassemi Nejad, J.; Kim, J.-E.; Moon, J.-O.; Lee, J.-S.; Lee, H.-G. Supplementing with L-Tryptophan Increases Medium Protein and Alters Expression of Genes and Proteins Involved in Milk Protein Synthesis and Energy Metabolism in Bovine Mammary Cells. Int. J. Mol. Sci. 2021, 22, 2751. [Google Scholar] [CrossRef]
- Jeon, S.W.; Conejos, J.R.; Kim, J.; Kim, M.J.; Lee, J.E.; Lee, B.S.; Park, J.S.; Moon, J.O.; Lee, J.S.; Lee, H.G. Supplementing conjugated and non-conjugated L-methionine and acetate alters expression patterns of CSN2, proteins and metabolites related to protein synthesis in bovine mammary cells. J. Dairy Res. 2020, 87, 70–77. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.-E.; Lee, J.-S.; Park, J.-S.; Moon, J.-O.; Lee, H.-G. Phenylalanine and valine differentially stimulate milk protein synthetic and energy-mediated pathway in immortalized bovine mammary epithelial cells. J. Anim. Sci. Technol. 2020, 62, 263–275. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, S.; Zheng, N.; Zhang, Y.; Wang, S.; Zhou, X.; Wang, J. Combination of histidine, lysine, methionine, and leucine promotes β-casein synthesis via the mechanistic target of rapamycin signaling pathway in bovine mammary epithelial cells. J. Dairy Sci. 2017, 100, 7696–7709. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.G. Effects of L-Histidine and Sodium Acetate on beta-Casein Expression in Nutrient-Restricted Bovine Mammary Epithelial Cells. Animals 2021, 11, 1444. [Google Scholar] [CrossRef]
- Wang, M.; Xu, B.; Wang, H.; Bu, D.; Wang, J.; Loor, J.J. Effects of Arginine concentration on the in vitro expression of Casein and mTOR pathway related genes in mammary epithelial cells from dairy cattle. PLoS ONE 2014, 9, e95985. [Google Scholar] [CrossRef]
- Toerien, C.A.; Trout, D.R.; Cant, J.P. Nutritional stimulation of milk protein yield of cows is associated with changes in phosphorylation of mammary eukaryotic initiation factor 2 and ribosomal s6 kinase 1. J. Nutr. 2010, 140, 285–292. [Google Scholar] [CrossRef]
- Doelman, J.; Curtis, R.; Carson, M.; Kim, J.; Metcalf, J.; Cant, J. Essential amino acid infusions stimulate mammary expression of eukaryotic initiation factor 2Bε but milk protein yield is not increased during an imbalance. J. Dairy Sci. 2015, 98, 4499–4508. [Google Scholar] [CrossRef] [PubMed]
- Doelman, J.; Kim, J.J.; Carson, M.; Metcalf, J.A.; Cant, J.P. Branched-chain amino acid and lysine deficiencies exert different effects on mammary translational regulation. J. Dairy Sci. 2015, 98, 7846–7855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Wang, M.; Zhou, G.; Chen, L.; Ding, L.; Bu, D.; Loor, J. Arginine Supply Impacts the Expression of Candidate microRNA Controlling Milk Casein Yield in Bovine Mammary Tissue. Animals 2020, 10, 797. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-E.; Lee, H.-G. Amino Acids Supplementation for the Milk and Milk Protein Production of Dairy Cows. Animals 2021, 11, 2118. https://doi.org/10.3390/ani11072118
Kim J-E, Lee H-G. Amino Acids Supplementation for the Milk and Milk Protein Production of Dairy Cows. Animals. 2021; 11(7):2118. https://doi.org/10.3390/ani11072118
Chicago/Turabian StyleKim, Jung-Eun, and Hong-Gu Lee. 2021. "Amino Acids Supplementation for the Milk and Milk Protein Production of Dairy Cows" Animals 11, no. 7: 2118. https://doi.org/10.3390/ani11072118
APA StyleKim, J.-E., & Lee, H.-G. (2021). Amino Acids Supplementation for the Milk and Milk Protein Production of Dairy Cows. Animals, 11(7), 2118. https://doi.org/10.3390/ani11072118





